Abstract
Background and Purpose
To determine the accuracy of susceptibility-weighted magnetic resonance imaging (SWI) for the detection of arteriovenous shunting (AVS) in vascular malformations of the brain (BVM).
Methods
We retrospectively identified 60 patients who had been evaluated for known or suspected BVM by both SWI and digital subtraction angiography (DSA), without intervening treatment, during a 3-year period. SWI images were retrospectively assessed by two independent reviewers for the presence of AVS as determined by the presence of signal hyperintensity within a venous structure in the vicinity of the BVM. Discrepancies were resolved by consensus among a panel of three neuroradiologists. Accuracy parameters of SWI for the detection of AVS were calculated utilizing DSA as the reference standard.
Results
A total of 80 BVMs were identified in the 60 patients included in our study. Of the 29 BVMs with AVS on DSA, 14 were untreated AVMs, 10 were previously-treated AVMs, and 5 were untreated dural arteriovenous fistulas. Overall, SWI was 93% sensitive and 98% specific for the detection of AVS in BVMs, with excellent inter-observer agreement (kappa 0.94). In the 14 previously-treated AVMs, SWI was 100% sensitive and specific for the detection of AVS. In the 28 BVMs associated with intracerebral hemorrhage, SWI was 100% sensitive and 96% specific for the detection of AVS.
Conclusions
SWI is accurate for the detection of arteriovenous shunting in vascular malformations of the brain and, for some patients, SWI may offer a non-invasive alternative to angiography in screening for or follow-up of treated BVMs.
Keywords: Susceptibility-weighted imaging (SWI), Arteriovenous shunting, Arteriovenous malformation (AVM), Developmental venous anomalies (DVA)
Introduction
Susceptibility weighted imaging (SWI) is a magnetic resonance imaging (MRI) technique which combines phase and magnitude signal to produce high resolution images of the cerebral venous system.1 In SWI images, veins appear hypointense due to deoxyhemoglobin and arteries are hyperintense due to time-of-flight effects and lack of T2* effects.1,2 Therefore, using SWI, it is possible to simultaneously and distinctly evaluate the arterial and venous systems of the brain.
We hypothesized that there would be abnormal hyperintense signal on the SWI images within the veins draining high-flow vascular malformations of the brain (BVM) due to arterialized blood flow in these veins from arteriovenous shunting (AVS). To test this hypothesis, we retrospectively reviewed all the brain MRI studies performed at our institution during a 3-year period that included a SWI sequence in patients with either known or suspected BVMs, and compared the performance of SWI in detecting AVS against the gold standard of catheter digital subtraction angiography (DSA).
Materials and Methods
Patient Selection
The study was approved by our hospital's institutional review board and conducted in compliance with the Health Insurance Portability and Accountability Act. Using our institution's radiology information system (RIS) search software, we retrospectively identified all patients who underwent a brain MRI study which included SWI as well as DSA for evaluation of a known or suspected BVM at our institution during a three-year period (January 1st, 2007-December 31st, 2009). Patient exclusion criteria were (1) a time interval between the MRI and DSA examinations greater than 365 days, or (2) endovascular or surgical treatment for the BVM between the MRI and DSA examinations. Both the MRI and DSA examinations were performed as standard of care procedures at the discretion of the clinical providers.
Image Acquisition
MRI studies were performed on either 1.5 or 3-Tesla scanners (Symphony 1.5-Tesla and Trio 3-Tesla, Siemens AG, Munich, Germany) and included an FDA approved SWI sequence. At 1.5-Tesla, the SWI scanning parameters were: flip angle, 15 degrees; TE, 40 msec; TR, 50 msec; slice thickness, 2mm; and in-plane resolution of 1 × 1 mm. At 3-Tesla, the SWI scanning parameters were: flip angle, 15 degrees; TE, 20 msec; TR, 27 msec; slice thickness, 2.0 mm; and in-plane resolution of 0.9 × 0.9 mm.
