Abstract
The neurobiological bases of increased vulnerability to substance abuse remain obscure. We report here that rats that were selectively bred for greater drug-seeking behavior exhibited higher levels of FGF2 gene expression. We then asked whether a single FGF2 administration (20ng/g, s.c.) on postnatal day 2 (PND2) can have a lifelong impact on drug-taking behavior, spatial and appetitive learning and the dopaminergic system. Indeed, early life FGF2 enhanced the acquisition of cocaine self-administration in adulthood. However, early life FGF2 did not alter spatial or operant learning in adulthood. Furthermore, early life FGF2 did not alter gene expression in the dopaminergic system in adulthood. These results suggest that elevated levels of FGF2 may lead to increased drug-taking behavior without altering learning. Thus, FGF2 may be an antecedent of vulnerability for drug-taking behavior and may provide clues to novel therapeutic approaches for the treatment of addiction.
Keywords: Neurotrophin, Dentate Gyrus, Self-administration, Postnatal
INTRODUCTION
The neural and molecular factors that predispose individuals to the initial vulnerability to drug-seeking behavior remain largely unknown. This first step that can lead to addiction has antecedents that are likely distinct from those leading to long-term use or relapse. Animal models have related individual differences in acquisition of drug-taking behavior to novelty-seeking and stress-responsiveness (Kabbaj et al., 2000; Kabbaj et al., 2001; Lucas et al., 1998; Rouge-Pont et al., 1998). For example, animals that exhibit high levels of reactivity to their environment (High Responders- HRs) spontaneously display high levels of drug-taking behavior in the initial stages. By contrast, animals that display low levels of reactivity to a novel environment (Low Responders- LRs) display low levels of drug-seeking behavior, unless they are stressed (Kabbaj et al., 2001). Thus, there seems to be two distinct paths to drug-taking behavior based on individual differences in emotionality.
We have previously assessed the role of the fibroblast growth factor (FGF) family in emotional behavior in rodents (Turner et al., 2006; Turner et al., 2008a; Turner et al., 2008b). FGF2 is the most well-described member of the family and plays a significant role in cell survival, proliferation, differentiation and migration (Ford-Perriss et al., 2001; Itoh and Ornitz, 2004; Reuss and von Bohlen und Halbach, 2003). Furthermore, FGF2 is expressed by neuronal and glial cells in mesolimbic circuitry, as well as the hippocampus. Finally, other investigators have assessed FGF2 and emotionality in the context of animal studies following various stress protocols (Bland et al., 2006; Bredy et al., 2003; Fumagalli et al., 2005; Molteni et al., 2001).
Though neurotrophic factors are best understood for the role they play in the development and maintenance of synaptic connections in the brain, emerging evidence suggests that neurotrophins contribute to substance abuse vulnerability (Graham et al., 2007). Stewart and colleagues have previously demonstrated that repeated injections of amphetamine increased FGF2 immunoreactivity in the ventral tegmental area (VTA) and substantia nigra (SN), which persisted for up to one month following the last amphetamine injection (Flores and Stewart, 2000). Here, FGF2 expression was strongly and positively correlated with the magnitude of locomotor sensitization. Furthermore, repeated injections of amphetamine resulted in increased dendritic growth of ventral tegmental dopaminergic neurons, and FGF2 was found to be essential for the morphological changes (Mueller et al., 2006). Finally, Flores et al. (2000) have demonstrated that immunoneutralization of FGF2 during the induction phase completely blocked the development of behavioral sensitization to amphetamine. These findings demonstrate that FGF2 is directly involved in the development of psychostimulant sensitization and, therefore, may be a key factor underlying the molecular mechanisms of addiction.
To this end, we wondered whether animals selectively bred for individual differences in response to novelty exhibit differences in FGF2 (Kabbaj et al., 2000; Stead et al., 2006b). Specifically, we assessed whether selectively bred HR and LR rats differ in FGF2 gene expression. Finally, we sought to determine whether FGF2 may play a causative role in the predisposition to self-administer cocaine. Here, we administered FGF2 to outbred animals during the neonatal period and assess cocaine self-administration in adulthood.
