Abstract
We report here for the first time that the specific MAPK kinase (MEK) inhibitor, PD-98059, not only completely knocked out granulocyte-macrophage colony-stimulating factor (GM-CSF)- stimulated MAPK activity, but it also partially (~70%) inactivated the ribosomal kinase p70S6K. Since a connection between the two major signaling pathways, Ras/MEK/MAPK and PI3-K/p70S6K was suspected, experiments were designed to prove a molecular crosstalk between them. First, p70S6K protein was detected in anti-MAPK immunoprecipitates; MAPK protein was similarly co-immunoprecipitated with anti-p70S6K antibodies, indicating a close spatial proximity of both signaling molecules. Second, p70S6K enzymatic activity was found in anti-MAPK immunopcomplexes and, likewise, MAPK activity was present in anti-p70S6K immunoprecipitates, being the latter activity higher in samples derived from GM-CSF-treated cells than in untreated samples. Since an upstream activator of p70S6K, phosphatidylinositol (PI)3-kinase, has been associated with cell movement in phagocytic cells, we studied whether MAPK had a role in neutrophil migration. Our data showed that functional chemotaxis was partially inhibited by PD-98059, as well as by rapamycin, a specific inhibitor of mTOR (upstream of p70S6K), and almost totally (~90%) by a combination of the two. In summary, a molecular connection between the MAPK and the p70S6K pathways exists, with the former exerting a positive feedback on the latter upon GM-CSF stimulation, and this leads to non-proliferative responses such as chemotaxis.
Keywords: p70S6K ribosomal kinase, MAP kinase, chemotaxis, cell signaling, GM-CSF, neutrophils
INTRODUCTION
Ribosomal p70 S6 kinase (p70S6K) was first identified as an enzyme that catalyzes the phosphorylation of the S6 protein [1–3], a component of the 40S subunit of eukaryotic ribosomes, and plays a role in the control of translation of mRNAs that possess a polypyrimidine tract at their 5′ ends [4] and in protein synthesis [5]. p70S6K was described as necessary for the progression through the cell cycle, specifically for the G1 -> S transition [6]. IL-3, EPO, and IL-2-induced activation of p70S6K [7–9], as well as cellular proliferation are inhibited with rapamycin, an immunosuppressant drug that targets a rapamycin associated protein (FRAP/mTOR) and dephosphorylates p70S6K [3,10]. However, the finding of a cell line that proliferates in the presence of rapamycin suggests the existence of p70S6K-independent pathways of cell cycle progression [5,6,8].
Ribosomal p70S6K has at least seven sites of phosphorylation, the most critical residues being Thr229, Thr389, Thr421 and Ser424, residing in the acidic carbonyl terminus, as well as within the central catalytic domain [3]. Thr389 is phosphorylated by FRAP/mTOR that is strongly inhibited by rapamycin. Upstream phosphorylation of p70 S6 kinase involve a complex network of effector molecules. The earliest known regulatory events involves the activation of phosphatidylinositol (PI)-3 kinase (PI3K) which phosphorylates PI and similar molecules at the D-3 position of the inositol ring, thus releasing two messengers: phosphatidylinositol-3,4 bis-phosphate (PI-3,4-P2) and phosphatidylinositol-3,4,5 triphosphate (PI-3,4,5-P3) [11]. These signals cause the recruitment of the phosphoinositide-dependent protein kinase (PDK1) that phosphorylates the serine/threonine kinase protein kinase B (PKB)/Akt [12], that in some cell lines activates p70S6K [13], or directly phosphorylates the catalytic domain of p70S6K at its Thr229 residue causing its activation [14].
The cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF) is particularly effective in priming neutrophil functionality. The GM-CSF receptor is linked to cytosolic tyrosine kinases that are involved in activation of intracellular signal transduction [15]. This involves, among others, phosphorylation and activation of the mitogen-activated protein (MAP) kinases [16] (p42mapk or ERK2 and p38-MAPK). Although phosphorylation of p90rsk, a known MAPK substrates, is known to occur, little information exists regarding other ribosomal kinases in neutrophil GM-CSF signaling, particularly PI3-kinase and p70S6K.
We have recently demonstrated [17] that p42-MAPK is involved in hematopoietic differentiation of leukemic cells and it is also highly active in terminally differentiated neutrophil migration in response to GM-CSF and the chemoattractant IL-8. We undertook this study to provide an insight into the mechanism as to why GM-CSF is more effective in activating p42-MAPK in mature cells. We report here for the first time an upregulation of the ribosomal p70S6K activity in response to GM-CSF and provide evidence for a connection between the MAPK and the p70S6K pathways leading to non-proliferative responses such as chemotaxis.