Catheter angiography was performed using a biplanar neuroangiographic unit (Axiom Artis, Siemens AG, Munich, Germany) with transfemoral arterial access followed by selective catheterization and contrast injection (Optiray 320, Covidien, Hazelwood, MO) of the vessels of interest.
Image Analysis
Using the conventional contrast-enhanced MRI and MR angiography (MRA, without inclusion of the SWI sequence), BVMs were defined by the presence of enlarged vascular structures within the brain parenchyma or the meninges. Two experienced neuroradiologists, blinded to the results of DSA and clinical characteristics, independently reviewed the SWI sequences after multiplanar reformatting of the original trans-axial SWI slices using the Emageon Ultravisual Viewer (Amicas, Inc., Boston, MA) embedded within our hospital's clinical information system (Clinical Desktop, BJC Healthcare, St. Louis, MO), to assess for the presence of AVS, as determined by the presence of signal hyperintensity within at least 1 venous structure draining the BVM being evaluated. Differences in reader interpretation were resolved by consensus using a panel including an additional board certified neuroradiologist.
Subsequently, the DSA examinations were reviewed in conjunction with an experienced interventional neuroradiologist to correlate the presence of SWI signal hyperintensity within a given venous structure draining the BVM with the presence of AVS within the same structure in the catheter angiogram.
Medical Record Review
Medical records were reviewed for patient age, sex, time interval between MRI and DSA examinations, hemorrhage on initial head CT, and prior surgical or endovascular treatments for BVMs.
Statistical Analysis
Statistical analysis was performed using MedCalc 11.1 software package (MedCalc Software, Mariakerke, Belgium). Inter-observer agreement for the presence of AVS on SWI was determined with the kappa statistic. Standard diagnostic accuracy parameters of SWI for the prediction of AVS in BVMs were calculated utilizing DSA as the reference standard.
Results
MRI with SWI and DSA was available for 69 patients with known or suspected BVM. Of these, 8 were excluded because of time interval between the MRI and DSA examinations greater than 365 days, and 1 was excluded because of radiosurgery between the MRI and DSA examinations. Inclusion criteria were met by the remaining 60 patients (32 females and 28 males) with a mean age of 42 years (median 46.5 years, range 6 months to 81 years). Thirty-five patients underwent MRI in a 1.5-Tesla scanner (58.3%) and 25 in a 3-Tesla scanner (41.7%). The mean time interval between the MRI and DSA examinations was 38 days (median 3 days, range 0-301 days). Twenty-four patients (40%) presented with intracerebral hemorrhage (ICH) on an initial non-contrast CT (NCCT) of the head.
Results of DSA Examinations
A total of 80 BVMs were identified in these 60 patients. Fifty-one BVMs did not have AVS on DSA (63.7%) which, includes 4 previously treated AVMs, 23 abnormally prominent normal-variant cortical or deep veins (atypical DVAs), and 24 typical DVAs with a medusa head appearance. Twenty-nine BVMs had AVS on DSA (36.3%), including 14 untreated AVMs (48.3%), 10 previously-treated AVMs (34.5%), and 5 untreated dural arteriovenous fistulas (DAVF, 17.2%).
A total of 28 BVMs were identified in a sub-analysis performed on patients presenting with acute ICH. Of these, 3 were ruptured AVMs with AVS on DSA, 7 were atypical DVAs and 18 were typical DVAs, 12 of which had distinct associated cavernomas.
Accuracy of SWI for the Detection of AVS in BVMs
Overall, the presence of SWI signal hyperintensity within at least 1 vein draining the BVM was 93% sensitive and 98% specific for the detection of AVS (Table 1). There was excellent inter-observer agreement for the detection of AVS on SWI, with a kappa statistic of 0.94 (95% confidence interval: 0.92-0.96). In the 14 previously-treated AVMs, SWI was 100% sensitive and 100% specific for the detection of AVS (Table 1), with perfect inter-observer agreement (kappa statistic 1.0). In the 28 BVMs associated with ICH, SWI was 100% sensitive and 96% specific for the detection of AVS (Table 1), also with perfect inter-observer agreement (kappa statistic 1.0).
