Abstract
Background
Metastatic dormancy, or the ability of cancer cells to survive but not progress in metastatic environments, is now recognized to be a common occurrence in cancer.
Summary
From a clinical perspective, this phenomenon is common in metastatic well-differentiated thyroid cancer, whereby patients often present with distant metastases that remain stable for years after removal of the primary tumor and subsequent treatment. Experimental data suggest that metastases can develop throughout the life of a cancer and that progression in the distant environment depends on the biology of the cancer cells that metastasize as well as that of the various microenvironments they encounter. A firm understanding of how thyroid cancer cell progression is regulated in different metastatic environments is necessary to devise effective therapies targeting progressive metastatic thyroid cancer.
Conclusion
In this review, current models of metastatic progression and factors that regulate late-stage metastatic progression that are particularly relevant for thyroid cancer are discussed.
Introduction
In 1889, Dr. Stephen Paget proposed the “seed and soil” hypothesis of cancer metastases, positing that the development of metastases in specific organs depended both on the cancer cell and the presence of a “congenial soil” at the metastatic site (1). Research regarding how and when metastases occur in the life of a tumor and how metastatic cancer cells survive and progress in distant sites has expanded on this hypothesis. One concept that has gained traction is that small micrometastatic lesions or individual cancer cells can survive in a quiescent state in metastatic niches without progression, a state known as metastatic dormancy (2,3). Experimental and clinical data, such as detection of tumor cells from circulation and bone marrow in patients thought cured from solid tumors, now support the notion that metastatic dormancy is not only possible, but it is a common event. These results challenge the “step-wise” model of metastatic progression whereby cancer cells progressively dedifferentiate prior to metastasizing and grow rapidly in the metastatic niche. They further suggest that in many cases, clinical “recurrences” may in fact represent progression of micrometastatic disease that was already present at diagnosis and not eradicated with initial therapy. Indeed it has been demonstrated that quiescent cancer cells may have markers of “stemness” with the ability to self-renew, have a slow rate of growth, and are relatively treatment resistant (4). From a clinical perspective, metastatic dormancy may be particularly common in well-differentiated thyroid cancer, a disease in which many individuals with biochemically and anatomically defined distant metastases often enjoy prolonged disease stability without therapy. In support of this concept are data that vascular invasion, even when within the tumor, is associated with metastases at diagnosis (5). Importantly, identifying mechanisms by which metastases escape from dormancy and progress in metastatic sites may be crucial in identifying appropriate targets for patients with progressive metastatic disease. These concepts and their potential impact on how we approach developing new therapies for thyroid cancer are the focus of this review.
Models of Metastatic Progression
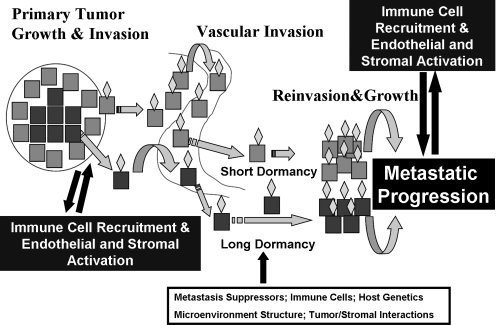
The long-held step-wise model of cancer metastasis is shown in the short dormancy component of Fig. 1. In this model, through serial genetic and epigenetic events, cancer cells become increasingly aggressive and gain the ability to invade into local structures, including blood vessels. This process may involve epithelial-to-mesenchymal transition (EMT) whereby the more differentiated epithelial cells gain functions typical of more primitive mesenchymal cells that may also have stem cell features (6–8). These cells gain access to the circulating blood vessels, evade the immune response, and are either degraded or reach a target organ. Factors that draw cancer cells to specific sites of metastatic deposition are uncertain but likely include circulatory patterns, lymphatic drainage patterns, and specific cancer cell–tissue interactions mediated by chemokines and other molecules. In thyroid cancer there appears to be organ specificity of either metastasis formation or progression as evidenced by the propensities of medullary thyroid cancer cells to metastasize to liver and bone and follicular and papillary cancer to metastasize to lungs and more rarely bone and brain. In this short dormancy model, once cancer cells have reached their target organs, they rapidly attach to the extracellular matrix, reinvade into the local tissue, develop a nutrient supply through angiogenesis, and grow and invade in the metastatic niche. Clinically, this model may apply to cancers with a very aggressive metastatic clinical course, such as anaplastic or poorly differentiated thyroid cancers.