MATERIALS & METHODS
Animals
Sprague-Dawley rats from Charles River (Wilmington, MA) were bred and maintained at the University of Michigan animal facilities in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). The animals were maintained under conditions of constant temperature and a 12:12 light:dark cycle with free access to food and water in their homecage (43 × 21.5 × 25.5 cm Plexiglas cages). On the day after birth (PND2) litters were culled to four males and four females and assigned to treatment groups. The pups were then injected with either FGF2 (20ng/g in 50μl in 0.1M PBS w/ 1% BSA, s.c.; Sigma) or vehicle (0.1M PBS w/ 1% BSA). Group assignments were counterbalanced within each litter. Previous studies have shown that the dose and timing of FGF2 used crosses the blood brain barrier and alters hippocampal neurogenesis (Cheng et al., 2002; Cheng et al., 2001; Wagner et al., 1999). Since drugs of abuse are also known to alter hippocampal neurogenesis (Eisch and Harburg, 2006; Yamaguchi et al., 2004; Yamaguchi et al., 2005), we wondered whether altering hippocampal neurogenesis early in development by FGF2 injection would alter drug-taking behavior in adulthood. Males were housed two per cage following weaning (PND21) and handled for two days prior to all behavioral experiments. For operant learning and cocaine self-administration, animals were housed on a reversed cycle 12:12h light:dark cycle (lights on at 1800), with testing starting on PND67 between 1300 and 1700. A separate group of animals were sacrificed (PND67) and brains snap frozen in isopentane (−80°C) for mRNA in situ hybridization of dopaminergic transcripts.
High responder (HR) and low responder (LR) rats were produced from our in-house Sprague-Dawley breeding colony (F6 generation) (for methods see Stead et al., 2006a). These animals were selectively bred based on differences in novelty-seeking behavior. HR animals exhibit a high locomotor response to a novel environment, and LR animals exhibit a low locomotor response to a novel environment. The animals were sacrificed (PND90) and brains were snap frozen in isopentane (−80°C). Brains were sectioned in series at 20 μm throughout the dorsal hippocampus and processed for FGF2 mRNA by in situ hybridization.
Morris Water Maze (MWM)
Animals were tested in the Morris Water Maze (PND67), as previously described, with the exception of four trials per day (Isgor et al., 2004). The water maze was an opaque-white circular tank 1.5 m in diameter and 0.5 m in height. The water was made opaque by the addition of nondairy creamer. A circular Plexiglas platform 10 cm in diameter was used as the escape platform. The tank was placed in the middle of a room that contained visual extra-maze cues. Animals were given four trials per day for 5 consecutive days to find a hidden platform. The order of animals was randomized between days and counterbalanced between groups. Animals started each trial from one of four randomized directions facing the wall with an inter-trial interval of 5-10 minutes. Each trial consisted of a maximum of two minutes. The latency to find the hidden platform (submerged 2 cm below water level) was scored for each animal on each trial. The animals were allowed to remain on the platform for 30 sec. If the animals did not locate the platform within 2 minutes, the animals were guided to the platform and allowed to remain there for 30 sec. Latency to find the hidden platform was averaged across trials for each day per animal and presented by group.
Operant Learning
MED Associates operant conditioning chambers (20.5 × 24.1 cm floor area, 29.2 cm high; MED Associates, St. Albans, VT) were used to assess operant learning for a food reward, banana-flavored food pellets (BioServe®, #F0059, Frenchtown, NJ). One hole was designated “active” and the other “inactive,” in a counterbalanced design. Each testing session began with illumination of the hole designated active (Kosten et al., 2000). Responses in the active hole resulted in delivery of one food pellet on a fixed ratio (FR1) schedule of reinforcement. There was a 10 sec timeout period following each pellet delivery. Each testing session was 30 min long or terminated when a maximum of 50 food pellets were delivered. Testing continued for 5 consecutive days.
