Abstract
Purpose
To quantify the association between siblings in age-related nuclear cataract, after adjusting for known environmental and personal risk factors.
Methods
All participants (probands) in the Salisbury Eye Evaluation (SEE) project and their locally resident siblings underwent digital slit lamp photography and were administered a questionnaire to assess risk factors for cataract including: age, gender, lifetime sun exposure, smoking and diabetes history, and use of alcohol and medications such as estrogens and steroids. In addition, blood pressure, body mass index, and serum antioxidants were measured in all participants. Lens photographs were graded by trained observers masked to the subjects' identity, using the Wilmer Cataract Grading System. The odds ratio for siblings for affectedness with nuclear cataract and the sibling correlation of nuclear cataract grade, after adjusting for covariates, were estimated with generalized estimating equations.
Results
Among 307 probands (mean age, 77.6 ± 4.5 years) and 434 full siblings (mean age, 72.4 ± 7.4 years), the average sibship size was 2.7 per family. After adjustment for covariates, the probability of development of nuclear cataract was significantly increased (odds ratio [OR] = 2.07, 95% confidence interval [CI], 1.30–3.30) among individuals with a sibling with nuclear cataract (nuclear grade ≥ 3.0). The final fitted model indicated a magnitude of heritability for nuclear cataract of 35.6% (95% CI: 21.0%–50.3%) after adjustment for the covariates.
Conclusions
Findings in this study are consistent with a genetic effect for age-related nuclear cataract, a common and clinically significant form of lens opacity.
Age-related cataract is the leading cause of blindness in the world1 and the leading cause of low vision in the United States,2 where it consumes approximately 60% of the Medicare budget for vision.3 It has been suggested that any intervention that could delay cataract for even 10 years may reduce the number of cataract surgeries in the United States by 45%.4 The potential impact of cataract prevention may be appreciated when we remember that 1 in 20 Americans over the age of 40 years have undergone cataract surgery.2 In the face of current knowledge about cataract and its causes, smoking prevention has been suggested as the most effective prevention strategy.5 Still, the simple clinical observation that some individuals survive very late in life without visually significant lens opacity has recently prompted several investigations into the genetics of age-related cataract as an avenue of research for novel prevention strategies.
Available evidence from population-based studies6–9 suggests that cataract does in fact aggregate in families. However, such associations might result either from shared genes or shared environment. Twin studies provide a potentially powerful tool to distinguish between “nature” and “nurture,” and have been consistent with the idea of a genetic influence on age-related cataract.10,11 However, such studies are dependent on assumptions about the shared environment of different types of twins, and such assumptions are inherent in the models used to analyze these data.
A practical difficulty in studies of the genetics of cataract, as with all age-related diseases, is that unaffected status at the time of examination may mean that the individual is truly unaffected or that he or she has simply not manifested the phenotype yet. Misattribution can thus be a serious problem in all but the oldest populations. An additional difficulty in performing studies to ascertain the degree of heritability of age-related cataract in the United States is that general access to cataract surgical services leads to systematic censorship of the most affected persons in the population, with attendant loss of power and possible introduction of bias.
In the current investigation, we studied the heritability of nuclear cataract in a cohort of older sibships recruited through the Salisbury Eye Examination (SEE) on Maryland's Eastern Shore. We attempted to overcome some of the mentioned difficulties in studying the genetics of age-related cataract. To distinguish between familial aggregation of cataract due to environmental and genetic causes, information was collected on major personal and environmental cataract risk factors, including cigarette, alcohol, exogenous estrogen and other medication use, lifetime ultraviolet-B light exposure, serum antioxidant levels, history of diabetes and other relevant medical conditions, and body mass index (BMI). To minimize misattribution due to as yet unaffected individuals who will eventually manifest the phenotype, we chose to work in an older population, with the minimum age for probands of 72 years. Finally, to reduce the impact of censored data from bilaterally pseudophakic individuals, we relied on previous study photographs and records from operating ophthalmologists to assign cataract grades for individuals without a native lens in either eye at the time of enrollment in the study.