MATERIALS AND METHODS
Materials and antibodies
GM-CSF was from R&D Systems (Minneapolis, MN); PMA, PKA inhibitor, calphostin, anti-mouse IgG (agarose beads); electrophoresis chemicals were from Bio-Rad Laboratories (Richmond, CA); [γ-32P]ATP (30 Ci/mmol) was from Amersham Pharmacia Biotech (Arlington Heights, IL); PD-98059 was from BioMol (Plymouth Meeting, PA); rapamycin was from Calbiochem (San Diego, CA); ion-exchange chromatography cellulose phosphate paper was from Whatman (Hillsboro, OR); “Transwell” 5-μm polycarbonate membrane dishes were from from Costar (Cambridge, MA); anti-p70S6 kinase (H-9) was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA); anti-p42 MAPK was from Zymed Laboratories Inc. (San Francisco, CA), anti-PY (PY20 clone), MAPK peptide substrate APRTPGGRR, p70S6 kinase peptide substrate KKRNRTLTK, MAPK (Erk2), active, purified full-length recombinant (214 units/mg) and rat p70S6K partially purified enzyme (20 units/ml) were from Upstate Biotechnology Inc. (Lake Placid, NY).
Cells
Peripheral blood neutrophils were isolated as previously described [18]. Between 50–55 ml of blood were collected from the antecubital vein of healthy individuals (who signed an Institutional Review Board-approved consent form) using sodium citrate as anticoagulant. Blood was mixed with 15 ml of 6% dextran, allowed to settle, and the plasma and buffy coat were removed and spun down at 800xg for 5 min. The pellet was resuspended in 35 ml of saline and centrifuged again for 15 minutes at 10°C in a Ficoll-Histopaque discontinous gradient. Neutrophils were recovered and contaminating erythrocytes were lysed by hypotonic shock. Cells were washed and the pellet was resuspended in Hanks Balanced Salt Solution (HBSS). Our experience has indicated that using this protocol, neutrophil aggregation (i.e., the hallmark for neutrophil activation) does not occur. Viability is usually >98% as per trypan blue exclusion. Cells were resuspended at the concentration of 1.0 × 107 cells/ml in fresh Hanks Balanced Salt Solution (HBSS) at the time of the experiment.
Preparation of cell extracts, immunoprecipitation and Western blotting
Cells were stimulated with GM-CSF and lysed in lysis buffer (12 mM Tris-HCl, pH 7.2, 0.75 mM NaCl, 100 μM sodium orthovanadate, 10 mM phenylmethylsulfonyl fluoride, 5μg/ml each of aprotinin, pepstatin A and leupeptin, and 0.12% Triton X-100). Immunoprecipitation was carried out as reported previously [19]. The immunoprecipitation efficiency of antibodies was monitored by Western blotting of the immunoprecipitates probed with the same antibody used in the immunoprecipitation step. Immune complexes were resuspended in a final volume of 30 μl of lysis buffer supplemented with 10% glycerol.
Ribosomal p70S6 kinase assays
In immunocomplex kinase assay, cell lysates were immunoprecipitated with specific anti-p70S6K antibody (10 μg/ml) as indicated above. The phosphoacceptor peptide substrate was 150 μM of the S6 kinase substrate peptide KKRNRTLTK, prepared in freshly prepared kinase buffer (13.4 mM HEPES, pH 7.3, 25 mM MgCl2, 30 μM Na2VO3, 5 mM p-nitrophenyl phosphate, 2 mM EGTA, 2 μM cAMP-dependent kinase inhibitor TTYADFIASGRTGRRNAIHD, 21μCi of [γ-32P]ATP/ml (7 nM), and 68 μM unlabeled ATP). One μg of cAMP-dependent kinase inhibitor inhibits 2,000–6,000 phosphorylating units of PKA (equivalent to the transference of 2–6 nmol of phosphate from ATP). To initiate the phosphotransferase reaction, aliquots (20 μl) of kinase buffer containing the appropriate substrates were mixed 1:1 (v/v) with the cell lysates or immunocomplex beads. The reaction was carried out at room temperature for 10 min and terminated by blotting 20 μl of the reaction mixture onto P81 ion exchange chromatography cellulose phosphate papers. Filter squares were washed, dried, and counted for radioactivity. In some experiments, the purified, active enzymes (p70S6K and MAPK) were used as positive controls. For these experiments 0.1 units (1 unit = 1 nmol of phosphate incorporated into their respective substrates per minute) of p70S6K partially purified enzyme or p42-MAPK, purified, full-length recombinant Erk2 and mixed with [γ-32P]ATP as indicated above.
Chemotaxis in vivo functional assay
After incubation with inhibitors, neutrophils (5×105) in chemotaxis buffer (Hanks + 1mM CaCl2, 1 mM MgCl2 and 0.1% BSA) were placed on the upper chambers of 6.5-mm “Transwell” dishes that are separated from the lower chambers by a 5-μm pore Nucleopore polycarbonate membrane. IL-8 was added in 0.6 ml chemotaxis buffer to the lower chamber. The dishes were incubated for 1.5 hours at 37°C under a 5% CO2 atmosphere and aliquots of the cells that have migrated to the lower chambers were counted on a microscope using a hemocytometer for cell number. The rest of the cells in the bottom wells were harvested, lysed and for the measurement of p70S6K kinase assay in vitro as indicated above. Viability at the end of the migration assay in both “Transwell” chambers remained >97±2%, even in the presence of MEK inhibitor or rapamycin, ruling out a toxic effect. Maintenance of the experiment for 3 hours increased proportionally the number of migrating cells but viability decreased to >75±3%.