Cocaine Self-Administration
Animals were individually housed during the self-administration paradigm. An intravenous catheter (Dow Corning, Midland, MI) was implanted under anesthesia in the right jugular vein. The catheter exited into a connector (a modified 22 gauge cannula; Plastics One, Roanoke, VA). A plastic blocker was placed over the opening of the connector during a recovery period of 4-5 days. Prior to and after self-administration training, catheter patency was checked using Penthotal (0.15-0.2 ml, i.v.). Cocaine HCl was dissolved in a saline solution. Gentamicin Sulfate (1 mg/kg, GentaVed 100 ml, Vedco) was used to flush the catheter daily. The self-administration chambers were equipped with two holes, one “active,” (Med Associates, St. Albans, VT) and another “inactive” hole. An infusion pump (Razel Scientific Instruments, Stamford, CT) was activated by nose pokes into the “active” hole. Immediately following activation of the active hole, responses made during this 20 sec period were recorded, but did not result in reactivation of the pump. Rats were trained to self-administer cocaine (0.25 mg/kg/infusion, i.v.) on a fixed ratio (FR1) schedule of reinforcement during daily 3 h self-administration sessions over 9 days. A maximum of 60 infusions was allowed per session.
mRNA in situ hybridization
For dopaminergic transcripts, tissue was sectioned at −20°C at 10μm and sliced in series throughout the brain and stored at −80°C. Sections were taken every 200 μm and mounted on slides. In situ hybridization methodology has been previously described elsewhere (Kabbaj et al., 2000). The sequences of rat mRNA used for generating probes were complementary to the following RefSeq database nos. FGF2 (NM_019305, 716-994); D1 (M35077, 420-900); D2 (M36831, 25-520); TH (M10244, 89-363); DAT (M80233, 719-1251). All cDNA segments were extracted (Qiaquick Gel Extraction Kit, Qiagen, Valencia, CA), subcloned in Bluescript SK (Stratagene, LA Jolla, CA.) and confirmed by nucleotide sequencing. Exposure times for each probe were as follows: FGF2 (7 days), D1 (24 hours), D2 (24 hours), TH (24 hours), DAT (24 hours). Brain section images were captured from film with a CCD camera (TM-745, Pulnix) using MCID. Radioactive signals were quantified using computer-assisted optical densitometry software (Scion Image). Data from multiple sections per animal were averaged resulting in a mean integrated optical density value for each animal and then averaged for each group.
Statistical Analyses
A Student’s t test was used to analyze gene expression by mRNA in situ hybridization. Operant learning, Morris water maze and cocaine self-administration were analyzed by a two-way repeated measures ANOVA with Fisher post hoc comparisons. Nose pokes were analyzed by a three-way repeated measures ANOVA.
RESULTS
First, we assessed whether animals selectively bred for differences in novelty-seeking behavior would differ in FGF2 gene expression. HR-bred animals exhibited increased FGF2 gene expression in the dentate gyrus compared to LR-bred animals by mRNA in situ hybridization [t(9) = 2.46, p < 0.05], see Table 1. These findings were also specific to the dentate gyrus, as differences were not observed in CA1 [t(9) = 1.56, p > 0.05], CA2 [t(9) = 0.56, p > 0.05] or CA3 [t(9) = 0.51, p > 0.05] of the hippocampus. Thus, FGF2 gene expression was increased basally in the dentate gyrus of animals that naturally exhibit an enhanced acquisition of cocaine self-administration. These results suggest that alterations in FGF2 may influence the vulnerability to drug-taking behavior.