Materials and Methods
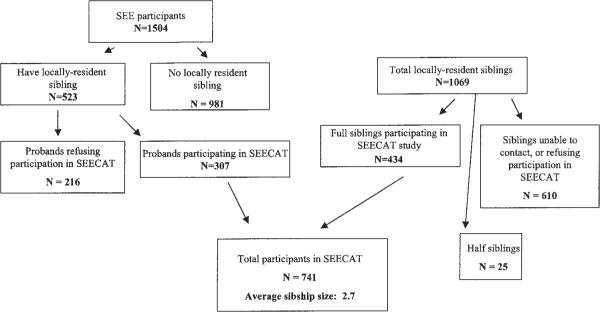
The Salisbury Eye Evaluation (SEE) initially recruited 2520 persons aged 65 to 84 years on a population basis from Medicare roles in the Salisbury area.12 This cohort has been observed with regular measurement of various cataract risk factors through a total of four rounds of visits to date, with lens photographs having been obtained at rounds 1, 2 and 4. At rounds 3 and 4, all surviving subjects (n = 1504) were administered a family history questionnaire, and were eligible for the SEE Cataract Genetics (SEECAT) study if they had one or more full or half siblings living within 100 miles of Salisbury or Baltimore (Fig. 1). Consent was obtained from eligible SEE participants (probands) to contact their siblings. These siblings were then sent letters describing the study and containing stamped, self-addressed postcards that they could return to study headquarters if they did not want to be contacted further. Siblings from whom no card was received after 2 weeks were contacted by telephone and administered the family history questionnaire. Interested eligible siblings were invited to study headquarters.
Figure 1.
Participants in the Salisbury Eye Evaluation Study of Cataract Genetics (SEECAT).
After giving informed consent, all probands and siblings gave full medical and ophthalmologic histories and also answered questionnaires regarding their age; gender; lifetime ultraviolet-B exposure (methodology documented elsewhere13); medical history including diabetes; and use of tobacco, alcohol, estrogen supplements, and other prescription and over-the-counter drugs. In addition, height was measured in stocking feet with a height board, and weight was obtained in kilograms on a digital scale. Seated blood pressure was measured (average of two readings) in the right arm with a mercury sphygmomanometer. Three 7-mL EDTA tubes of blood were drawn by sterile venipuncture, two for later DNA analysis, and one that was centrifuged within 8 hours for 20 minutes at 2200 rpm, with plasma being drawn off, labeled, and frozen at −20° C. Plasma samples were shipped in batches of 100 on dry ice to the laboratory for measurement of antioxidant levels.
α-Carotene, β-carotene, β-cryptoxanthin, lycopene, lutein, zeaxanthin, retinol, and α-tocopherol were measured in 100 μL of plasma by high-performance liquid chromatography using a modified method from the Nutrition Laboratory, Inorganic Toxicology and Nutrition Branch, Division of Laboratory Sciences, National Center of Environmental Health, Centers for Disease Control and Prevention (Atlanta, GA).14 The internal standards used were tocol (Hoffmann-La Roche, Nutley, NJ) at 300 and 325 nm and all-trans-ethyl-β-apo-8′-carotenoate (purified sample courtesy of Fred Khachik, U. S. Department of Agriculture) at 450 nm. Quality control was assessed by repeated analysis of pooled human plasma control samples run at the beginning and end of each analysis. Standard curves were run periodically, using standard reference material 986C (National Institute of Standards and Technology, Gaithersburg, MD). The mobile phase consisted of one pump in acetonitrile with 0.1% triethylamine and a second pump in ethanol with 0.1% triethylene. A gradient method was applied by varying the solvent concentrations from 85% acetonitrile-triethylamine to 50% acetonitrile-triethylamine and again to 85% acetonitrile-triethylamine.