RESULTS
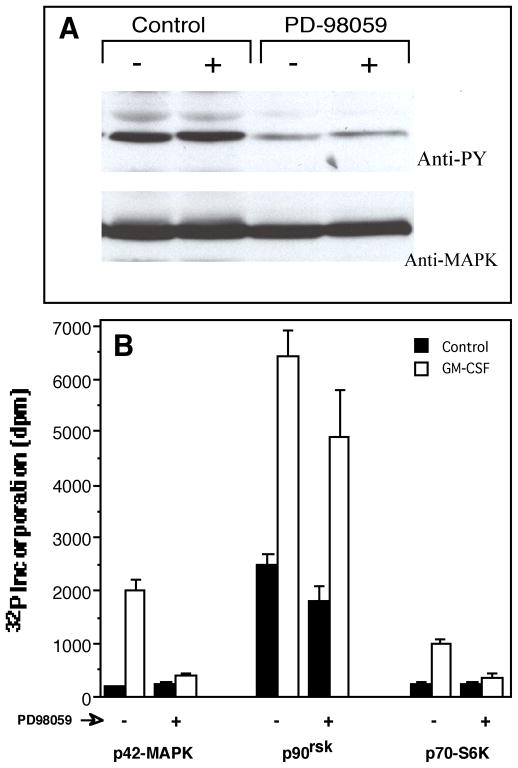
While studying the effect of the MAPK kinase (MEK) inhibitor PD-98059 on MAPK and p90rsk kinases in myelocytic cell lines expressing the neutrophilic phenotype, we observed that the inhibitor had effects on cell signaling other than those described for the Ras/MEK pathway. As known, PD-98059 inhibits MAPK tyrosyl phosphorylation, and, accordingly, we have observed a profound inhibitory effect in differentiated HL-60 cells (Fig. 1A). Further, PD-98059 inhibited ~90% of MAPK enzymatic activity and, partially (~50%), that of its substrate p90rsk (Fig. 1B). Remarkably, the MEK inhibitor PD-98059 had a collateral effect on p70S6K activity, where it inhibited ~70% of its enzymatic activity. This effect was not a result of cell death since viability assays were routinely conducted with 0.4% Trypan Blue Stain in cells treated with the MEK inhibitor and were 94 ± 3%. The observed effect of PD-98059 on p70S6K was the first evidence indicative of a crosstalk between the Ras/MAPK and the PI3-K/p70S6K pathways.
Figure 1. Inhibition of p70S6K activity by the MEK inhibitor PD-98059.
(A) Neutrophil phenotype-expressing cells dHL-60 (HL-60 cells continuously cultured in the presence of 1.25% DMSO for three days) were incubated with 25 μM PD-98059 for 30 minutes, followed by a short (5 min.) incubation with (+) or without (−) 270 pM GM-CSF and cell lysates were generated. Resulting proteins were analyzed by Western blotting with anti-phosphotyrosine antibodies (PY) and subsequently by anti-MAPK to confirm that equal amount of protein was loaded in each lane. (B) dHL-60 cells were incubated with 25 μM PD-98059 for 30 minutes and cell lysates were generated as in (A). These were split into three sets that were used for in vitro kinase assays against MAPK peptide substrate APRTPGGRR (left group of bars); p90rsk peptide substrate RRLSSLRA (middle group of bars); and p70S6K peptide substrate KKRNRTLTK (right group of bars). Results are the mean ± SE of four independent experiments performed in duplicate.
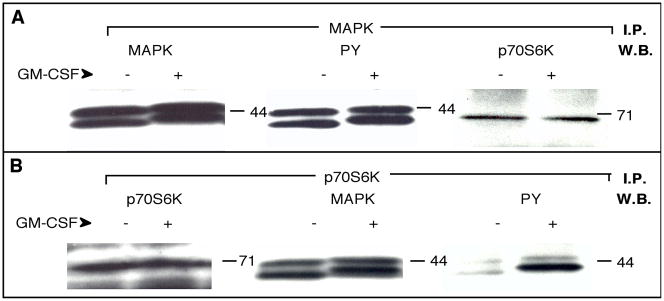
To explore this point in depth, we conducted MAPK immunoprecipitation of neutrophil lysates derived from control and GM-CSF-treated cells. MAPK was readily detectable in Western blots derived from these immunoprecipitates (Fig. 2A, left panel). When these blots were stripped and reprobed with anti-PY antibodies, a significant change in phosphotyrosine (seen as a mobility shift) could be observed at 3 minutes and an even greater change at 5 minutes of GM-CSF stimulation (Fig. 2A, middle panel). Although this has been documented elsewhere, we report here for the first time that p70S6K could be co-immunoprecipitated with anti-p42-MAPK antibodies (Fig. 2A, right panel). To completely confirm the molecular association between p70S6K and MAPK, we performed the converse experiment, i.e., immunoprecipitation with anti-p70S6K and immunoblotting with anti-MAPK. As seen in Fig. 2B, p70S6K protein was brought down with the antibodies (left panel) but so was MAPK (middle). Note also that the activity of MAPK was intact throughout the experiment, since samples derived from GM-CSF-stimulated cells had a higher level of phosphotyrosine than controls (right panels).
