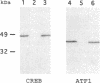
Abstract
The mammalian transcription factor CREB is thought to activate cAMP-inducible genes in a variety of differentiated cell types and is probably involved in other signalling pathways. Undifferentiated F9 embryonal carcinoma (UF9) cells are refractory to cAMP and become cAMP-responsive following differentiation to endoderm like cells. It has been proposed that UF9 cells contain a negative regulator(s) of the cAMP-response that might act through direct interaction with CREB. We have used a protein blotting assay and 32P-labelled CREB to probe for CREB-binding proteins in nuclear extracts from F9 cells and to examine their abundance during differentiation. We find that ATF1 (a protein that is highly homologous to CREB) and a novel polypeptide(s) of ∼100 kDa (CBP100) are the major CREB-binding proteins in extracts from UF9 cells. As expected ATF1 is detected due to leucine zipper-dependent heterodimerisation with CREB. In contrast CBP100 interacts with CREB independently of the leucine zipper. The total amount of ATF1 and the amount of ATF1 that is complexed with CREB are substantially reduced following differentiation. In addition, ATF1 mRNA levels are lower in differentiated F9 cells indicating that a pretranslational mechanism contributes to the decreased ATF1 protein levels observed. CBP100 levels are also reduced or CBP100 is modified upon differentiation. We discuss the potential roles of ATF1 and CBP100 in regulating CREB activity during differentiation of F9 embryonal carcinoma cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz L. A., Gilman M. Z. Two distinct forms of active transcription factor CREB (cAMP response element binding protein). Proc Natl Acad Sci U S A. 1990 Jul;87(14):5258–5262. doi: 10.1073/pnas.87.14.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz L. A., Riabowol K. T., Gilman M. Z. Multiple sequence elements of a single functional class are required for cyclic AMP responsiveness of the mouse c-fos promoter. Mol Cell Biol. 1989 Oct;9(10):4272–4281. doi: 10.1128/mcb.9.10.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weih F., Schmidt A., Fournier R. E., Schütz G. A cyclic AMP response element mediates repression of tyrosine aminotransferase gene transcription by the tissue-specific extinguisher locus Tse-1. Cell. 1990 Jun 1;61(5):905–916. doi: 10.1016/0092-8674(90)90201-o. [DOI] [PubMed] [Google Scholar]
- Carey M. Mechanistic advances in eukaryotic gene activation. Curr Opin Cell Biol. 1991 Jun;3(3):452–460. doi: 10.1016/0955-0674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Carr D. W., Scott J. D. Blotting and band-shifting: techniques for studying protein-protein interactions. Trends Biochem Sci. 1992 Jul;17(7):246–249. doi: 10.1016/0968-0004(92)90402-u. [DOI] [PubMed] [Google Scholar]
- Daniel F., Morello D., Le Bail O., Chambon P., Cayre Y., Kourilsky P. Structure and expression of the mouse beta 2-microglobulin gene isolated from somatic and non-expressing teratocarcinoma cells. EMBO J. 1983;2(7):1061–1065. doi: 10.1002/j.1460-2075.1983.tb01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P. K., Karl K. A., Colicos M. A., Prywes R., Kandel E. R. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Simon M. C., Roeder R. G. Multiple sequence elements in the c-fos promoter mediate induction by cAMP. Genes Dev. 1989 Feb;3(2):198–211. doi: 10.1101/gad.3.2.198. [DOI] [PubMed] [Google Scholar]
- Flint K. J., Jones N. C. Differential regulation of three members of the ATF/CREB family of DNA-binding proteins. Oncogene. 1991 Nov;6(11):2019–2026. [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Laoide B. M., Schlotter F., Sassone-Corsi P. Transcriptional antagonist cAMP-responsive element modulator (CREM) down-regulates c-fos cAMP-induced expression. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5448–5452. doi: 10.1073/pnas.88.12.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes N. S., Mellström B., Benusiglio E., Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992 Jan 2;355(6355):80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Menzel P., Leonard J., Fischer W. H., Montminy M. R. Characterization of motifs which are critical for activity of the cyclic AMP-responsive transcription factor CREB. Mol Cell Biol. 1991 Mar;11(3):1306–1312. doi: 10.1128/mcb.11.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Greenblatt J. Roles of TFIID in transcriptional initiation by RNA polymerase II. Cell. 1991 Sep 20;66(6):1067–1070. doi: 10.1016/0092-8674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler J. P., Lustbader J. W., Chen C. Y. Identification of multiple nuclear factors that interact with cyclic adenosine 3',5'-monophosphate response element-binding protein and activating transcription factor-2 by protein-protein interactions. Mol Endocrinol. 1991 Feb;5(2):256–266. doi: 10.1210/mend-5-2-256. [DOI] [PubMed] [Google Scholar]
- Hurst H. C., Masson N., Jones N. C., Lee K. A. The cellular transcription factor CREB corresponds to activating transcription factor 47 (ATF-47) and forms complexes with a group of polypeptides related to ATF-43. Mol Cell Biol. 1990 Dec;10(12):6192–6203. doi: 10.1128/mcb.10.12.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst H. C., Totty N. F., Jones N. C. Identification and functional characterisation of the cellular activating transcription factor 43 (ATF-43) protein. Nucleic Acids Res. 1991 Sep 11;19(17):4601–4609. doi: 10.1093/nar/19.17.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. Complex inhibitions. Curr Biol. 1991 Aug;1(4):224–226. doi: 10.1016/0960-9822(91)90063-3. [DOI] [PubMed] [Google Scholar]