A full ocular examination including dilation of the pupil was performed by an optometrist (HB), and slit lamp (D1 digital camera; Nikon, Melville, NY; and Photograph Slit-lamp SL-7E; Topcon, Paramus, NJ) and digital retroillumination camera (Marcher Instruments Ltd., Hereford, UK) photographs were obtained through a dilated pupil using slit lamp and ambient light parameters that have been described in detail elsewhere.15 These photographs were then graded by a team of five trained and experienced graders after an initial period of standardization. The Wilmer Cataract Grading System was used for all grading. Briefly, a decimal grade for nuclear opalescence between 0.1 and 4.0 was assigned by two graders for each phakic eye on the basis of digital slit lamp photographs with reference to four photographic standards (representing grades of 1.0, 2.0, 3.0, and 4.0). Nuclear color was not considered in assigning this grade. The final nuclear grade for an eye consisted of the arithmetic mean between the grades assigned by the two graders, unless the grades differed by more than 0.2 units, in which adjudication involving at least one senior investigator (NGC, SKW) was conducted. All grading was performed under subdued lighting on one of two cathode ray tube (CRT) computer screens which had initially been standardized against one another (Do It Interactive, Inc., Baltimore, MD).
In the circumstance in which a digital lens photograph could not be obtained in either eye (insufficient media clarity, inability to comply with the photographic protocol, bilateral pseudophakia), an attempt was made to assign a cataract subtype(s) for one or both eyes based either on photographs from a previous round of SEE or information obtained from the surgeon's preoperative clinical evaluation of the lens in the subject's chart. A bilaterally pseudophakic subject was deemed to be affected by nuclear cataract for the purposes of analysis if either eye had nuclear grade ≥3 on a previously graded SEE photograph or had nuclear cataract graded by the surgeon as 2+ or greater (on a scale of 4), or characterized as “dense,” “significant,” or similar terminology in the chart. Clinician grades may come several years after the most recent previous study photograph and so frequently provided new information.
Senior graders reviewed bilateral photographs for 20 subjects in whom both digital and film images were captured before beginning the study. Interobserver and intraobserver agreement in comparing digital and film images of the same eye was similar to that observed in previous testing using film images alone.
This protocol was approved in its entirety by the Joint Committee on Clinical Investigations, the Institutional Review Board for the Johns Hopkins University School of Medicine. The study was performed in accord with the Declaration of Helsinki.
Statistical Methods
Nuclear cataract was treated in both quantitative (Wilmer decimal grade 0.1–4.0) and binary fashion (with an individual defined as “affected” if the photograph grade was ≥3 or the assigned grade was “present” in either eye). The degree of family association for nuclear cataract was assessed in the binary analysis by the odds ratio (OR) comparing the odds of being affected by nuclear cataract for those with an affected sibling to those without, after adjustment for covariates. In the quantitative analysis, familial association was measured by the heritability (h2), which is twice the residual correlation between siblings after adjustment for the various cataract risk factors. Let yij denote the maximum nuclear cataract grade for sibling j in family i, let zij = 1 if yij ≥ 3 and = 0 otherwise, and let xij denote a vector of covariates (including an intercept). In the analysis of the quantitative measure, we assumed a linear model, E(yij | xij) = β′xij, with constant residual correlation between siblings corr(yij, yik | xij, xik) = ρ. Note that the heritability is twice the residual sibling correlation: h2 = 2ρ. The parameters β and ρ were estimated by generalized estimating equations,16,17 using the package GEEPACK version 0.2–4 (available at http://cran.r-project.org/src/contrib/Descriptions/geepack.html) with the R statistical system version 1.7.1.18
In the analysis of the binary measure, we followed the approach of Liang and Beaty19 and assumed a logistic model, logit [Pr(zij = 1 | xij)] = θ′xij, with constant log odds ratio ln OR(zij, zik | xij, xik) = γ. The parameters θ and γ were again estimated by GEE, as described by Liang et al.20 Calculations were performed in R version 1.7.1, using the package GEESIBSOR, available from the authors.