Figure 2. Co-immunoprecipitation of p70S6K and MAPK proteins.
(A) Neutrophils (1×107 cells/ml) were incubated in the presence (+) or the absence (−) of 300 pM GM-CSF for 5 minutes. Lysates were immunoprecipitated (I.P.) with anti-MAPK (ERK1+2) antibodies and Western blots (W.B.) derived from immunocomplex beads were subjected to probing with anti-MAPK (left panel) followed by stripping of the antibody and re-probing with anti-PY (middle panel). Shown in both cases are the regions of the blot around the 44 kDa protein marker. Finally, the blot was stripped and re-probed with anti-p70S6K antibodies (right panel, showing the region of the blot around the 71 kDa marker). (B) Neutrophils immunoprecipitated with anti-p70S6K (C-18) antibodies followed by Western blotting with the same antibody (left panel, showing the region of the blot around the 71 kDa protein marker) and subsequent re-probing with anti-MAPK (middle panel, showing the 44-kDa region of the blot). Finally, the blot was probed again with anti-PY (PY20) (right panel, shown again is the region ~ 44 kDa). Results are typical among three other experiments.
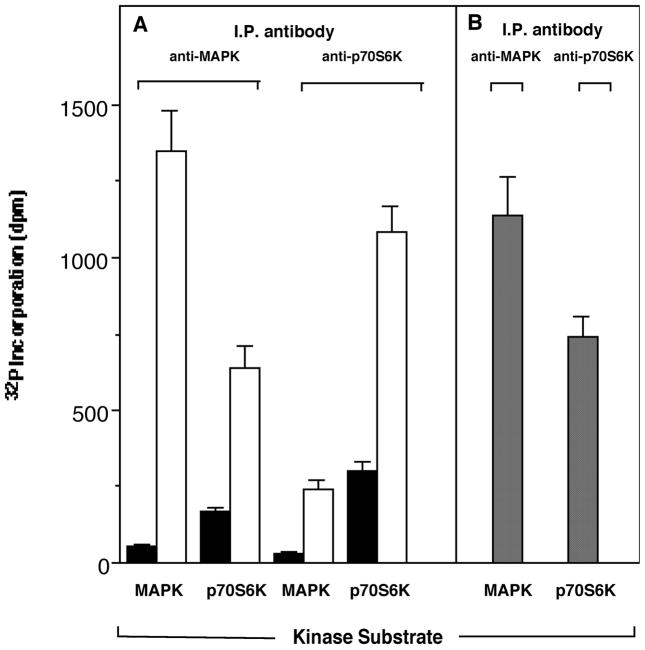
The presence of p70S6K and MAPK in its phospho form in co-immunoprecipitates begged the question as to their enzymatic activity. Therefore we next measured kinase activity in immunocomplexes. As seen in Fig. 3, activity of the enzyme followed a pattern similar to that discussed above with the presence of proteins. The activity brought down by the antibodies was substantial in these experiments, and at equivalent levels of pure, active enzymes included in the assays as positive controls (Fig. 3, right panel). These data indicate that MAPK pathway provides a positive feedback on p70S6K in GM-CSF-stimulated cells.
Figure 3. Co-immunoprecipitation of p70S6K and MAPK enzymatic activities.
(A) Neutrophil suspensions (1×107 cells/ml) were either untreated (solid bars) or stimulated with GM-CSF (empty bars). Cells lysates were prepared from those cells and divided into two sets. The first was immunoprecipitated (“I.P. antibody”) with anti-MAPK (ERK2) and the second with anti-p70S6K antibody. Immunocomplex beads from both sets were assayed for kinase activity against a “Kinase Substrate”: either MAPK peptide substrate (APRTPGGRR) or p70S6K peptide substrate (KKRNRTLTK). (B) Same as above only this time the starting material was recombinant pure enzyme instead of cell lysates (specific activities: p70S6K = 20 units/ml, MAPK = 214 units/mg).
