Abstract
Objective
In adults, the ratio of plant sterols to cholesterol in plasma correlates with dietary cholesterol absorption. We hypothesized that this correlation could be validated in children with Smith Lemli-Opitz syndrome (SLOS), a cholesterol synthesis disorder.
Study design
We obtained measurements of cholesterol absorption by a direct radioisotope cholesterol absorption method during 9 SLOS subject visits. We measured plasma sterols in 22 SLOS subjects and 16 controls, as well as dietary intake of cholesterol and sitosterol (n=11 SLOS).
Results
The correlations of 2 plasma plant sterol ratios (sitosterol/cholesterol and campesterol/cholesterol) with direct cholesterol absorption measurement were poor (R= −0.33 and R= −0.25, respectively), significantly lower than the published correlation in adults (R=+0.73) (P<0.02).
Conclusions
Although the ratios of plant sterols to cholesterol in plasma has been used as a surrogate for cholesterol absorption in adults and children, these ratios may not accurately reflect cholesterol absorption in children with SLOS. These ratios should not be used as a surrogate for cholesterol absorption in children without further validation.
Keywords: sitosterol, phytosterols, gas chromatography, dietary cholesterol, dietary phytosterols, plant sterols, clinical study, children
INTRODUCTION
The Smith-Lemli-Opitz syndrome (SLOS) is an autosomal recessive condition with multiple malformations and mental retardation (OMIM# 270400) caused by mutations in the gene (DHCR7) encoding the final enzyme in the cholesterol biosynthetic pathway, 7-dehydrocholesterol Δ7-reductase (DHCR7; E.C. 1.3.1.21)(1). This enzyme defect results in low concentrations of cholesterol in plasma and tissues and high concentrations of the precursors: 7-dehydrocholesterol and its derivative 8-dehydrocholesterol. These abnormal concentrations likely contribute to the clinical features. Even though hypercholesterolemia is a well-known risk factor for cardiovascular disease, it is important to remember that cholesterol is essential for normal development and function. For example, cholesterol is a necessary component of cell membranes, a substrate for bile acids and steroid hormones, a signal for synaptogenesis (2), a major component of myelin, and a necessary component for the activation of sonic hedgehog proteins (3).
Monitoring the absorption of dietary cholesterol is important in SLOS, because dietary cholesterol is widely used as a potential treatment. The absorption of dietary cholesterol in children with SLOS has been demonstrated indirectly by the increase in plasma cholesterol after cholesterol consumption (4–8). In addition, we recently directly measured cholesterol absorption in children with SLOS (9). Due to the risk of radiation exposure, this method is used only twice in the SLOS children and not at all in controls in our studies, limiting the utility of this method in testing potential treatments. A stable isotope method using 13C-cholesterol administered orally and D7-cholesterol administered intravenously has been reported in children to measure cholesterol absorption (10). The stable isotopes, however, are expensive and the instrumentation needed for isotope ratio mass spectrometric analysis is not widely available. Furthermore, because the measurements can only be done invasively with an intravenous catheter and multiple blood samples, this technique is limited to older, larger children. A simpler and lower risk method available to children of all ages is needed in the study of SLOS as well as other conditions affecting cholesterol metabolism.
A correlation between the ratios of plant sterols to cholesterol in plasma (μmol plant sterols/mmol cholesterol) and direct measure of cholesterol absorption has been described for adults (11–13). There are no published data showing a similar correlation in children. If the correlation between the ratios of plant sterols to cholesterol in plasma and cholesterol absorption were validated in SLOS children, then this method could allow for simple, relatively non-invasive monitoring of dietary cholesterol absorption. We obtained measurements of cholesterol absorption by a direct radioisotope cholesterol absorption method during 9 SLOS subject visits. We also measured the ratios of plant sterols to cholesterol in plasma (μg sitosterol/mg cholesterol and μg campesterol/mg cholesterol) to determine if these ratios correlated with the direct cholesterol absorption measurement.