FIG. 1.
A model of metastatic progression in cancer. Primary tumor growth and invasion occur through the gain of genetic or epigenetic changes in the primary tumor often in cells that have a change in character through the process of epithelial to mesenchymal transition (blue cells). Individual cells, or groups of cells, that have gone through this transition, as well as those that have not (perhaps earlier in the life of the cancer), gain access to blood vessels through incompletely defined mechanisms. Some of the cells are targeted to specific organs (gold diamonds) and enter their new microenvironment. Cells that have dedifferentiated are likely able to modify the premetastatic niche to allow for proliferation and invasion with short latency in the metastatic site. Cells shed into the circulation that are more differentiated (red cells) likely enter a period of prolonged dormancy controlled by a number of factors that may be released over time through changes in the tumor cells or the metastatic microenvironment. In both cases, metastatic progression at the metastatic site likely requires interactions with immune cells, endothelial cells, and the stroma.
More recently a second model has been proposed and experimentally verified (long dormancy component of Fig. 1 and reviewed by Aguirre-Ghiso [2], Nguyen et al. [3], and Klein and Hölzel [9]). In this model, individual or small clusters of differentiated epithelioid cancer cells are capable of gaining access to the circulation at an earlier stage than previously believed in the life of a tumor. These cells are “shed” into blood vessels and some of them are able to localize to specific organs and escape through the local vasculature into the metastatic tissue as noted above. However, once these cells arrive in the metastatic niche, they do not grow. Indeed, even nontransformed cells have been demonstrated to be capable of surviving long periods of time without growth in the lungs following tail vein injections in mice (10). This period of stability is regulated both by the expression of genes intrinsic to the cancer cells, by the metastatic microenvironment, or by other factors such as the immune system (see following text). It is possible that these cells may exhibit markers of “stemness” or reflect a slow-growing, treatment-resistant subpopulation of cells that remain in a quiescent phase in a G0–G1 arrest (11). Loss of dormancy can occur through a number of mechanisms intrinsic to the cancer cells, due to cells recruited to the local environment, and/or due to genetic or epigenetic changes in the microenvironment. How cancer cells and the microenvironment communicate likely involves a combination of secreted proteins and microvesicles such as exosomes that contain genetic information (12).
It seems likely that both the early and late metastatic models occur in patients with thyroid cancer and that rates of progression may vary depending on the biology of the metastatic cells from the primary tumor and the specific metastatic site. For example, patients with anaplastic thyroid cancer or large widely invasive papillary or follicular cancer tend to progress rapidly in a variety of microenvironments with a short period of dormancy. In contrast, patients with metastatic well-differentiated thyroid cancer typically enjoy prolonged periods of metastatic dormancy followed by slow rates of subsequent progression. Predictors of progression in distant metastases from well-differentiated thyroid cancer include the histology of the primary tumor, the size and location of the metastases at diagnosis and degree of metabolic activity based on [18F]fluorodeoxyglucose positron emission tomography scanning (13,14). Certain mutations, such as BRAF V600E (15) or p53 (16), or activation of phosphoinositide 3 (OH) kinase pathways (17) may predict a more aggressive course as well, but this has not yet been clearly implicated in progression of metastatic disease (18). It is of interest that clinical trial populations enriched for patients with progressive metastatic disease have a particularly high incidence of BRAF mutations that are persevered in metastatic tissues (19,20). However, it has also been shown that thyroid cancer cells gain additional genetic or epigenetic changes in metastatic lesions, consistent with the possibility of late-stage genomic progression of thyroid cancer cells (19). These data underscore the need to directly evaluate thyroid cancer metastatic tissues to devise more effective therapeutic approaches.
Regulators of Metastatic Progression
There are many regulators of metastatic progression once cells have reached the metastatic microenvironment. Several of the most important in relation to thyroid cancer that might be therapeutically relevant are reviewed in the following sections.