With both analyses, standard errors were obtained by the robust sandwich estimator.16 Covariates were chosen by stepwise selection. A covariate was retained in the model if the corresponding probability was less than 0.1.
Results
A total of 307 probands and their 434 full siblings (total n = 741) participated in the study, forming a total of 274 sibships of one to eight individuals and an average size of 2.7. (Fig. 1) The number of sibships is smaller than the number of probands, because some of the individuals recruited from the SEE study as probands were siblings of other probands. Probands were significantly older than their siblings, and were more likely to have nuclear cataract, bilateral pseudophakia and several other risk factors for cataract, though none of these differences except the greater nuclear cataract prevalence remained significant after age adjustment (Table 1). Approximately 30% of subjects were black. Cataract grades could be assigned for 64% and 72% of bilaterally pseudophakic/aphakic probands and siblings, respectively (Table 1). Among persons with nuclear cataract, 49% had pure nuclear, 23% nuclear mixed with posterior subcapsular cataract (PSC), 17% nuclear mixed with cortical, and 11% nuclear mixed with PSC and cortical opacity.
Table 1.
Characteristics of Participants in the Salisbury Eye Evaluation Study (Probands) and Their Siblings, with Regard to Demographics, Cataract Risk Factors, Nuclear Cataract Status, and Pseudophakia
| Characteristics | Probands | Siblings | Unadjusted Difference | Age-Adjusted Difference |
|---|---|---|---|---|
| Mean age (y) | 77.6 ± 4.5 | 72.4 ± 7.4 | <0.001 | — |
| Gender (female) | 180/307 (58.6) | 254/434 (58.5) | 1.0 | — |
| Race (black) | 86/307 (28.0) | 119/434 (27.4) | — | — |
| Mean body mass index | 28.4 ± 5.5 | 29.4 ± 6.1 | 0.016 | 0.34 |
| Smoking status (%) (never/former/current) | 45/42/12 | 43/47/10 | 0.43 | 0.86 |
| Alcohol status (%) (never/former/current) | 30/48/21 | 29/45/25 | 0.51 | 0.49 |
| Systolic or diastolic hypertension (%) | 198/302 (66) | 239/434 (55) | 0.005 | 0.065 |
| Diabetes (%) | 77/302 (25) | 83/434 (19) | 0.046 | 0.097 |
| Current or recent steroid use (%) | 22/276 (8) | 41/432 (9) | 0.59 | 0.24 |
| Bilateral pseudophakia (%) | 78/307 (25.4) | 69/434 (15.9) | 0.002 | 0.83 |
| Able to assign cataract grade (among pseudophakes) | 50/78 (64.1) | 50/69 (72.5) | 0.29 | 0.61 |
| Nuclear cataract in either eye (%)* | 141/274 (51) | 121/406 (30) | <0.001 | 0.036 |
Includes persons with nuclear cataract grade ≥3.0 on SEE round 3 photographs and also persons determined to be "affected" by nuclear cataract on the basis of clinical records and/or old photographs.
When probands and siblings were considered together in a regression model with nuclear cataract grade as a continuous outcome, age was found to be positively associated with the presence of nuclear cataract, as was female gender, white race, and a history of smoking. BMI, alcohol use, presence of diabetes, use of steroids and exogenous estrogens, and the various serum antioxidants were not significantly associated with nuclear cataract (Table 2).
Table 2.