METHODS
Subjects
Twenty-two subjects with SLOS were enrolled in this study, 2 of whom were siblings. Nineteen were admitted to the Oregon Health & Science University (OHSU) General Clinical Research Center (GCRC) for 1-wk visits as part of our ongoing research. In 3 of the 22 subjects, we measured the plasma sterols in blood samples that had been shipped to us between visits. Prior to visits of 5 of the children the parents were instructed to feed their child an essentially cholesterol-free diet at home for 2–3 weeks before the admission; the dietary cholesterol intake ranged from 1.9 to 4.3 mg/kg/d. Thirteen children received a high cholesterol diet usually with added egg yolk; the cholesterol content ranged from 14.2 to 50.0 mg/kg/d. Four children received a high cholesterol diet with simvastatin. Due to the common finding of feeding difficulties in SLOS, 8 of the children were fed via gastrostomy-tube. Sixteen control children were enrolled for blood samples; 4 were siblings of the SLOS children and 12 were patients who required a blood draw for other reasons. The OHSU Institutional Review Board approved these studies and written informed consent was obtained.
Plasma Sterol Analysis
Plasma samples were saponified and extracted into hexane by the same procedures described previously (7, 14). Concentrations of the trimethylsilyl ether derivatives of individual plasma sterols were measured by capillary-column gas chromatography (GC) with a CP-Wax 57 column (25M, 0.32mmID; Chrompack-Varian, Walnut Creek CA). Internal standard (5α-cholestane) and authentic standard of cholesterol were used for calibration. The most abundant plant sterols in plasma are campesterol and sitosterol. Because 7-dehydrocholesterol and campesterol co-elute on GC, campesterol was measured by gas chromatography-mass spectrometry (GCMS) as described earlier (15). Because plasma sitosterol and campesterol are transported with cholesterol in lipoproteins, the absolute concentrations of sitosterol and campesterol (μg/dL) were adjusted for concentration of cholesterol (mg/dL) (analyzed simultaneously from the same sample) and are expressed as a ratio: plasma plant sterol/cholesterol (μg plant sterol /mg of cholesterol).
Food Analysis
During each 1-week visit, all food given to subjects was weighed before and after consumption, and exact amounts of food consumed were calculated by GCRC bionutrition staff. The study dietitian calculated the cholesterol content of the food consumed during each visit using the program Food Processor (ESHA Research, Salem, OR). Additionally, a composite, made up of 10% of the total gram weight of food consumed, was blended and an aliquot was analyzed for cholesterol content by GC. Cholesterol measured in food aliquots was highly correlated with the cholesterol calculated from weighed samples (mg cholesterol/g food) (R=0.989). Because the database of plant sterol composition of foods is less comprehensive than that of cholesterol, the 3 predominant dietary plant sterols (sitosterol, campesterol and stigmasterol) were not calculated by the study dietitian and were only measured in the food aliquot by GC. Sitosterol represented 70 ± 5% of the total plant sterols, and there was a close correlation of sitosterol with total plant sterols (R=0.99) indicating that sitosterol, the most abundant plant sterol in the diet, is proportional to the total plant sterols consumed.
Cholesterol absorption
Direct measurement of dietary cholesterol absorption was obtained by fecal dual isotope ratio method during 9 SLOS subject visits (one child was evaluated twice during separate admissions). The concentrations of plasma plant sterols and cholesterol were available for each visit. This method was described previously (9). The dosing plan was reviewed and approved by the OHSU Radiation Safety Committee. In brief, a radioactive test meal was provided with an age based dose of the radioisotopes 4-14C cholesterol and 5,6-3H sitostanol, and carrier cholesterol (0.4mg) in canola oil mixed with cholesterol from egg yolk (14.5 ± 1.0mg/kg). This cholesterol mix was added to appropriate food or formula and administered to the child. The same dietary protocols were used for children who were gastrostomy-tube fed or orally fed. Radioactivity of blood samples obtained at 24 and 48 h after the meal verified the absorption of the 14C -cholesterol. Stools collected over the 4 to 6 days following the meal were pooled, homogenized with equal amounts of water, and frozen until analyzed. The radioactivity of an aliquot of the stool (0.5 to 1.0g) was determined according to the method described previously (9, 14). Although 13 cholesterol absorption tests were initially completed, 4 were excluded due to low recovery in the stools of the radioactive sitostanol which is unabsorbed by the digestive tract. This suggests incomplete collection of the stools during the visit.