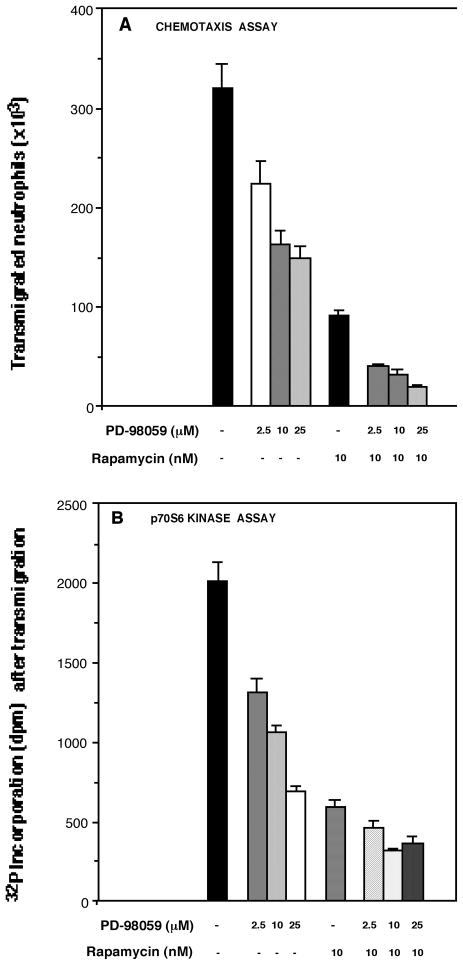
To further investigate if the observed effects on p70S6K had any bearing on the functionality of mature neutrophils, we tested the specific p70S6K inhibitor, rapamycin, on their ability to suppress chemotaxis. To this, we used a “Transwell” setting (see material and methods) intended to measure neutrophil functional chemotaxis in vivo. Rapamycin, a specific inhibitor of the mammalian target of rapamycin (mTOR/FRAP) effectively (>66%) blocked the migration of GM-CSF-primed neutrophils towards IL-8 (Fig. 4A), indicating a strong role of p70S6K/mTOR in neutrophil chemotaxis. Further, we found that the MEK inhibitor, PD-98059 was also effective in inhibiting neutrophil migration due to GM-CSF/IL-8 (Fig. 4A). Finally, a combination of the two inhibitors virtually abolished (~90%) cell migration (Fig. 4A), pointing again to a crossinteraction between the two major signaling pathways. We also harvested neutrophil cells once they had migrated to the lower chamber of the “Transwell” device after 1.5 hours. The status of the p70S6K enzymatic activity (normalizing for same amount of protein) was tested on those migrated cells, and is shown in Fig. 4B. A correlation of kinase activity with migration was observed (compare Fig. 4A and 4B): those cells that had impaired migration due to pretreatment with the inhibitors had also a diminished intrinsic activity once migration had occured.
Figure 4. The effect of MEK and p70S6K inhibition on chemotaxis and on enzyme activity after chemotaxis.
(A) Neutrophils were resuspended in chemotaxis buffer (see Methods) and preincubated with the indicated concentrations of the MEK inhibitor PD-98059, rapamycin, or both, for 20 min each at 25°C. Cells were then primed with 1 nM GM-CSF for 15 min and placed on the upper chambers of 6.5-mm “Transwell” dishes at the density of 5×105 per well. The lower chambers contained 50 nM IL-8 as the chemoattractant. The dishes were incubated for 1.5 hours at 37°C to allow cells to migrate to the lower dishes. Aliquots from those were taken out and counted on a microscope using a hemocytometer. Shown is the mean of two independent experiments in duplicate. (B) Once the cells had migrated to the lower dishes, they were also harvested, lysed and processed for p70S6K in vitro enzymatic assays as described earlier. Protein was measured in cells extracts and normalized so all samples contained the same amount of protein regardless of number of cells encountered in wells. Further, kinase assay controls were run in parallel with no substrate peptide added to the reaction mixture and radioactivity was subtracted from test samples with the p70S6K peptide substrate.
DISCUSSION
Our data support the idea of molecular crosstalk between PI3-K/PKB(Akt)/p70S6K and the Ras/Raf/ERK pathways in mature cells. PI3K activity is activated by GM-CSF in a Jak-2-dependent manner [20]. One example of cooperation between the PI3-K/PKB(Akt)/p70S6K pathway and the ERK signaling cascades is that the MAPK cascade shortens the duration of PKB activation and glycogen synthase kinase inhibition [21]. Further, changes in the balance between the PI3-K pathway and the ERK and p38-MAPK pathways could determine particular phenotypes of visceral and vascular cells [22]. It is only now that we are beginning to understand how multiple cooperating pathways mediate the effects of Ras on progression through the cell cycle [23] and in principle, whatever is found in exponentially growing cells might or might not be applicable to others, like mitosis-lacking, fully mature cells.
In addressing the molecular mechanism of chemotaxis, the data presented here have indicated that the specific MAPK kinase (MEK) inhibitor, PD-98059, partially inactivates p70S6K. This came as a surprise since PD-98059 has traditionally been considered a specific inhibitor of MEK. It inhibits phosphorylation activation of MEK1 by upstream kinases, while it is a poor inhibitor of MEK once it is activated (phospho form) or the constitutively active MEK1 mutant [24]. PD-98059 is an inefficient inhibitor of MEK2 [24,25]. Thus, our data point to the direction of a crosstalk between MEK and p70S6K pathways.