Association of Various Covariates with Nuclear Cataract Grade among a Population of Older Persons and Their Siblings in Salisbury, Maryland
| Covariate | Beta Coefficient (95% Confidence Interval) | Standard Error | P |
|---|---|---|---|
| Age (per year) | 0.045 (0.036–0.054) | 0.0046 | <0.0001 |
| Female gender | 0.212 (0.092–0.332) | 0.061 | <0.0001 |
| White race | 0.289 (0.150–0.428) | 0.071 | <0.0001 |
| Smoking (per pack-year) | 0.0025 (0.0005–0.0045) | 0.0010 | 0.014 |
The odds of being affected by nuclear cataract was elevated by twofold among those with an affected sibling (OR 2.08, 95% CI 1.39– 3.10), a significant association that persisted after adjusting for personal and environmental risk factors for cataract measured in our study (OR 2.07, 95% CI 1.30–3.30; Table 3). The heritability of nuclear cataract in this population was 35.6% (95% CI: 21.0%–50.3%), suggesting that some 36% of the variance in nuclear cataract grade can be attributed to genetic causes (Table 3).
Table 3.
Odds Ratio of Development of Nuclear Cataract among Persons with a Sibling Having Nuclear Cataract Compared with Those without Affected Siblings and Adjusted and Unadjusted Heritability of Nuclear Cataract
| Nuclear Cataract | Unadjusted | Adjusted* |
|---|---|---|
| OR | 2.08 (1.39–3.10) | 2.07 (1.30–3.30) |
| Heritability | 54.9% (31.5–78.3) | 35.6% (21.0–50.3) |
Adjusted for age, gender, race, body mass index, use of tobacco and alcohol, blood pressure, diabetes, use of steroids, total serum carotenoids, and lifetime ultraviolet-B light exposure. Only age, gender, race, and smoking status were significantly associated in the model and were used for the final adjustment.
Discussion
Our results provide evidence that genetics plays a significant role in nuclear cataract, the most prevalent type of cataract in European-derived populations,21 and the cataract subtype most commonly requiring surgery.22 The finding that nuclear cataract is genetically as well as environmentally determined in consistent with previous population-based investigations7,8 and twin studies.10 The fact that several independent lines of investigation in different populations have supported the heritability of nuclear cataract suggests that this finding is comparatively robust. The 36% heritability estimate for nuclear cataract in our study, an index of the proportion of cataract variation controlled by genetic causes, is comparable to that which has been reported in other studies. Hammond et al.10 reported a heritability figure of 48% in their twin study of nuclear cataract, and Heiba et al.8 estimated that a single major gene may account for 35% of nuclear cataract variation in the Beaver Dam Eye Study population.
In the regression model used in the present study, advancing age, female gender, white race, and smoking history were associated with nuclear cataract grade. All these factors have been identified previously in epidemiologic studies.23 Certain other covariates that have been found to be positively or negatively associated with nuclear cataract in the past, including alcohol intake, BMI, serum antioxidant status, and steroid use,21 were not found to predict nuclear cataract outcome significantly in our models. The list of covariates found to be predictive in our model included factors that have been most robust across numerous studies, whereas the associations with nutrition,24 alcohol intake, and BMI have been either less consistent or of uncertain directionality.21 Although steroid use has consistently been associated with development of cataract in many studies, regular users of steroids were relatively rare in this population.
The shortcomings of this study in providing reliable information about the heritability of nuclear cataract must be acknowledged. In the first place, our population was selected at baseline to be 65 years and older, and had reached a mean age in the mid-70s by the time of the present study. This is more than a decade older than the population studied by Hammond et al.10, for example, with a mean age of 62 years. There are both advantages and disadvantages to working with a population in the eighth and ninth decades of life in performing genetic studies on ocular diseases of aging. Although the chances of misclassification of affection status due to a younger individual having failed yet to manifest the phenotype of interest is reduced; however, the impact of environmental as opposed to genetic influences may be magnified in an older population. In addition, the effect of bilateral pseudophakia, where the opportunity directly to examine the lens phenotype has been lost, is increased among older individuals. Finally, the effects of survivorship, whereby persons living longer may be healthier than those dying younger, and of bias introduced by the inability of sicker and more physically impaired individuals to participate, are exaggerated in an older group.