Statistical analysis
Data are expressed as mean ± SD. Correlations of the ratios of plant sterols to cholesterol with directly measured cholesterol absorption in children were determined by linear mixed modeling that accounted for the correlation within sibling groups and were compared to published correlations in adults with normal cholesterol. A T-test was used to compare concentrations of sitosterol in plasma.
RESULTS
Cholesterol absorption
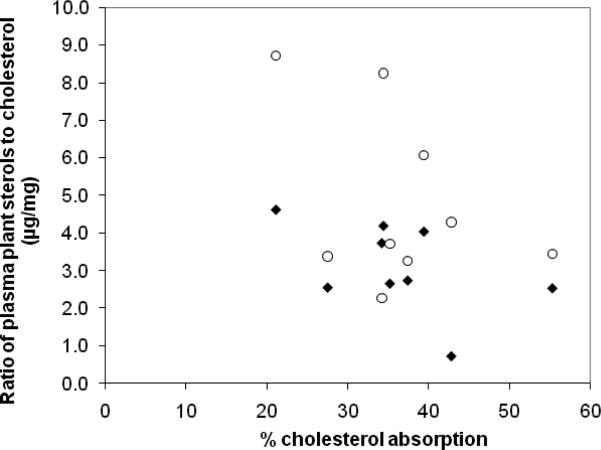
Nine cholesterol absorption test results are reported in Figure 1. None of these were reported in our previous study (9). The mean plasma cholesterol specific activity (dpm/mg cholesterol) for 24 and 48 h after the test meal was similar to values reported earlier (Table 1)(9). 3H-sitostanol was not detected in any of the plasma samples. In subject 1, 14C-cholesterol was not detected in the usual plasma sample volume of 0.2ml probably due to the very low concentration of cholesterol and an inadequate blood sample from the infant. There was a negative correlation between the ratio of sitosterol to cholesterol in plasma and direct measure of cholesterol absorption (R= −0.33) (Figure 1)(Table 3; online). Although the number of test results is small, this correlation was significantly lower (p<0.02) than the correlation of 0.73 that has been published for adults (11). The correlation of the ratio of campesterol to cholesterol in plasma with cholesterol absorption (R=-0.25) was also significantly lower (p<0.01) than 0.73.
Figure 1.
The correlation coefficients of ratios of sitosterol to cholesterol in plasma (filled diamonds) (R= −0.33) and the ratios of campesterol to cholesterol in plasma (open circles) (R= -0.25) (μg plant sterol/mg cholesterol) with the fractional cholesterol absorption (percentage of the amount given) were poor. These were significantly lower than similar correlations in adults R= + 0.73, (11).
Table 1.
Radioactive test meal and specific activity of plasma cholesterol in the direct measure of cholesterol absorption.
| Plasma cholesterol* |
||||||
|---|---|---|---|---|---|---|
| subject | age | wt | Cholesterol in test meal | 24 hr | 48 hr | |
| yr | kg | mg | μCi | dpm/mg | dpm/mg | |
| 1 | 0.4 | 4.3 | 60 | 1.11 | ND† | ND† |
| 2 | 0.5 | 5.5 | 81 | 1.21 | 421 | 389 |
| 3 | 1 | 6.4 | 83 | 1.16 | 167 | 193 |
| 5a | 1.9 | 9.7 | 135 | 1.22 | 300 | 379 |
| 5b | 2.4 | 10.3 | 142 | 1.22 | 332 | 288 |
| 6 | 2.9 | 13.7 | 212 | 2.27 | 281 | 290 |
| 7 | 3.1 | 10.9 | 154 | 3.6 | 446 | 450 |
| 16 | 9.3 | 20 | 312 | 3.66 | 301 | 459 |
| 17 | 10.2 | 20.5 | 324 | 3.7 | 256 | 361 |
|
| ||||||
| mean | 313 | 351 | ||||
| SD | 89.1 | 89.7 | ||||
specific activity as dpm of14 Ccholesterol/mgcholesterol
ND not detected
Plasma sitosterol concentrations in SLOS and controls
To determine if the lack of correlation was due to differences in the ratios of plant sterols to cholesterol in plasma in children with SLOS compared to controls, plasma sitosterol and cholesterol concentrations were measured in 22 SLOS children and 16 controls having a similar age range. (Table 2). The mean plasma sitosterol concentration of the SLOS children was significantly lower than controls (P<0.001). Values in the literature of sitosterol concentrations for children are similar to ours, ranging from 187 to 289μg/dL(16–19). The ratios of sitosterol to cholesterol in the children with SLOS were higher (but not significantly higher) than the ratios in control children. These ratios are similar to those in the literature ranging from 1.23 to 2.17 for children (19–22) and 1.04 to 1.70 for adults(23–26).