Table 1. Accuracy of SWI for the Detection of Arteriovenous Shunting in BVMs.
| Parameter: | All BVMs, n=80 (95% CI) | BVMs w ICH, n=28 (95% CI) | Treated BVMs,* n=14 (95% CI) |
|---|---|---|---|
| Sensitivity: | 93.1 (75.8-98.8) | 100.0 (31-100) | 100.0 (65.5-100) |
| Specificity: | 98.0 (88.2-99.9) | 96.0 (77.7-99.8) | 100 (39.6-100) |
| PPV: | 96.4 (79.8-99.8) | 75.0 (21.9-98.7) | 100 (65.5-100) |
| NPV: | 96.2 (85.7-99.3) | 100.0 (82.8-100) | 100 (39.6-100) |
| Positive LR: | 47.5 (6.8-331.5) | 25 (3.7-170.6) | ∞ |
| Negative LR: | 0.07 (0.02-0.27) | 0 | 0 |
| Accuracy: | 96.3 | 96.4 | 100 |
| Prevalence: | 36.3 | 10.7 | 71.4 |
All the previously-treated BVMs were AVMs. SWI: susceptibility-weighted imaging; BVM: vascular malformation of the brain; n=number of BVMs; CI: confidence interval; w: with; ICH: intracerebral hemorrhage; PPV: positive predictive value; NPV: negative predictive value; LR: likelihood ratio; AVM: arteriovenous malformation.
Discussion
BVMs can be characterized as either high-flow or low-flow malformations. The high-flow BVMs are either pial/parenchymal AVMs or DAVFs, and are associated with risk for ICH and non-hemorrhagic complications3,4 such as ischemic steal phenomena,5 mass effect6 or venous hypertension, with resultant focal neurologic deficits and/or seizures.7-9 High-flow BVMs often require surgery,10,11 gamma-knife radiosurgery,12 endovascular embolization or a combination of two or more of these techniques.13,14 Conversely, low-flow BVMs are predominantly DVAs without AVS. Although DVAs alone do not increase the risk of hemorrhage, they are often associated with cavernomas, which can bleed.15-18. It is not always possible to distinctly identify an associated cavernoma in patients with DVAs and ICH wherein the large amounts of blood products can obscure the cavernomas on almost all MRI sequences, particularly the SWI sequences where there is a blooming artifact from the blood products, this is also evident in our study, where we could identify distinct associated cavernomas in only 12 of the 18 patients with DVAs who presented with hemorrhage. DVAs are usually observed without treatment, although associated hemorrhagic cavernomas may sometimes be resected.19-21
Accurate differentiation between high-flow and low-flow BVMs on neuroimaging is crucial. Most patients with suspected BVMs undergo non-invasive evaluation with conventional MRI or MRA, which can reliably differentiate between a typical DVA with a pathognomonic “medusa-head” appearance from radially-oriented small veins converging to a central draining vein, and a typical AVM or DAVF. However, it is often difficult to differentiate between an atypical DVA with complex vascular anatomy or abnormally prominent normal-variant cortical or deep veins and a high-flow BVM with these techniques.22-25 These studies are also less reliable in the presence of ICH.26,27 Although diagnostic accuracy can be improved by time-resolved MRA with high temporal resolution, this approach is technically difficult.28,29 Hence, invasive evaluation with DSA is often required to differentiate between atypical DVAs and AVMs/AVFs, and to evaluate for a BVM in the setting of acute ICH.