Metastasis suppressors
Metastasis suppressor genes are genes whose products inhibit the metastatic process and proliferation, but do not suppress tumor formation (21). Loss of metastasis suppressor gene expression or function could potentially result in loss of growth restraint in the metastatic niche thereby allowing for cancer progression. Thus, these genes may serve as “gatekeepers” of metastatic progression. More than 20 metastatic suppressor genes have now been described with varying levels of supportive evidence (reviewed by Horak et al. [21]). Several of these have been studied in thyroid cancer, including NM23 (22–24), KiSS-1 (25), RCAN1-4 (26,27), and others. These proteins, in general, seem to predominate in single metastatic cells where they maintain quiescence. The mechanisms responsible for metastasis suppression vary for the different suppressors. In several cases, in vivo suppression may involve functions on the host tissues in addition to those in the cancer cells (28). For example, cancer cells overexpressing a KiSS-1 and over-secreting its protein products (Kisspeptins) appear to have reduced ability to proliferate in the lungs, suggesting that secreted KiSS-1 may induce factors that maintain dormancy in the lung metastatic niche (29). It is of interest that RCAN1-4 and the tetraspanin KAI-1 (CD82) have also been shown to block tumor-induced angiogenesis through effects on the host rather than the cancer cell (30–33). Thus, metastasis suppressors have potential to restrain metastatic progression through effects in the cancer cells as well as the microenvironment. Delivery of metastasis suppressors is one potential therapeutic option that is being explored.
Oncogenes and oncogenic pathways
Oncogenes are well-known inducers of cell growth and dedifferentiation. They can induce chromosomal instability and EMT, activate pathways that lead to degradation of local intracellular matrix proteins, and also induce recruitment of bone marrow progenitor cells that may facilitate angiogenesis (34). Indeed, it has been experimentally proven that breast cells display oncogene-dependent proliferation and invasion in the pulmonary metastatic niche (10). This may also be true in bone marrow where breast cancer cells have been isolated in patients thought to be cured of breast cancer clinically. This situation is akin to thyroid cancer patients in whom metastatic disease can be identified by thyroglobulin measurement or iodine scanning but not detected using conventional cross-sectional imaging for many years. While, as noted above, BRAF V600E is associated with a greater likelihood of local invasion and nodal metastases, whether or not particular oncogenes regulate metastatic dormancy is uncertain (15). This represents an important area of research when considering targeting oncogenes with therapeutic intent in patients with progressive distant metastases.
Angiogenesis
Once cancer cells proliferate in the metastatic site, they have potential to grow to a point where they can no longer be supported by the vascular bed of the organ to provide nutrients. The hypoxic state that develops leads to increased expression of hypoxia inducible factor 1 (HIF1) and subsequent release of pro-angiogenic factors that lead to blood vessel formation (35). This may also be supported by release of signals that recruit bone marrow–derived macrophages, which further support new blood vessel formation. Factors that limit the ability of cancers to respond to hypoxia or that reduce endothelial cell response to angiogenic factors may limit the rate of progression of cancers. The specific factors and requirements for angiogenesis and blood vessel formation may vary in different organs. Many of the current treatments that have shown disease-stabilizing effects in metastatic thyroid cancer are thought to inhibit metastasis-related angiogenesis by inhibiting responses to vascular endothelial growth factor (VEGF) (36). However, whether or not VEGF receptors are the primary target of the compounds clinically has not been proven. Finally, there are concerns that short-term treatment has potential to induce neovascularization and subsequent tumor progression or that the compounds may not be equally active in all metastatic niches (37).
Extracellular matrix
The extracellular matrices (ECMs) in different metastatic niches are unique, but in all cases the matrix presents a physical and chemical barrier between metastatic cancer cells and the cellular environment that surrounds them. The ECM is composed of collagens, laminins, and other components that create a three-dimensional scaffold to which metastatic cancer cells must attach for metastases to occur (38). The matrix components likely play an important role in maintaining metastatic dormancy. Data from three-dimensional model systems have defined a critical role for activation of β1 integrin and Rho GTPase signaling by the extracellular matrix and other factors, such as tetraspanins, in enabling cell survival, proliferation, and cytoskeletal changes required for the switch from dormancy to proliferation and invasion (39,40). Initiation of cell movement–related pathways and secretion of proteinases to degrade surrounding matrix proteins required for invasion involves a coordinated and complex signaling program (41). Finally, the physical barriers to invasion also relate to physical factors, such as the relative stiffness of the tissue and other features. Models to study these physical factors are underway in many laboratories (42). Evidence from several groups has implicated β1 integrin regulators such as transforming growth factor β and urokinase plasminogen activator receptor (43–45), as well as downstream pathways such as Src kinase (SRC), focal adhesion kinase (FAK), and pre activated kinase (PAK) in thyroid cancer progression (46,47). Whether or not these potential regulators of the dormancy-progression switch are viable therapeutic targets has not yet been reported.