Dietary cholesterol, sitosterol or fat intake and plasma sitosterol
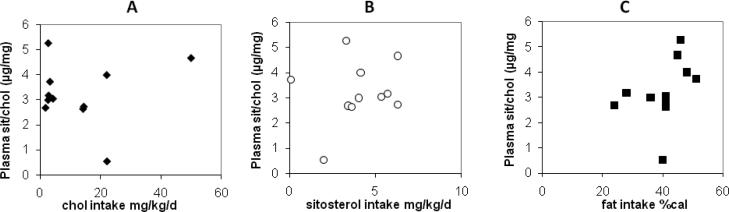
During 11 of the 1-week visits we measured dietary consumption of cholesterol, sitosterol, campesterol, stigmasterol, and fat. Due to study protocol, dietary cholesterol had a wide range: some children were fed an essentially cholesterol free diet and others were fed a high cholesterol diet. There was also a wide range of the consumption of dietary sitosterol (0.1 to 6.3 mg/kg/d). All children had an adequate consumption of dietary fat: the percent of total kilocalories from fat ranged from 24 to 48. There were no strong correlations among any of these dietary components with ratios of sitosterol to cholesterol in plasma Figure 2). Due to the small sample size, however, we are unable to rule out possible weak correlations between diet and plant sterol concentrations.
Figure 2.
Correlations between each dietary component and the ratio of sitosterol to cholesterol were low:cholesterol intake (Panel A, mg/kg/d, filled diamonds) R=0.10; dietary sitosterol intake (Panel B, mg/kg/d, open circles) R=0.19; and dietary fat intake (Panel C, % of total calories, filled squares R=0.40,.
DISCUSSION
Determination of cholesterol absorption in relation to whole body cholesterol metabolism is of interest in both clinical and fundamental respects, especially in children with SLOS. Methods developed to directly measure cholesterol absorption use radioisotopes (27) or stable isotopes (28). Due to the limitations and risks of these methods, we evaluated the correlation between the ratios of plant sterols to cholesterol in plasma (used as a surrogate for cholesterol absorption in adults) and directly measured cholesterol absorption to determine whether if this ratio would be a reliable surrogate for cholesterol absorption in children with SLOS. We did not find a strong correlation between the ratios of plant sterols to cholesterol in plasma and directly measured cholesterol absorption in children with SLOS.
Four of the 22 children in our study were administered statins with a high cholesterol diet as a potential treatment. There are conflicting data in the literature regarding the effect of statins on the absorption of dietary cholesterol (29, 30). There were no significant differences of concentration or ratio of sitosterol to cholesterol in this small subgroup. It may be prudent, however, to monitor dietary cholesterol absorption in the children with SLOS provided with high cholesterol diets and statins in research trials.
Dietary plant sterols have been shown to affect the absorption of cholesterol (31–33) and the plasma concentration of plant sterols(19). In addition, low amount of dietary fat could potentially limit fat and sterol absorption by limiting bile acid secretion. Despite the wide range of dietary cholesterol and of dietary plant sterol intake there appears to be no strong effect of these dietary components on the ratios of plasma sitosterol to cholesterol in plasma. We also considered the possibility that a low rate of bile acid synthesis in SLOS might account for the lack of correlation. In an earlier report, we quantified bile acid synthesis by the sterol balance method in SLOS and control children on a very low cholesterol diet (14)and found no significant difference in total bile acid synthesis. Another possible reason for lack of a correlation could be inaccurate cholesterol absorption determination. This seems highly unlikely since the mean fractional cholesterol absorption of the 9 test meals (36 ± 10%) was similar to our previously reported values (27 ± 7%). These percents are lower than those reported for adults, 43.7 ± 8.4% (34), there are no data, however, of direct measure of cholesterol absorption in healthy children that could be used for comparison. Furthermore, our approach to ensuring adequate collections and our internal controls (see methods) makes it highly likely that the direct cholesterol absorption measures were accurate. Perhaps the lack of correlation is specific to SLOS. The depressed cholesterol synthesis in SLOS may have a unique effect on the intestinal mucosa cells that alters the absorption and processing of sterols.