Table 1. Average integrated optical density values of FGF2 mRNA by in situ hybridization in the hippocampus of selectively bred HR and LR animals.
| Region | n | HR ± s.e.m. | n | LR ± s.e.m. |
|---|---|---|---|---|
| CA1 | 5 | 2827.6 ± 226.5 | 6 | 2489.8 ± 65.4 |
| CA2 | 5 | 19662.1 ± 2375.6 | 6 | 18185.1 ± 1390.2 |
| CA3 | 5 | 2569.7 ± 174.5 | 6 | 2422.7 ± 218.4 |
| DG | 5 | 18378.6 ± 1268.5* | 6 | 15048.3 ± 650.4 |
p < 0.05 versus LR, Student’s t test
For this and all subsequent results, outbred rats were injected with either FGF2 (20ng/g, s.c.) or vehicle on postnatal day (PND) 2. In terms of cocaine self-administration, there was a significant main effect of day [F(8,88) = 5.559, p < 0.0001], but no main effect of group (FGF2 or vehicle) [F(1,88) = 2.812, p > 0.05]. There was, however, a significant day by group interaction [F(8,88) = 2.137, p < 0.05], with FGF2-injected animals exhibiting a higher number of infusions on days 3 and 4 than vehicle controls (p < 0.05), see Figure 1. Within group comparisons revealed significantly more drug infusions from days 3-8 relative to day 1 in the FGF2-injected group compared to the vehicle group. The vehicle group showed a greater number of infusions than the FGF2-injected group only on days 8 and 9. Furthermore, both groups discriminated between the active and inactive nose holes, suggesting that all of the animals learned the operant response. Thus, animals treated with FGF2 early in life acquired cocaine self-administration at a faster rate than vehicle controls.
Figure 1.
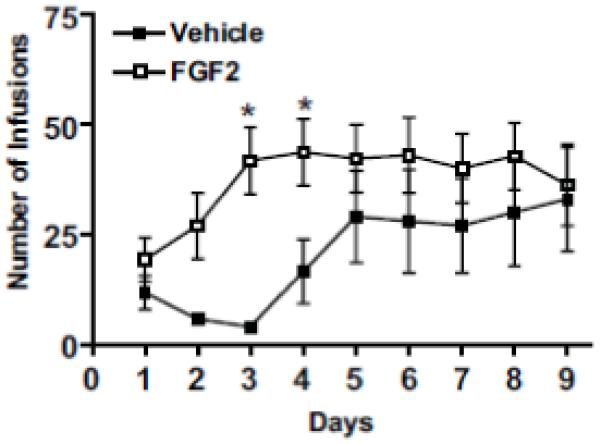
Early life FGF2 effects on cocaine self-administration in adulthood. (a) FGF2-injected animals exhibited enhanced acquisition of cocaine self-administration on days 3 and 4 compared to vehicle controls. All values are mean ± SEM (n = 8 per group). *p < 0.05.
An analysis of nose pokes into the active versus inactive holes in both FGF2-injected rats and vehicle controls during the acquisition of cocaine self-administration was also performed (data not shown). There were significant main effects of group [F(1,22) = 6.98, p < 0.05], and hole (active versus inactive) [F(1,22) = 23.08, p < 0.001]. In addition, significant interactions between group and training day (1-9) [F(8,176) = 2.605, p < 0.05], as well as between hole and training day [F(8,176) = 3.944, p < 0.0001], were also observed. Post hoc Fisher comparisons indicated that the FGF2-injected animals showed significantly higher levels of nose pokes than controls into the active hole as compared to the inactive hole (p<0.05) from Days 3-9. Similar significant increases in the number of responses were seen in the vehicle group only after Day 5 (p<0.05).