In an attempt to reduce the impact of one of these problems inherent in cataract studies in an older population, the high prevalence of bilateral pseudophakia, we assigned grades for approximately one of eight probands in our study, based on photographs from previous rounds of SEE and preoperative clinical notes by ophthalmic surgeons in the area. Although this effort was successful in obtaining information for most of the bilaterally pseudophakic subjects, several assumptions were made: that a grade ≥2+ (on a scale of 4) or the notation of “dense” by a clinician was equivalent to a grade of ≥3.0 in a standardized cataract grading scale as implemented by trained graders and that clinical notations across different nonstandardized ophthalmologists and over time were equivalent. Obviously, these assumptions are not entirely valid and introduced an unavoidable degree of noise into our primary outcome measurement. We decided that this inevitable lack of precision was nonetheless acceptable, given the alternative of reduced power, and possible introduction of bias, from the systematic censoring of a large number of the most severely affected persons in the population that would have been necessary without some imputation strategy. Our use of the current approach is to some extent validated by the fact that our results were similar in magnitude and direction whether the estimated grades were included or not, although confidence intervals were of course somewhat tighter with the use of the additional data.
The rate of participation among eligible subjects in this study was modest, and in the case of siblings of SEE participants, fell below 50%. It is possible that the low participation resulted in bias, though estimates of heritability, the principle outcome for the study, would be biased only if persons whose cataract status was concordant with that of their siblings were more or less likely to participate than those with discordant phenotypes. Because we have no information regarding the lens status of nonparticipating siblings, we are unable to exclude this possibility, but it is not immediately obvious that factors would lead to such differential participation between concordant and discordant sibships. The relatively low rates of participation also resulted in a smaller total sample size and thus wider confidence intervals around our heritability estimate.
Finally, the conclusions reached by this study regarding genetic influences on nuclear cataract must to some extent depend on the assumptions inherent in the statistical models used. In the first place, by defining the residual correlation between siblings as a measure of the association due to genetic influences, we have implicitly assumed that there are no additional, unmeasured personal or environmental risk factors, the sharing of which between siblings may actually have accounted for residual correlation not due to measured factors. This assumption is in some ways difficult either to defend or attack. By definition, putative unknown risk factors cannot be enumerated or their existence discounted. It can only be said that in the present study we assessed nearly all factors shown in the past two decades of cataract epidemiology to impact on the development and progression of lens opacity. It is also possible that genetic influences on nuclear cataract is mediated through an individual's response to the effects of smoking and other such risk factors—that is, through gene–environment interactions. In a model such as the one used here, adjusting on such factors may actually lead to an underestimate of the heritability of nuclear cataract. The statistical model used in this report has no way to account for the impact of possible gene–environment interactions.
Studies of the genetics of age-related cataract are in their infancy. Though a small number of genes have been reported to be associated with cataract in limited populations, particularly in Japan (galactokinase,25 glutathione-S-transferase26), the reported impact been modest, and these findings have generally not been replicated in other populations.27 Though known, Mendelian-inherited forms of congenital cataract provide several potential candidate genes for age-related cataract28; however, given the very different phenotype and age of onset between these two types of cataract, the applicability of these candidates is far from clear. The immediate future direction of cataract genetic studies will probably involve genome-wide scans, based either on individual studies or consortiums between studies in an effort to increase power. Large, population-based studies of cataract in which investigators have not concentrated on the collection of families, but have obtained blood samples, are likely to be in the position to contribute best through case–control studies of positional candidates in areas highlighted by genome-wide scans. It is to be hoped that such efforts will take the field from where it is now, where most information about cataract has come from traditional, population-based studies not organized to explore genetic issues, toward a better understanding of the genes contributing to cataract development. The minimal data currently available from genetic studies of other ocular diseases of aging, such as age-related macular degeneration, suggest that such genes may be numerous, and their individual impact modest.