Immune surveillance
It has been known for many years that cytotoxic CD8+ T lymphocytes are able to kill cancer cells and that they, along with other components of the immune response are capable of maintaining tumor cells in a dormant state (48,49). These types of data have led to experiments and clinical trials using tumor cells or tumor antigens to induce specific populations of cytotoxic T cells for treatment with evidence of clinical benefit in some tumor types (50). Lymphocyte infiltrate has been demonstrated to occur commonly in papillary thyroid cancer (PTC), particularly those with RET/PTC mutations, and inflammation has been associated with the development and prognosis of PTC in population studies (51,52). It has been recently reported that the specific types of infiltrating lymphocytes influence tumor size and local metastatic spread (53). These data, when considered with evidence that macrophages and some lymphocytes may also promote tumor formation and progression in thyroid cancer demonstrate that effects of cancer-associated immune infiltrates cannot be generalized and may be highly regulated (54–56). Despite this complexity, the field of tumor vaccines to induce cytotoxic immune responses to tumor-specific antigens remains a promising avenue of research for selective therapy for cancers (57).
Cancer cell impact on the premetastatic niche: exosome-mediated gene and protein transfer
Another important concept is that single cancer cells or small clusters of cancer cells may release signals that modify tissues to better accept cancer cells (reviewed by Peinado et al. [58]). This ability to create a “premetastatic” niche that will be more conducive to implantation and progression may be mediated locally by proteins released by cancer cells. One intriguing mechanism is the release of exosomes and other microvesicles from cancer cells (59). Exosomes are small (<100 nm) lipid bilayer microvesicles derived from the fusion of intracellular microvesicular bodies with the cytosolic membrane. Exosomes contain DNA, mRNA, microRNAs, and proteins, some of which can be enriched in comparison to the parental cells. The formation and release of exosomes from cells is tightly controlled by cells, primarily via member of the RAB family of small G proteins (60). After being released from cells, exosomes travel either locally or in peripheral blood, fuse to recipient cells and deliver their cargo (61). It has been demonstrated, for example, that cancer cell–derived exosomes can deliver oncogenes to recipient non–cancer cells in vitro (39,62). Although the methods are laborious, it has been shown that cancer cell–derived exosomes and microvesicles can be isolated from the circulating blood of cancer patients, suggesting that exosome-mediated information transfer can occur in patients (63). It has been hypothesized that cancer cells may influence the metastatic niche before their arrival through this mechanism, creating a premetastatic niche. In addition to this “long-distance” communication, exosome-delivered cancer cell information represents another method by which cancer cells influence their local microenvironment for successful survival and growth. The role of exosome and microvesicles in the premetastatic or metastatic environment is incompletely defined. However, it seems likely that this may be a regulated cancer cell function that could be inhibited or exploited to influence the rate of cancer progression in metastatic locations.
Clinical Implications and Summary
Metastatic progression of cancer is a multifaceted and complex process. In poorly differentiated or anaplastic thyroid cancer, both local and metastatic progression can occur rapidly, suggesting in these cases that the cancer cells have achieved a very aggressive nonregulated state prior to metastasizing. However, in well-differentiated thyroid cancer, the presence of vascular invasion even in small tumors predicts the presence of distant metastases. These metastatic lesions are often located in the lymph nodes or lungs, are identified based on thyroglobulin elevations, are typically small and multiple, and tend to remain stable for years or decades. These data suggest that most thyroid cancer distant metastases may occur early and remain dormant in the metastatic niche, especially in the lymph nodes and lungs, and further suggest that the latency between the metastatic event and subsequent progression is regulated by the cancer cells and the host environment. From a clinical perspective, if this hypothesis is correct it implies that late-stage progression is not likely to be related to the development of new secondary metastases but rather to changes in growth pattern of cells that lie dormant in metastatic locations. Clinically nearly all patients placed on clinical trials have evidence of progression in one or more metastatic environment. Thus, to design more effective therapies or preventive strategies, a clear understanding of metastatic dormancy and progression is needed. This is a challenging task that will involve obtaining tissue samples from primary tumors and stable and progressive metastatic tissues in individual patients, the development of in vitro and in vivo models to assess metastatic dormancy and progression, and the identification of targetable regulators that are not intrinsic to survival of the host. Taking thyroid cancer research out of the thyroid into the new frontier of metastatic location has potential to have major impact on the future therapies for patients with progressive and life-threatening forms of thyroid cancer.