Since an inhibition of MAPK leads to partial inhibition of the p70S6K response, a link should exist between the two signaling pathways. In order to propose that p70S6K (or other members of its pathway, namely PDK1 or PI3-K) are substrates for MAPK action, first a putative MAP kinase phosphorylation site must be present in the former molecule (Table I). The table depicts several amino acid sequences in ribosomal p70S6Ks matching a phosphorylation motif recognized by MAPK. We found 13, 15 and 17 putative sites that MAPK could potentially phosphorylate in p70S6Kβ, p70S6Kα1 and p70S6Kα2, respectively. For comparison, p90rsk, a known true substrate of MAPK had 12 phosphorylation sites.
Table I. Putative phosphorylation sites on human ribosomal kinases.
Listed are consensus sites of MAPK (at zero mismatches) [ST]-x(3,5)-P found in p70S6Kβ Homo sapiens (NP_003943), p70S6Kα1 H. sapiens (189508), p70S6Kα2 H. sapiens (189510) and p90rsk H. sapiens (2008108A). MAPK phosphorylates Ser/Thr residues that are followed by Pro which is 3–5 amino acids downstream [34]. A search using NetPhos 2.0 found Ser and Thr phosphorylation sites in several human ribosomal kinases, and those that matched the consensus [ST]-x(3,5)-P were selected for inclusion in the table.
| p70S6Kα1 | p70S6Kα2 | p70S6Kβ | p90rsk |
|---|---|---|---|
| 77, TSVNRGP | 54, TSVNRGP | 67, SVNVGP | 54, SEKADP |
| 256, TIEYMAP | 233, TIEYMAP | 245, TIEYMAP | 225, TVEYMAP |
| 288, TGAPP | 265, TGAPP | 277, TGSPP | 335, TPPFKP |
| 387, TRQTP | 311, SRLGAGP | 376, TRQTP | 359, TPKDSP |
| 390. TPVDSP | 364, TRQTP | 379, TPVDSP | 363, SPGIP |
| 412, TYVAP | 367, TPVDSP | 401, TYVAP | 452, SKRDP |
| 441, SPRTP | 389, TYVAP | 436, SPLKFSP | 577, TANFVAP |
| 444, TPVSP | 418, SPRTP | 449, SPSLP | 612, TPFANGP |
| 447, SPVKFSP | 421, TPVSP | 451, SLPEP | 676, TQKDKLP |
| 462, SASTANP | 424, SPVKFSP | 456, TELP | 707, SSKPTP |
| 464, STANP | 439, SASTANP | 469, STTAP | 708, SKPTP |
| 465, TANPQTP | 441, STANP | 470, TTAPL | 711, TPQLKP |
| 470, TPVEYP | 442, TANPQTP | 471, TAPLP | |
| 489, SGEASAP | 447, TPVEYP | ||
| 493, SAPLP | 466, SGEASAP | ||
| 470, SAPLP | |||
| 492, SKRP | |||
It is also noteworthy to point out that the greatest abundance of phosphorylation sites was observed in all C-termini, clustered around Pro-rich regions. As an illustration, p70S6Kβ had the stretch 449SPSLPEPTELPLPPLLPPPPSTTAPLPIRPP in the C-end. Also, the ExPaSy ScanProsite program indicated that most of the [ST]-x(3,5)-P sites considered are not located in the protein kinase ATP binding or active-site signatures, indicating that phosphorylation, if present, would occur in an allosteric site that could potentially alter the activity of the substrate (p70S6K).
As indicated above, the data shown in this report indicates that MAPK exerts a positive feedback on p70S6K leading to an increased chemotactic response in neutrophils. Chemotaxis is a crucial physiological event in the leukocyte’s mission of fighting off bacteria. It is known that chemotaxis and cell motility are heavily dependent on activation of the phosphatidylinositol-(PI)-3-kinase (PI3-K)/PKB/p70S6K pathway. There are conflicting reports on the interaction between PI3-Kinase and Ras/MAPK pathways. Integrin-mediated adhesion is followed by an increase of PI3-K leading to activation of Raf-1, Mek-1 and Erk-2 [26]. Similarly, PDK1 homologues in yeast activate the Pkc1-MAPK pathway [27] and PI 3-K has been implicated in gastrin-mediated MAPK signaling upstream of Shc phosphorylation [28].
Conversely, Akt activation inhibits the Raf-MEK-ERK pathway in differentiated myotubes but not in myoblasts [29] and a Raf phosphorylation at a highly conserved serine residue in its regulatory domain by Akt inhibits the Raf-MEK-ERK signaling pathway in the human breast cancer line HEK293 [30]. As for an interaction directly involving p70S6K, early reports [31] indicated that ERK/MAPK phosphorylates recombinant p70S6K in vitro, although that did not lead to an increase in enzymatic activity suggesting that in vivo additional help from other kinases might be needed to fully activate p70S6K. Nitric oxide donors enhance thrombin-mediated activation of p70S6K and this effect is associated with cellular motility [32]. The same group has shown that p70S6K colocalizes with actin stress fibers and that p70S6K is present in the actin arc, a structure found near the forward edge of migrating 3T3 fibroblasts [33]. Also, S6K1 was shown to be necessary for proliferation in fibroblast growth factor (FGF-2) treated SCLC cancer cells and additional stimulation of MEK pathway correlated with activation of SK2, a recently described homologue of SK1 [34].