In view of such a complex model, what likely practical benefits can we expect from cataract genetic studies? It seems unlikely that this ubiquitous, late-onset disease with an existing surgical cure will provide a practical forum for resource-intensive strategies such as gene therapy or population or clinic-based screening of potentially affected persons. A more likely scenario is that studies of the cataract genetic studies will eventually yield knowledge of the protein pathways involved in lens opacity, so that discovery of anticataract agents may proceed in a rational fashion, rather than through the current process of hit or miss. To be practical, such agents probably should be delivered on a mass basis as supplements, which sets a high standard indeed for safety and low cost. However, the last century of medical progress provides at least two examples of the power of supplementation—fluoridated water29 and iodized salt,30—interventions that should serve as models for future work in the area of cataract prevention.
Acknowledgments
Supported by Grants R-01 16294 from the National Institute of Aging and K-23 EY00388 from the National Eye Institute and by Research to Prevent Blindness (NC, SKW).
Footnotes
Disclosure: N. Congdon, None; K.W. Broman, None; H. Lai, None; B. Munoz, None; H. Bowie, None; D. Gilbert, None; R. Wojciechowski, None; C. Alston, None; S.K. West, None
References
- 1.Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Org. 1995;73:115–121. [PMC free article] [PubMed] [Google Scholar]
- 2.Eye Disease Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Ellwein LB, Urato CJ. Use of eye care and associated charges among the Medicare population: 1991–1998. Arch Ophthalmol. 2002;120:804–811. doi: 10.1001/archopht.120.6.804. [DOI] [PubMed] [Google Scholar]
- 4.Kupfer C. Bowman lecture. The conquest of cataract: a global challenge. Trans Ophthalmol Soc UK. 1985;104:1–10. [PubMed] [Google Scholar]
- 5.McCarty CA, Nanjan MB, Taylor HR. Attributable risk estimates for cataract to prioritize medical and public health action. Invest Ophthalmol Vis Sci. 2999;41:3720–3725. [PubMed] [Google Scholar]
- 6.McCarty CA, Mukesh BN, Fu CL, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999;128:446–465. doi: 10.1016/s0002-9394(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 7.The Framingham Offspring Eye Study Group Familial aggregation of lens opacities: The Framingham Eye Study and the Framingham Offspring Eye Study. Am J Epidemiol. 1994;140:555–564. [PubMed] [Google Scholar]
- 8.Heiba IM, Elston RC, Klein BEK, Klein R. Genetic etiology of nuclear cataract: evidence for a major gene. Am J Med Gen. 1993;47:1208–1214. doi: 10.1002/ajmg.1320470816. [DOI] [PubMed] [Google Scholar]
- 9.Heiba IM, Elston RC, Klein BE, Klein R. Evidence for a major gene for cortical cataract. Invest Ophthalmol Vis Sci. 1995;36:227–235. [PubMed] [Google Scholar]
- 10.Hammond CJ, Snieder H, Spectotr TD, Gilbert CE. Genetic and environmental factors in age-related nuclear cataract in monozygotic and dizygotic twins. N Engl J Med. 2000;342:1786–1790. doi: 10.1056/NEJM200006153422404. [DOI] [PubMed] [Google Scholar]
- 11.Hammond CJ, Duncan DD, Snieder H, et al. The heritability of age-related cortical cataract: the twin eye study. Invest Ophthalmol Vis Sci. 2001;42:601–605. [PubMed] [Google Scholar]
- 12.West SK, Munoz B, Schein OD, Duncan DD, Rubin GS. Racial differences in lens opacities: the Salisbury Eye Evaluation (SEE) project. Am J Epidemiol. 1998;148:1033–1039. doi: 10.1093/oxfordjournals.aje.a009579. [DOI] [PubMed] [Google Scholar]