Several authors have reported ratios of plant sterol to cholesterol in children as a surrogate for cholesterol absorption based on studies in adults (20–22, 35, 36). Our results call into question this assumption. This surrogate may be found to be valid in normal control children, but not in SLOS; we cannot determine the validity in normal healthy children in the present study, as we could not measure cholesterol absorption directly in that group. Unless a correlation of the ratio of plant sterols to cholesterol in plasma with cholesterol absorption is demonstrated in children, reports using the plant sterol ratios as a surrogate for cholesterol absorption in children should be interpreted with caution.
Supplementary Material
ACKNOWLEDGMENTS
We thank the OHSU General Clinical Research Center staff: particularly Martha McMurry and Dr. Dawn Peters (statistician) and Jean O`Malley (statistician). We thank all health care providers who assisted in the care of these patients and for subject referrals to us. Dr. Melanie Gillingham provided control samples, and Dr. Jean-Baptiste Roullet provided guidance and useful discussion. We especially appreciate the willingness of the children and families to participate in the study.
Source of funding: National Heart, Lung, and Blood Institute (RO1 HL-073980), the General Clinical Research Center Grant (MO1 RR-000334), and the Oregon Clinical and Translational Research Institute, Grant (UL1 RR024140) from the National Center for Research Resources, a component of the National Institutes of Health (NIH,) and NIH Roadmap for Medical Research.
ABBREVIATIONS
- SLOS
Smith Lemli-Opitz syndrome
- GC
gas chromatography
- GCMS
gas chromatography mass spectrometry
- OHSU
Oregon Health & Science University
REFERENCES
- 1.Battaile KP, Steiner RD. Smith-Lemli-Opitz syndrome: the first malformation syndrome associated with defective cholesterol synthesis. Mol Genet Metab. 2000;71:154–62. doi: 10.1006/mgme.2000.3020. [DOI] [PubMed] [Google Scholar]
- 2.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–7. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 3.Porter JA, Ekker SC, Park WJ, von Kessler DP, Young KE, Chen CH, et al. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell. 1996;86:21–34. doi: 10.1016/s0092-8674(00)80074-4. [DOI] [PubMed] [Google Scholar]
- 4.Nwokoro NA, Mulvihill JJ. Cholesterol and bile acid replacement therapy in children and adults with Smith-Lemli-Opitz (SLO/RSH) syndrome. Am J Med Genet. 1997;68:315–21. doi: 10.1002/(sici)1096-8628(19970131)68:3<315::aid-ajmg13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Irons M, Elias ER, Abuelo D, Bull MJ, Greene CL, Johnson VP, et al. Treatment of Smith-Lemli-Opitz syndrome: results of a multicenter trial. Am J Med Genet. 1997;68:311–4. [PubMed] [Google Scholar]
- 6.Elias ER, Irons MB, Hurley AD, Tint GS, Salen G. Clinical effects of cholesterol supplementation in six patients with the Smith-Lemli-Opitz syndrome (SLOS) Am J Med Genet. 1997;68:305–10. doi: 10.1002/(sici)1096-8628(19970131)68:3<305::aid-ajmg11>3.0.co;2-x. see comments. [DOI] [PubMed] [Google Scholar]
- 7.Merkens LS, Connor WE, Linck LM, Lin DS, Flavell DP, Steiner RD. Effects of dietary cholesterol on plasma lipoproteins in Smith-Lemli-Optitz syndrome. Pediatr Res. 2004;56:726–32. doi: 10.1203/01.PDR.0000141522.14177.4F. [DOI] [PubMed] [Google Scholar]