Acknowledgment
The author wishes to acknowledge funding from the National Cancer Institute (P01 CA124570 and R01 CA152066).
Disclosure Statement
The author discloses the following commercial relationships: Veracyte, Inc.: Advisory Board; Astra-Zeneca: Consultant; clinical trials participation with the following: Exelixis, Esai, and Bayer/Onyx.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breasts. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 2.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen DX. Bos PD. Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 4.Shachaf CM. Kopelman AM. Arvanitis C. Karlsson A. Beer S. Mandl S. Bachmann MH. Borowsky AD. Ruebner B. Cardiff RD. Yang Q. Bishop JM. Contag CH. Felsher DW. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 5.Gardner RE. Tuttle RM. Burman KD. Haddady S. Truman C. Sparling YH. Wartofsky L. Sessions RB. Ringel MD. Prognostic importance of vascular invasion in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:309–312. doi: 10.1001/archotol.126.3.309. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji T. Ibaragi S. Hu G-F. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69:7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mani SA. Guo W. Liao M-J. Eaton EN. Ayyanan A. Zhou AY. Brooks M. Reinhard F. Zhang CC. Shipitsin M. Campbell LL. Polyak K. Brisken C. Yang J. Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A. Settleman J EMT cancer stem cells, drug resistance. an emerging axis of evil in the war on cancer. Oncogene. 29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein CA. Hölzel D. Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle. 2006;5:1788–1798. doi: 10.4161/cc.5.16.3097. [DOI] [PubMed] [Google Scholar]
- 10.Podsypanina K. Du YC. Jechlinger M. Beverly LJ. Hambardzumyan D. Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusumbe AP. Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res. 2009;69:9245–9253. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 12.Skog J. Wurdinger T. van Rijn S. Meijer DH. Gainche L. Sena-Esteves M. Curry WT., jr. Carter BS. Krichevsky AM. Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins RJ. Wan Q. Grewal RK. Reibke R. Gonen M. Strauss HW. Tuttle RM. Drucker W. Larson SM. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 14.Durante C. Haddy N. Baudin E. Leboulleux S. Hartl D. Travagli JP. Caillou B. Ricard M. Lumbroso JD. De Vathaire F. Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 15.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 16.Fagin JA. Matsuo K. Karmakar A. Chen DL. Tang SH. Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paes JE. Ringel MD. Dysregulation of the phosphatidylinositol 3-kinase pathway in thyroid neoplasia. Endocrinol Metab Clin North Am. 2008;37:375–387. doi: 10.1016/j.ecl.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ringel MD. Molecular markers of aggressiveness of thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2009;16:361–366. doi: 10.1097/MED.0b013e32832ff2cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricarte-Filho JC. Ryder M. Chitale DA. Rivera M. Heguy A. Ladanyi M. Janakiraman M. Solit D. Knauf JA. Tuttle RM. Ghossein RA. Fagin JA. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloos RT. Ringel MD. Knopp MV. Hall NC. King M. Stevens R. Liang J. Wakely PE., Jr. Vasko VV. Saji M. Rittenberry J. Wei L. Arbogast D. Collamore M. Wright JJ. Grever M. Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horak CE. Lee JH. Marshall JC. Shreeve SM. Steeg PS. The role of metastasis suppressor genes in metastatic dormancy. APMIS. 2008;116:586–601. doi: 10.1111/j.1600-0463.2008.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zafon C. Obiols G. Castellvi J. Tallada N. Galofre P. Gemar E. Mesa J. Simo R. nm23-H1 immunoreactivity as a prognostic factor in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:3975–3980. doi: 10.1210/jcem.86.8.7710. [DOI] [PubMed] [Google Scholar]
- 23.Arai T. Watanabe M. Onodera M. Yamashita T. Masunaga A. Itoyama S. Itoh K. Sugawara I. Reduced nm 23-H1 messenger RNA expression in metastatic lymph nodes from patients with papillary carcinoma of the thyroid. Am J Pathol. 1993;142:1938–1944. [PMC free article] [PubMed] [Google Scholar]