- 13.Duncan DD, Munoz B, Bandeen-Roche K, West SK. Assessment of ocular exposure to ultraviolet-B for population studies. Salisbury Eye Evaluation Project Team. Photochem Photobiol. 1997;66:701–709. doi: 10.1111/j.1751-1097.1997.tb03210.x. [DOI] [PubMed] [Google Scholar]
- 14.Sowell AL, Huff DL, Yeager PR, Caudill SP, Gunter EW. Simultaneous determination of retinol, α-tocopherol, lutein/zeaxanthin, β-cryptoxanthin, lycopene, trans-β-carotene, and four retinyl esters in serum by reversed-phase high-performance liquid chromatography with multi-wavelength detection. Clin Chem. 1994;40:411–416. [PubMed] [Google Scholar]
- 15.West SK, Munoz B, Wang F, Taylor H. Measuring progression of lens opacities for longitudinal studies. Curr Eye Res. 1993;12:123–132. doi: 10.3109/02713689308999480. [DOI] [PubMed] [Google Scholar]
- 16.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 17.Prentice RL, Zhao LP. Estimating equations for parameters in means and covariances of multivariate discrete and continuous responses. Biometrics. 1991;47:825–839. [PubMed] [Google Scholar]
- 18.Ihaka R, Gentleman R. R: A language for data analysis and graphics. J Comp Graph Stat. 1996;5:299–314. [Google Scholar]
- 19.Liang KY, Beaty TH. Measuring familial aggregation by using odds-ratio regression models. Genet Epidemiol. 1991;8:361–370. doi: 10.1002/gepi.1370080602. [DOI] [PubMed] [Google Scholar]
- 20.Liang KY, Zeger SL, Qaqish B. Multivariate regression analyses for categorical data (with discussion) J R Stat Soc B. 1992;1:3–40. [Google Scholar]
- 21.Congdon NC, Taylor HR. Age-related cataract. In: Weale RA, West SK, Johnson GJ, Minassian DC, editors. The Epidemiology of Eye Disease. Arnold; London: 2003. pp. 106–107. [Google Scholar]
- 22.Lewis A, Congdon NG, West SK, Munoz B, Bowie H, Lai H. Cataract surgery and cataract sub-type in a defined, older population: the SEECAT Study. Br J Ophthalmol. doi: 10.1136/bjo.2004.045484. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West SK, Valmadrid CT. Epidemiology of risk factors for age-related cataract (review) Surv Ophthalmol. 1995;39:323–334. doi: 10.1016/s0039-6257(05)80110-9. [DOI] [PubMed] [Google Scholar]
- 24.Congdon NG, West KP., Jr. Nutrition and the eye (review) Curr Opin Ophthalmol. 1999;10:464–473. doi: 10.1097/00055735-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Okano Y, Asada M, Fujimoto A, et al. A genetic factor for age-related cataract: identification and characterization of a novel galactokinase variant, “Osaka,” in Asians. Am J Hum Genet. 2001;68:1036–1042. doi: 10.1086/319512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekine Y, Hommura S, Harada S. Frequency of glutathione-S-transferase 1 gene deletion and its possible correlation with cataract formation. Exp Eye Res. 1995;60:159–163. doi: 10.1016/s0014-4835(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 27.Alberti G, Oguni M, Podgor M, et al. Glutathione S-transferase M1 genotype and age-related cataracts. Lack of association in an Italian population. Invest Ophthalmol Vis Sci. 1996;37:1167–1173. [PubMed] [Google Scholar]
- 28.Congdon NG. Cataract genetics. Bull World Health Org. 2001;79:258–259. [PMC free article] [PubMed] [Google Scholar]
- 29.Mouradian WE, Wehr E, Crall JJ. Disparities in children's oral health and access to dental care. JAMA. 2000;284:2525–2631. doi: 10.1001/jama.284.20.2625. [DOI] [PubMed] [Google Scholar]
- 30.Van der Haar F. The challenge of the global elimination of iodine deficiency disorders (review) Eur J Clin Nutr. 1997;51:S3–S8. [PubMed] [Google Scholar]