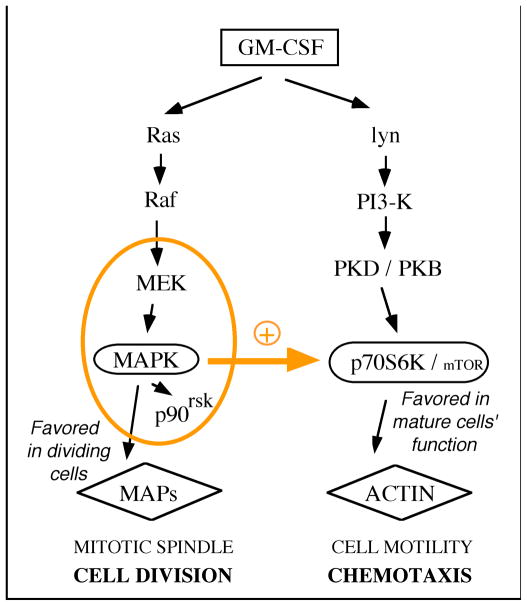
Based on the data described here, we propose the model presented in Fig. 5. The figure indicates that MAPK exerts a positive feedback on p70S6K/mTOR in terminally differentiated cells, such as the neutrophil. Upon commitment to differentiation of myeloid cells, the normal transduction pathway is altered in a way that allows the cell to fully respond to an external signal, when most needed to react to acute signals (e.g., fMet-Leu-Phe from bacteria or GM-CSF from host) during chemotaxis towards the infection site.
Figure 5. A differentiation-stage dependency of GM-CSF cell signaling model.
GM-CSF activates the Ras/MAPK and the PI3-K/p70S6K pathways in hematopoietic cells, but at different rates. In rapidly dividing cells (e.g., myeloid progenitors, leukemic cells) the former pathway is favored, while in mature cells (e.g., neutrophils) the latter pathway is more relevant. According to the data presented here, the MAPK pathway exerts a positive feedback on the p70S6K pathway, leading to an increased chemotaxis response in terminally differentiated cells. This molecular crosstalk becomes important in cells incapable of mitotic division (such as the neutrophil).
Acknowledgments
The authors thank Dr. Cassandra C. Paul for help setting up the migration experiments. This work has been supported in part by the National Institutes of Health grant HL056653 and American Heart Association (National program) 0250417N (J.G.-C.).
Abbreviations
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- MAPK
mitogen-activated protein kinase
- MEK
MAPK kinase
- p70S6K
ribosomal p70 S6 kinase
- mTOR/FRAP
mammalian target of rapamycin
- PI3K
phosphatidyl-inositol (PI)-3 kinase
References
- 1.Nemenoff RA, Price DJ, Mendelsohn MJ, Carter EA, Avruch J. An S6 kinase activated during liver regeneration is related to the insulin-stimulated S6 kinase in H4 hepatoma cells. J Biol Chem. 1988;263:19455–19460. [PubMed] [Google Scholar]
- 2.Proud CG. p70 S6 kinase: an enigma with variations. Trends Biochem Sci. 1996;21:181–185. [PubMed] [Google Scholar]
- 3.Pullen N, Thomas G. The modular phosphorylation and activation of p70S6K. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 4.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′ TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawasome H, Papst P, Webb S, Keller GM, Johnson GL, Gelfand EW. Targeted disruption of p70(s6k) defines its role in protein synthesis and rapamycin sensitivity. Proc Natl Acad Sci USA. 1998;95:5033–5038. doi: 10.1073/pnas.95.9.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheid MP, Charlton L, Pelech SL, Duronio V. Role of p70 S6 kinase in cytokine-regulated hematopoietic cell survival. Biochem Cell Biol. 1996;74:595–600. doi: 10.1139/o96-064. [DOI] [PubMed] [Google Scholar]
- 7.Calvo V, Crews CM, Vik TA, Bierer BE. Interleukin 2 stimulation of p70 S6 kinase activity is inhibited by the immunosuppressant rapamycin. Proc Natl Acad Sci USA. 1992;89:7571–7575. doi: 10.1073/pnas.89.16.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvo V, Wood M, Gjerston C, Vik T, Bierer BE. Activation of 70-kDa S6 kinase, induced by the cytokines interleukin-3 and erythropoietin and inhibited by rapamycin, is not an absolute requirement for cell proliferation. Eur J Immunol. 1994;24:2664–2671. doi: 10.1002/eji.1830241115. [DOI] [PubMed] [Google Scholar]
- 9.Reif K, Burgering BMT, Kantrell A. Phosphotidylinositol 3-kinase links the interleukin-2 receptor to protein kinase B and p70 S6 kinase. J Biol Chem. 1997;272:14426–14433. doi: 10.1074/jbc.272.22.14426. [DOI] [PubMed] [Google Scholar]
- 10.Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schreiber SL. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377:441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 11.Fukui Y, Ihara S, Nagata S. Downstream of phosphotidylinositol-3 kinase, a multifunctional signaling molecule, and its regulation in cell responses. J Biochem. 1998;124:1–7. doi: 10.1093/oxfordjournals.jbchem.a022067. [DOI] [PubMed] [Google Scholar]
- 12.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB. 3-phosphoinositide-dependent protein kinase-1 (PDK-1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 13.Conus NM, Hemmings BA, Pearson RB. Differential regulation by calcium reveals distinct signaling requirements for the activation of Akt and p70S6k. J Biol Chem. 1998;273:4776–4782. doi: 10.1074/jbc.273.8.4776. [DOI] [PubMed] [Google Scholar]