- 24.Arai T. Yamashita T. Urano T. Masunaga A. Itoyama S. Itoh K. Shiku H. Sugawara I. Preferential reduction of nm23-H1 gene product in metastatic tissues from papillary and follicular carcinomas of the thyroid. Mod Pathol. 1995;8:252–256. [PubMed] [Google Scholar]
- 25.Ringel MD. Hardy E. Bernet VJ. Burch HB. Schuppert F. Burman KD. Saji M. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP kinase in thyroid cancer cells. J Clin Endocrinol Metab. 2002;87:2399–2402. doi: 10.1210/jcem.87.5.8626. [DOI] [PubMed] [Google Scholar]
- 26.Stathatos N. Bourdeau I. Espinosa AV. Saji M. Vasko VV. Burman KD. Stratakis CA. Ringel MD. KiSS-1/G protein-coupled receptor 54 metastasis suppressor pathway increases myocyte-enriched calcineurin interacting protein 1 expression and chronically inhibits calcineurin activity. J Clin Endocrinol Metab. 2005;90:5432–5440. doi: 10.1210/jc.2005-0963. [DOI] [PubMed] [Google Scholar]
- 27.Espinosa AV. Shinohara M. Porchia LM. Chung YJ. McCarty S. Saji M. Ringel MD. Regulator of calcineurin 1 modulates cancer cell migration in vitro. Clin Exp Metastasis. 2009;26:517–526. doi: 10.1007/s10585-009-9251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodenstine TM. Welch DR. Metastasis suppressors and the tumor microenvironment. Cancer Microenviron. 2008;1:1–11. doi: 10.1007/s12307-008-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nash KT. Phadke PA. Navenot J-M. Hurst DR. Accavitti-Loper MA. Sztul E. Vaidya KS. Frost AR. Kappes JX. Peiper SC. Welch DR. Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J Natl Cancer Inst. 2007;99:309–321. doi: 10.1093/jnci/djk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryeom S. Baek K-H. Rioth MJ. Lynch RC. Zaslavsky A. Birsner A. Yoon SS. McKeon F. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell. 2008;13:420–431. doi: 10.1016/j.ccr.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Minami T. Yano K. Miura M. Kobayashi M. Suehiro J. Reid PC. Hamakubo T. Ryeom S. Aird WC. Kodama T. The Down syndrome critical region gene 1 short variant promoters direct vascular bed-specific gene expression during inflammation in mice. J Clin Invest. 2009;119:2257–2270. doi: 10.1172/JCI35738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baek KH. Zaslavsky A. Lynch RC. Britt C. Okada Y. Siarey RJ. Lensch MW. Park IH. Yoon SS. Minami T. Korenberg JR. Folkman J. Daley GQ. Aird WC. Galdzicki Z. Ryeom S. Down's syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009;459:1126–1130. doi: 10.1038/nature08062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iiizumi M. Bandyopadhyay S. Watabe K. Interaction of Duffy antigen receptor for chemokines and KAI1: a critical step in metastasis suppression. Cancer Res. 2007;67:1411–1414. doi: 10.1158/0008-5472.CAN-06-3801. [DOI] [PubMed] [Google Scholar]
- 34.Reddy KB. Nabha SM. Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/a:1023781114568. [DOI] [PubMed] [Google Scholar]
- 35.Keith B. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. C.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman SI. Advances in chemotherapy of differentiated epithelial and medullary thyroid cancers. J Clin Endocrinol Metab. 2009;94:1493–1499. doi: 10.1210/jc.2008-0923. [DOI] [PubMed] [Google Scholar]
- 37.Ebos JM. Lee CR. Cruz-Munoz W. Bjarnason GA. Christensen JG. Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barkan D. Green JE. Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 46:1181–1188. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nazarenko I. Rana S. Baumann A. McAlear J. Hellwig A. Trendelenburg M. Lochnit G. Preissner KT. Zoller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 40.Barkan D. Kleinman H. Simmons JL. Asmussen H. Kamaraju AK. Hoenorhoff MJ. Liu ZY. Costes SV. Cho EH. Lockett S. Khanna C. Chambers AF. Green JE. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Baker EL. Bonnecaze RT. Zaman MH. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys J. 2009;97:1013–1021. doi: 10.1016/j.bpj.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasko V. Espinosa AV. Scouten W. He H. Auer H. Liyanarachchi S. Larin A. Savchenko V. Francis GL. de la Chapelle A. Saji M. Ringel MD. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007;104:2803–2808. doi: 10.1073/pnas.0610733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riesco-Eizaguirre G. Rodriguez I. De la Vieja A. Costamagna E. Carrasco N. Nistal M. Santisteban P. The BRAFV600E oncogene induces transforming growth factor {beta} secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. 2009;69:8317–8325. doi: 10.1158/0008-5472.CAN-09-1248. [DOI] [PubMed] [Google Scholar]