- Jones N. Transcriptional regulation by dimerization: two sides to an incestuous relationship. Cell. 1990 Apr 6;61(1):9–11. doi: 10.1016/0092-8674(90)90207-u. [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C., MacMillan E. M., Nomura T., Sarai A., Ramsay R. G., Aimoto S., Ishii S., Gonda T. J. Transactivation and transformation by Myb are negatively regulated by a leucine-zipper structure. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3088–3092. doi: 10.1073/pnas.89.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Q., Yun Y. D., Hoeffler J. P., Habener J. F. Cyclic-AMP-responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990 Dec;9(13):4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Leonard J., Serup P., Gonzalez G., Edlund T., Montminy M. The LIM family transcription factor Isl-1 requires cAMP response element binding protein to promote somatostatin expression in pancreatic islet cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6247–6251. doi: 10.1073/pnas.89.14.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Liu F., Green M. R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell. 1990 Jun 29;61(7):1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
- Macgregor P. F., Abate C., Curran T. Direct cloning of leucine zipper proteins: Jun binds cooperatively to the CRE with CRE-BP1. Oncogene. 1990 Apr;5(4):451–458. [PubMed] [Google Scholar]
- Maekawa T., Matsuda S., Fujisawa J., Yoshida M., Ishii S. Cyclic AMP response element-binding protein, CRE-BP1, mediates the E1A-induced but not the Tax-induced trans-activation. Oncogene. 1991 Apr;6(4):627–632. [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas as a model system for the study of embryogenesis and neoplasia. Cell. 1975 Jul;5(3):229–243. doi: 10.1016/0092-8674(75)90098-7. [DOI] [PubMed] [Google Scholar]
- Masson N., Ellis M., Goodbourn S., Lee K. A. Cyclic AMP response element-binding protein and the catalytic subunit of protein kinase A are present in F9 embryonal carcinoma cells but are unable to activate the somatostatin promoter. Mol Cell Biol. 1992 Mar;12(3):1096–1106. doi: 10.1128/mcb.12.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A., Brady H., Theill L. E., Karin M. Regulation of the pituitary-specific homeobox gene GHF1 by cell-autonomous and environmental cues. Nature. 1990 Jun 28;345(6278):829–832. doi: 10.1038/345829a0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Gonzalez G. A., Yamamoto K. K. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990 May;13(5):184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- Nichols M., Weih F., Schmid W., DeVack C., Kowenz-Leutz E., Luckow B., Boshart M., Schütz G. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. EMBO J. 1992 Sep;11(9):3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Gann A. A. Activators and targets. Nature. 1990 Jul 26;346(6282):329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- Ransone L. J., Wamsley P., Morley K. L., Verma I. M. Domain swapping reveals the modular nature of Fos, Jun, and CREB proteins. Mol Cell Biol. 1990 Sep;10(9):4565–4573. doi: 10.1128/mcb.10.9.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfuss R. P., Walton K. M., Loriaux M. M., Goodman R. H. The cAMP-regulated enhancer-binding protein ATF-1 activates transcription in response to cAMP-dependent protein kinase A. J Biol Chem. 1991 Oct 5;266(28):18431–18434. [PubMed] [Google Scholar]
- Sassone-Corsi P., Visvader J., Ferland L., Mellon P. L., Verma I. M. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988 Dec;2(12A):1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- Sheng M., Thompson M. A., Greenberg M. E. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991 Jun 7;252(5011):1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Tassios P. T., La Thangue N. B. A multiplicity of differentiation-regulated ATF site-binding activities in embryonal carcinoma cells with distinct sequence and promoter specificities. New Biol. 1990 Dec;2(12):1123–1134. [PubMed] [Google Scholar]
- Vallejo M., Miller C. P., Habener J. F. Somatostatin gene transcription regulated by a bipartite pancreatic islet D-cell-specific enhancer coupled synergetically to a cAMP response element. J Biol Chem. 1992 Jun 25;267(18):12868–12875. [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Wada T., Watanabe H., Usuda Y., Handa H. Different biological activities of the hetero- and homodimers formed by the 47- and 43-kilodalton proteins of transcription factor ATF/E4TF3. J Virol. 1991 Feb;65(2):557–564. doi: 10.1128/jvi.65.2.557-564.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Menzel P., Rivier J., Montminy M. R. Characterization of a bipartite activator domain in transcription factor CREB. Cell. 1990 Feb 23;60(4):611–617. doi: 10.1016/0092-8674(90)90664-z. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription factors: a new family gathers at the cAMP response site. Trends Genet. 1990 Mar;6(3):69–72. doi: 10.1016/0168-9525(90)90081-g. [DOI] [PubMed] [Google Scholar]