- 14.Pullen N, Dennis PB, Andjelkovic M, Duffner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 15.Downey GP, Butler JR, Brumell J, Borregaard N, Kjeldsen L, Sue-A-Quan AK, Grinstein S. Chemotactic peptide-induced activation of MEK-2, the predominant isoform in human neutrophils. Inhibition by wortmannin. J Biol Chem. 1996;271:21005–21011. doi: 10.1074/jbc.271.35.21005. [DOI] [PubMed] [Google Scholar]
- 16.Zu YL, Qi J, Gilchrist A, Fernandez GA, Vazquez-Abad D, Kreutzer DL, Huang CK, Sha’afi RI. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-α or FMLP stimulation. J Immunol. 1998;160:1982–1989. [PubMed] [Google Scholar]
- 17.Lehman JA, Paul CC, Baumann MA, Gomez-Cambronero J. MAP kinase upregulation after hematopoietic differentiation: role of chemotaxis. Am J Physiol Cell Physiol. 2001;280:183–191. doi: 10.1152/ajpcell.2001.280.1.C183. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Cambronero J, Huang CK, Gomez-Cambronero TM, Waterman WH, Becker EL, Sha’afi RI. Granulocyte-macrophage colony-stimulating factor-induced protein tyrosine phosphorylation of MAP Kinase in human neutrophils. Proc Natl Acad Sci USA. 1992;89:7551–7555. doi: 10.1073/pnas.89.16.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph D, Paul CC, Baumann MA, Gomez-Cambronero J. S6 kinase p90rsk in GM-CSF-stimulated proliferative & mature hemopoietic cells. J Biol Chem. 1996;271:13088–13093. doi: 10.1074/jbc.271.22.13088. [DOI] [PubMed] [Google Scholar]
- 20.Al-Shami A, Naccache PH. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Involvement of Jak2 in the stimulation of phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:5333–5338. doi: 10.1074/jbc.274.9.5333. [DOI] [PubMed] [Google Scholar]
- 21.Shaw M, Cohen P. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett. 1999;461:120–124. doi: 10.1016/s0014-5793(99)01434-9. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi K, Takahashi M, Kimura K, Nishida W, Saga H, Sobue K. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J Cell Biol. 1999;145:727–770. doi: 10.1083/jcb.145.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 24.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD-098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 25.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/Mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagaki M, Schmelzle T, Yamaguchi K, Irie K, Hall MN, Matsumoto K. PDK1 homologs activate the PKC1-Mitogen-activated protein kinase pathway in yeast. Mol Cell Biol. 1999;19:8344–8352. doi: 10.1128/mcb.19.12.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seva C, Kowalski-Chauvel A, Daulhac L, Barthez C, Vaysse N, Pradayrol L. Wormannin-sensitive activation of p70S6-kinase and MAP-kinase by the G protein-coupled receptor, G/CCKb. Biochem Biophys Res Comm. 1997;238:202–206. doi: 10.1006/bbrc.1997.7163. [DOI] [PubMed] [Google Scholar]
- 29.Rommel C, Clarke B, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-Mek-Erk pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (Protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay NK, Price DJ, Kyriakis JM, Pelech S, Sanghera J, Avruch J. An array of insulin-activated, proline-directed serine/threonine protein kinases phosphorylate the p70 S6 kinase. J Biol Chem. 1992;267:3325–3335. [PubMed] [Google Scholar]
- 32.Berven LA, Frew IJ, Crouch MF. Nitric oxide donors selectively potentiate thrombin-stimulated p70S6K activity and morphological changes in Swiss 3T3 cells. Biochem Biophys Res Comm. 1999;266:352–360. doi: 10.1006/bbrc.1999.1833. [DOI] [PubMed] [Google Scholar]
- 33.Berven LA, Crouch MF. Cellular function of p70S6K: A role in regulating cell motility. Immunol Cell Biol. 2000;78:447–451. doi: 10.1046/j.1440-1711.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 34.Pardo OE, Arcaro A, Salerno G, Tetley TD, Valovka T, Gout I, Seckl MJ. Novel cross talk between MEK and S6K2 in FGF-2 induced proliferation of SCLC cells. Oncogene. 2001;20:7658–7667. doi: 10.1038/sj.onc.1204994. [DOI] [PubMed] [Google Scholar]
- 35.Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]