- 8.Pappu AS, Steiner RD, Connor SL, Flavell DP, Lin DS, Hatcher L, et al. Feedback inhibition of the cholesterol biosynthetic pathway in patients with Smith-Lemli-Opitz syndrome as demonstrated by urinary mevalonate excretion. J Lipid Res. 2002;43:1661–9. doi: 10.1194/jlr.m200163-jlr200. [DOI] [PubMed] [Google Scholar]
- 9.Lin DS, Steiner RD, Flavell DP, Connor WE. Intestinal absorption of cholesterol by patients with Smith-Lemli-Opitz syndrome. Pediatr Res. 2005;57:765–70. doi: 10.1203/01.PDR.0000157723.98422.B5. [DOI] [PubMed] [Google Scholar]
- 10.Woollett LA, Wang Y, Buckley DD, Yao L, Chin S, Granholm N, et al. Micellar solubilisation of cholesterol is essential for absorption in humans. Gut. 2006;55:197–204. doi: 10.1136/gut.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilvis RS, Miettinen TA. Serum plant sterols and their relation to cholesterol absorption. Amer J Clin Nutr. 1986;43:92–7. doi: 10.1093/ajcn/43.1.92. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 13.von Bergmann K, Lutjohann D, Lindenthal B, Steinmetz A. Efficiency of intestinal cholesterol absorption in humans is not related to apoE phenotype. J Lipid Res. 2003;44:193–7. doi: 10.1194/jlr.m200319-jlr200. [DOI] [PubMed] [Google Scholar]
- 14.Steiner RD, Linck LM, Flavell DP, Lin DS, Connor WE. Sterol balance in the Smith-Lemli-Opitz syndrome. Reduction in whole body cholesterol synthesis and normal bile acid production. J Lipid Res. 2000;41:1437–47. [PubMed] [Google Scholar]
- 15.Fricke CB, Schroder M, Poulsen M, von Bergmann K, Wester I, Knudsen I, et al. Increased plant sterol and stanol levels in brain of Watanabe rabbits fed rapeseed oil derived plant sterol or stanol esters. British Journal of Nutrition. 2007;98:890–9. doi: 10.1017/S0007114507756532. [DOI] [PubMed] [Google Scholar]
- 16.Kempen HJ, de Knijff P, Boomsma DI, van der Voort HA, Gevers Leuven JA, Havekes L. Plasma levels of lathosterol and phytosterols in relation to age, sex, anthropometric parameters, plasma lipids, and apolipoprotein E phenotype, in 160 Dutch families. Metabolism. 1991;40:604–11. doi: 10.1016/0026-0495(91)90051-w. [DOI] [PubMed] [Google Scholar]
- 17.Ketomaki A, Gylling H, Simes MA, Vuirio A, Miettinen TA. Squalene and noncholesterol sterols in serum and lipoproteins of children with and without familial hypercholestrolemia. Pediatr Res. 2003;53:648–53. doi: 10.1203/01.PDR.0000055771.28409.40. [DOI] [PubMed] [Google Scholar]
- 18.Tammi A, Ronnemaa T, Rask-Nissila L, Miettinen TA, Gylling H, Valsta L, et al. Apolipoprotein E phenotype regulates cholesterol absorption in healthy 13-month-old children--The STRIP Study. Pediatr Res. 2001;50:688–91. doi: 10.1203/00006450-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Tammi A, Ronnemaa T, Valsta L, Seppanen R, Rask-Nissila L, Miettinen TA, et al. Dietary plant sterols alter the serum plant sterol concentration but not the cholesterol precursor sterol concentrations in young children (the STRIP Study). Special Turku Coronary Risk Factor Intervention Project. J Nutr. 2001;131:1942–5. doi: 10.1093/jn/131.7.1942. [DOI] [PubMed] [Google Scholar]
- 20.Jarvisalo M, Raitakari O, Gylling H, Miettinen TA. Cholesterol absorption and synthesis in children with type 1 diabetes. Diabetes Care. 2006;29:2300–4. doi: 10.2337/dc05-2235. [DOI] [PubMed] [Google Scholar]
- 21.Joki P, Suomalainen H, Jarvinen KM, Juntunen-Backman K, Gylling H, Miettinen TA, et al. Cholesterol precursors and plant sterols in children with food allergy. American Journal of Clinical Nutrition. 2003;77:51–5. doi: 10.1093/ajcn/77.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Siirtola A, Ketomaki A, Miettinen TA, Gylling H, Lehtimaki T, Holmberg C, et al. Cholesterol absorption and synthesis in pediatric kidney, liver, and heart transplant recipients. Transplantation. 2006;81:327–34. doi: 10.1097/01.tp.0000189173.46727.18. [DOI] [PubMed] [Google Scholar]