One possible interpretation of the more rapid acquisition of drug self-administration in the FGF2-injected group is that the rats learn more rapidly. Therefore, we assessed learning between the groups in two separate tasks. Spatial learning was assessed using the Morris Water maze. The FGF2-injected animals did not differ significantly from vehicle controls in the latency to find the hidden platform, see Figure 2a. There was a significant main effect of day in the Morris Water Maze [F(4,44) = 15.83, p < 0.001]. There was no main effect of group [F(1,44) = 3.4, p > 0.05]. There was also no group by day interaction [F(4,44) = 0.59, p > 0.05]. These results suggest that there are no differences on a spatial learning task between FGF2-injected animals and vehicle animals.
Figure 2.
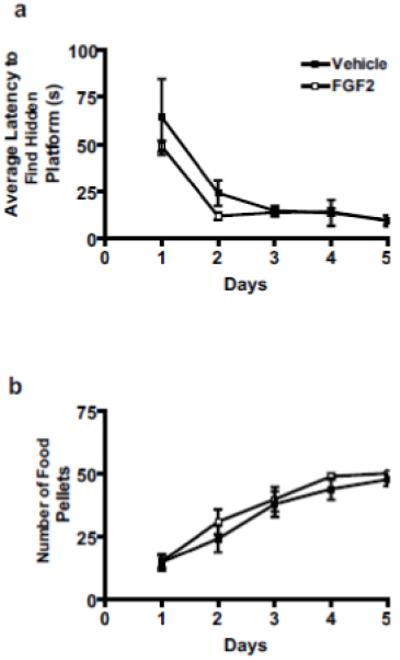
Early life FGF2 effects on spatial and appetitive learning in adulthood. Spatial learning was assessed in the Morris Water Maze, and appetitive learning was assessed by an operant learning task. (a) No differences were observed between FGF2-injected and vehicle animals in spatial learning. All values are mean ± SEM (n = 4 per group). (b) No differences were observed between FGF2-injected and vehicle animals in appetitive learning. All values are mean ± SEM (n = 14 per group).
Similarly, we did not find any differences on an appetitive learning task (ie. operant food reward). There was a significant main effect of day in the operant learning task [F(4,104) = 44.54, p < 0.001]. However, there was no main effect of group [F(1,104) = 0.74, p > 0.05]. There was also no group by day interaction [F(4,104) = 0.37, p > 0.05]. As depicted in Figure 2b, there were no differences in the amount of food consumed over the 5 day training period. Thus, there do not appear to be any differences in appetitive learning between FGF2-injected animals and vehicle animals.
An alternate hypothesis we examined is that the enhanced acquisition of cocaine self-administration in the FGF2-injected animals could be due to an altered mesolimbic reward circuit. To this end, we analyzed gene expression of dopaminergic transcripts by mRNA in situ hybridization in adult animals that received either vehicle or FGF2 early in life. As illustrated in Table 2, we assessed the dopamine D1 receptor, the dopamine D2 receptor, tyrosine hydroxylase (TH), and the dopamine transporter (DAT) in the caudate-putamen (CP), nucleus accumbens (NA), ventral tegmental area (VTA) and substantia nigra pars compacta (SN). There were no significant differences between the two groups for any of the transcripts in any of the regions. Thus, early life FGF2 treatment did not alter gene expression in the dopaminergic system in adulthood. However, it is possible that the responsiveness of the dopaminergic system to cocaine may be altered, and this has not yet been examined. Nor have we examined other indices of dopamine function.
Table 2. Average integrated optical density values of dopaminergic transcripts by mRNA in situ hybridization in various brain regions of FGF2-injected animals and vehicle controls.