- 45.Nowicki TS. Kummer NT. Iacob C. Suslina N. Schaefer S. Schantz S. Shin E. Moscatello AL. Tiwari RK. Geliebter J. Inhibition of uPAR and uPA reduces invasion in papillary thyroid carcinoma cells. Laryngoscope. 2010;120:1383–1390. doi: 10.1002/lary.20915. [DOI] [PubMed] [Google Scholar]
- 46.Schweppe RE. Kerege AA. French JD. Sharma V. Grzywa RL. Haugen BR. Inhibition of Src with AZD0530 reveals the Src-Focal Adhesion kinase complex as a novel therapeutic target in papillary and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2009;94:2199–2203. doi: 10.1210/jc.2008-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarty SK. Saji M. Zhang X. Jarjoura D. Fusco A. Vasko VV. Ringel MD. Group I p21-activated kinases regulate thyroid cancer cell migration, are overexpressed, activated in thyroid cancer invasion. Endocr Relat Cancer. 17:989–999. doi: 10.1677/ERC-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrar JD. Katz KH. Windsor J. Thrush G. Scheuermann RH. Uhr JW. Street NE. Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN-gamma in establishing and maintaining the tumor-dormant state. J Immunol. 1999;162:2842–2849. [PubMed] [Google Scholar]
- 49.Wieder T. Braumuller H. Kneilling M. Pichler B. Rocken M. T cell-mediated help against tumors. Cell Cycle. 2008;7:2974–2977. doi: 10.4161/cc.7.19.6798. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg SA. Restifo NP. Yang JC. Morgan RA. Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guarino V. Castellone MD. Avilla E. Melillo RM. Thyroid cancer and inflammation. Mol Cell Endocrinol. 2010;321:94–102. doi: 10.1016/j.mce.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Muzza M. Degl'Innocenti D. Colombo C. Perrino M. Ravasi E. Rossi S. Cirello V. Beck-Peccoz P. Borrello MG. Fugazzola L. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol. 2010;72:702–708. doi: 10.1111/j.1365-2265.2009.03699.x. [DOI] [PubMed] [Google Scholar]
- 53.French JD. Weber ZJ. Fretwell DL. Said S. Klopper JP. Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab. 2010;95:2325–2333. doi: 10.1210/jc.2009-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Visser KE. Eichten A. Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 55.Ryder M. Ghossein RA. Ricarte-Filho JC. Knauf JA. Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15:1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pufnock JS. Rothstein JL. Oncoprotein signaling mediates tumor-specific inflammation and enhances tumor progression. J Immunol. 2009;182:5498–5506. doi: 10.4049/jimmunol.0801284. [DOI] [PubMed] [Google Scholar]
- 57.Aldrich JF. Lowe DB. Shearer MH. Winn RE. Jumper CA. Kennedy RC. Vaccines, immunotherapeutics for the treatment of malignant disease. Clin Dev Immunol. 2010 doi: 10.1155/2010/697158. [EPub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peinado H. Lavotshkin S. Lyden D. The secreted factors responsible for pre-metastatic niche formation: Old sayings, new thoughts. Semin Cancer Biol. 2011 doi: 10.1016/j.semcancer.2011.01.002. [EPub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 59.Al-Nedawi K. Meehan B. Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- 60.Ostrowski M. Carmo NB. Krumeich S. Fanget I. Raposo G. Savina A. Moita CF. Schauer K. Hume AN. Freitas RP. Goud B. Benaroch P. Hacohen N. Fukuda M. Desnos C. Seabra MC. Darchen F. Amigorena S. Moita LF. Thery C. Rab27a, Rab27b control different steps of the exosome secretion pathway. Nature Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11–13. [DOI] [PubMed] [Google Scholar]
- 61.Simons M. Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Al-Nedawi K. Meehan B. Micallef J. Lhotak V. May L. Guha A. Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 63.Thery C. Amigorena S. Raposo G. Clayton A. Isolation, characterization of exosomes from cell culture supernatants, biological fluids. Curr Protoc Cell Biol Chapter. 2006;3(3.22) doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]