- 23.Berge KE, von Bergmann K, Lutjohann D, Guerra R, Grundy SM, Hobbs HH, et al. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J Lipid Res. 2002;43:486–94. [PubMed] [Google Scholar]
- 24.von Bergmann K, Sudhop T, Lutjohann D. Cholesterol and plant sterol absorption: recent insights. Amer J Cardiol. 2005;96:4. doi: 10.1016/j.amjcard.2005.03.014. [Review] [20 refs] [DOI] [PubMed] [Google Scholar]
- 25.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism: Clinical & Experimental. 1989;38:136–40. doi: 10.1016/0026-0495(89)90252-7. [DOI] [PubMed] [Google Scholar]
- 26.Nissinen MJ, Gylling H, Miettinen TA. Responses of surrogate markers of cholesterol absorption and synthesis to changes in cholesterol metabolism during various amounts of fat and cholesterol feeding among healthy men. Br J Nutrition. 2008;99:370–8. doi: 10.1017/S0007114507811998. [DOI] [PubMed] [Google Scholar]
- 27.Matthan NR, Lichtenstein AH. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 2004;174:197–205. doi: 10.1016/S0021-9150(03)00248-X. [Review] [63 refs] [DOI] [PubMed] [Google Scholar]
- 28.Woollett LA, Buckley DD, Yao L, Jones PJ, Granholm NA, Tolley EA, et al. Effect of ursodeoxycholic acid on cholesterol absorption and metabolism in humans. J Lipid Res. 2003;44:935–42. doi: 10.1194/jlr.M200478-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Vanhanen H, Kesaniemi YA, Miettinen TA. Pravastatin lowers serum cholesterol, cholesterol-precursor sterols, fecal steroids, and cholesterol absorption in man. Metabolism. 1992;41:588–95. doi: 10.1016/0026-0495(92)90050-k. [DOI] [PubMed] [Google Scholar]
- 30.Miettinen TA, Gylling H. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur J Clin Invest. 2003;33:976–82. doi: 10.1046/j.1365-2362.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 31.Ostlund RE, Jr., Racette SB, Okeke A, Stenson WF. Phytosterols that are naturally present in commercial corn oil significantly reduce cholesterol absorption in humans. American Journal of Clinical Nutrition. 2002;75:1000–4. doi: 10.1093/ajcn/75.6.1000. see comment. [DOI] [PubMed] [Google Scholar]
- 32.Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R, et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc. 2003;78:965–78. doi: 10.4065/78.8.965. [DOI] [PubMed] [Google Scholar]
- 33.Haney EM, Huffman LH, Bougatsos C, Freeman M, Steiner RD, Nelson HD. Screening and treatment for lipid disorders in children and adolescents: systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2007;120:e189–214. doi: 10.1542/peds.2006-1801. [DOI] [PubMed] [Google Scholar]
- 34.Connor WE, Lin DS. The intestinal absorption of dietary cholesterol by hypercholesterolemic (type II) and normocholesterolemic humans. J Clin Invest. 1974;53:1062–70. doi: 10.1172/JCI107643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedman M, Miettinen TA, Gylling H, Ketomaki A, Antikainen M. Serum noncholesterol sterols in children with heterozygous familial hypercholesterolemia undergoing pravastatin therapy. Journal of Pediatrics. 2006;148:241–6. doi: 10.1016/j.jpeds.2005.08.068. [DOI] [PubMed] [Google Scholar]
- 36.Mortaz M, Fewtrell MS, Cole TJ, Lucas A. Cholesterol metabolism in 8 to 12-year-old children born preterm or at term. Acta Paediatrica. 2003;92:525–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.