| Probe-Region | n | Vehicle ± s.e.m. | n | FGF2 ± s.e.m. |
|---|---|---|---|---|
| D1-NA | 10 | 51555 ± 5633 | 10 | 39930 ± 6432 |
| D1-CP | 10 | 158017 ± 20006 | 10 | 138437 ± 17812 |
| D2-NA | 9 | 23373 ± 4322 | 4 | 26487 ± 11951 |
| D2-CP | 9 | 148929 ± 20977 | 4 | 133942 ± 56219 |
| D2-VTA | 10 | 1757 ± 441 | 10 | 2566 ± 1026 |
| D2-SN | 10 | 5343 ± 1017 | 10 | 6004 ± 1173 |
| DAT-VTA | 10 | 13078 ± 1792 | 10 | 16458 ± 3355 |
| DAT-SN | 10 | 20592 ± 2178 | 10 | 22063 ± 2723 |
| TH-VTA | 8 | 36016 ± 3606 | 10 | 33580 ± 3673 |
| TH-SN | 9 | 32021 ± 1981 | 10 | 37231 ± 2261 |
DISCUSSION
This is the first study to show that administration of FGF2 influences the propensity to self-administer drugs of abuse. Here, we report that FGF2 administration early in development led to an enhanced acquisition of cocaine self-administration in adulthood. This is also consistent with our observation that animals selectively bred for increased novelty-seeking behavior (HR) showed increased basal expression of FGF2 in the hippocampus compared to animals selectively bred for decreased novelty-seeking behavior (LR). Importantly, the early life FGF2-induced enhancement of drug-taking behavior could not be attributed to alterations in the learning capacity of the animals. Together, these findings suggest that the FGF system may play a role in the vulnerability to drug abuse.
HR animals exhibited increased FGF2 gene expression in the dentate gyrus compared to LR animals. Furthermore, this effect was specific to the dentate gyrus, as this finding did not generalize to other subfields of the hippocampus. HR animals were previously known to exhibit a higher propensity to self-administer drugs of abuse in adulthood than LR animals (Kabbaj et al., 2001). Thus, animals that naturally exhibit differences in cocaine self-administration showed evidence of alterations in FGF2 gene expression.
Perhaps most interesting, early life FGF2 treatment (PND2) enhanced acquisition of cocaine self-administration in adult animals (PND67). To our knowledge, this is the first study to demonstrate that administration of FGF2 can alter drug-taking behavior. Importantly, this finding is in the same direction as expected for a HR-like phenotype, as HR animals are known to acquire self-administration at a faster rate than LR animals (Kabbaj et al., 2001). In fact, FGF2 animals resembled HR animals in the rate of acquisition of cocaine self-administration. Similarly, five daily infusions of another neurotrophic factor, brain-derived neurotrophic factor (BDNF), has recently been found to enhance cocaine self-administration (Graham et al., 2007).
Given the above results, we examined two hypotheses that could have explained the altered acquisition of cocaine self-administration in the FGF2-injected animals—that the animals learn more effectively than vehicle animals, or that cocaine’s rewarding properties may be enhanced by the treatment. Early life FGF2 administration (PND2) did not significantly influence Morris water maze learning in adulthood (PND67), nor did early life FGF2 (PND2) influence operant learning of a food reward (PND67). Thus, early life FGF2 did not appear to have profound effects on spatial or appetitive learning in adulthood.
Finally, early life FGF2 (PND2) did not permanently alter gene expression in the dopaminergic system in adulthood (PND67). Basal levels of D1, D2, TH and DAT mRNA remained unaltered in the caudate-putamen, nucleus accumbens, ventral tegmental area or substantia nigra. While FGF2 has been implicated in the development of dopamine neurons, our treatment did not result in long-term alterations in dopaminergic transcripts (Klejbor et al., 2006; Mueller et al., 2006; Timmer et al., 2007). Future studies should attempt to assess FGF2 gene expression in the mesolimbic pathway following early life FGF2.
In summary, HR animals that naturally exhibit enhanced acquisition of cocaine self-administration compared to LR animals also exhibit increased FGF2 gene expression compared to LR animals. Furthermore, animals injected with FGF2 early in life exhibited enhanced acquisition of cocaine self-administration in adulthood compared to vehicle controls. Finally, these changes were not related to alterations in spatial or appetitive learning or alterations in gene expression in the dopaminergic system. This study suggests that the FGF system, and more specifically FGF2, may be involved in the initial vulnerability to drug addiction.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the technical assistance of Tracy Simmons, James Stewart, Jennifer Fitzpatrick, Sharon Burke, Edny Gula and the statistical consultation provided by Brady West (CSCAR, University of Michigan). We would also like to thank Dr. Ceylan Isgor and Dr. Charles Neal Jr. for their assistance and advice in the conduct of this work. We would also like to thank Dr. Terry Robinson for his advice on design and analysis of this work. This work was supported by NIH Conte Center Grant #L99MH60398, NIDA Grant #5P01DA21633-02 to HA & SJW and NIDA Training Grant #T32DA007267-13 and #T32DA007268-14 to CAT.
REFERENCES
- Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–7. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–9. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Black IB, DiCicco-Bloom E. Hippocampal granule neuron production and population size are regulated by levels of bFGF. Eur J Neurosci. 2002;15:3–12. doi: 10.1046/j.0953-816x.2001.01832.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Tao Y, Black IB, DiCicco-Bloom E. A single peripheral injection of basic fibroblast growth factor (bFGF) stimulates granule cell production and increases cerebellar growth in newborn rats. J Neurobiol. 2001;46:220–9. doi: 10.1002/1097-4695(20010215)46:3<220::aid-neu1004>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–86. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Flores C, Samaha AN, Stewart J. Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine. J Neurosci. 2000;20:RC55. doi: 10.1523/JNEUROSCI.20-02-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Stewart J. Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat. Psychopharmacology (Berlin) 2000;151:152–65. doi: 10.1007/s002130000417. [DOI] [PubMed] [Google Scholar]
- Ford-Perriss M, Abud H, Murphy M. Fibroblast growth factors in the developing central nervous system. Clin Exp Pharmacol Physiol. 2001;28:493–503. doi: 10.1046/j.1440-1681.2001.03477.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Slotkin TA, Racagni G, Riva MA. Prenatal stress elicits regionally selective changes in basal FGF-2 gene expression in adulthood and alters the adult response to acute or chronic stress. Neurobiological Disorders. 2005;20:731–7. doi: 10.1016/j.nbd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–37. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–9. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berlin) 2001;158:382–7. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Klejbor I, Myers JM, Hausknecht K, Corso TD, Gambino AS, Morys J, et al. Fibroblast growth factor receptor signaling affects development and function of dopamine neurons - inhibition results in a schizophrenia-like syndrome in transgenic mice. J Neurochem. 2006;97:1243–58. doi: 10.1111/j.1471-4159.2006.03754.x. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Angulo JA, Le Moal M, McEwen BS, Piazza PV. Neurochemical characterization of individual vulnerability to addictive drugs in rats. Eur J Neurosci. 1998;10:3153–63. doi: 10.1046/j.1460-9568.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, et al. Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res Brain Res Rev. 2001;37:249–58. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- Mueller D, Chapman CA, Stewart J. Amphetamine induces dendritic growth in ventral tegmental area dopaminergic neurons in vivo via basic fibroblast growth factor. Neuroscience. 2006;137:727–35. doi: 10.1016/j.neuroscience.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Reuss B, von Bohlen, Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–57. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–7. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, et al. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006a;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, et al. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. J Neurosci. 2006b;26:345–53. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer M, Cesnulevicius K, Winkler C, Kolb J, Lipokatic-Takacs E, Jungnickel J, et al. Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J Neurosci. 2007;27:459–71. doi: 10.1523/JNEUROSCI.4493-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiatry. 2006;59:1128–35. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Turner CA, Calvo N, Frost DO, Akil H, Watson SJ. The fibroblast growth factor system is downregulated following social defeat. Neurosci Lett. 2008a;430:147–50. doi: 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008b;1224:63–8. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–16. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, et al. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci. 2004;1025:351–62. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Arai H, et al. Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse. 2005;58:63–71. doi: 10.1002/syn.20182. [DOI] [PubMed] [Google Scholar]