MRI and MRA also produce suboptimal results in follow-up examinations of patients with treated AVMs.26,27,30,31 Specifically, dilated draining veins or enlarged arteries may appear in the absence of AVS, since vessel caliber changes often take time to reverse after elimination of AVS.28 Likewise, there is often contrast enhancement in the treated AVM nidus from reactive gliosis.32
SWI offers unique advantages in the detection of AVS, given the intrinsic contrast between hyperintense rapidly-flowing oxygenated arterial blood and hypointense slowly-flowing deoxygenated venous blood on this sequence.33,34 This unique contrast between arteries and veins is observed regardless of vessel caliber and SWI does not require intravenous contrast administration or technically-demanding dynamic acquisition techniques. Recently, Fujiyama et al. utilized SWI measurements to detect quantitative changes in blood oxygenation within the anterior spinal veins after endovascular treatment of spinal DAVFs.35 Tsui et al. described faint SWI signal hyperintensity within a venous varix in a patient with a cerebral DAVF.36 Saini et al. also described other SWI findings in a DAVF but did not describe abnormal hyperintensity in a venous structure.37 However, our study is the first to evaluate the use of SWI in the management of BVMs in a large series of patients.
Our results confirm that SWI can reliably detect AVS in both de-novo and previously-treated high-flow BVMs. Given its high negative predictive value, most patients with complex BVMs and absent AVS on SWI images may not require further evaluation with DSA. For instance, in one of our patients, none of the 13 BVMs reported as AVMs on conventional contrast-enhanced MRI and MRA showed SWI signal hyperintensity, and all were confirmed to be cortical venous anomalies on subsequent DSA (Figure 1). In another patient, an abnormally-dilated vessel which had been diagnosed as a benign venous varix on conventional MRI and MRA studies, based primarily on its stability over several years, showed SWI signal hyperintensity within the “varix”, and on a subsequent DSA study, a parenchymal AVM draining into this dilated cortical vein was identified (Figure 2).
Figure 1.
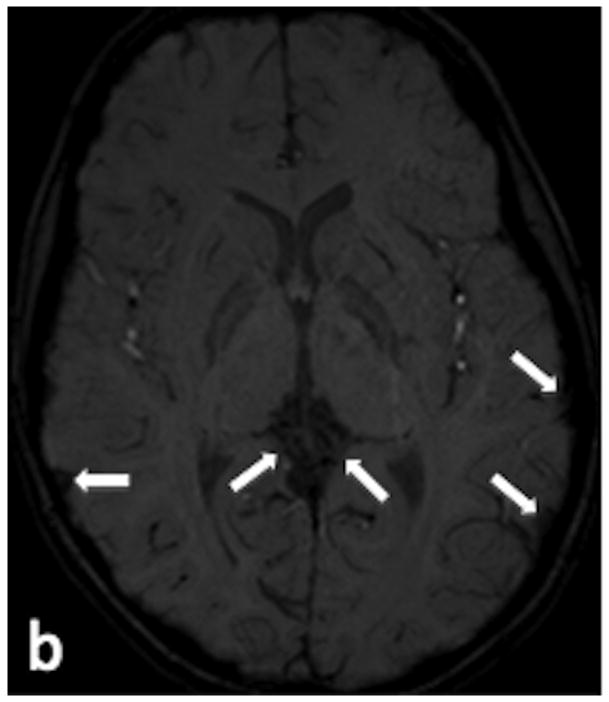
9 year-old female with seizures. (a) Axial T2-weighted image demonstrates numerous enlarged vessels overlying the cortical surface of the brain and in the region of the vein of Galen (arrows). (b) Axial SWI image demonstrates no signal hyperintensity within any of the enlarged vessels (arrows), indicating absent AVS. (c) Venous phase of a left common carotid angiogram in the lateral projection demonstrates numerous cortical and deep venous anomalies (arrows), none of which demonstrated AVS.
Figure 2.
9 year-old female presents for evaluation of persistent headaches. (a) Axial T2-weighted image demonstrates enlarged vascular structures overlying the surface of the right temporal lobe (arrow). (b) Axial SWI image demonstrates hyperintensity within these enlarged vascular structures (arrow), indicating AVS. (c) Arterial phase of a right common carotid angiogram in the lateral projection demonstrates a right Sylvian fissure AVM (arrowhead), with early draining veins anteriorly into the superior sagittal sinus (white arrows) and posteriorly into the right transverse sinus (black arrows).
Importantly, our study suggests that SWI can differentiate between high and low-flow BVMs in the setting of acute ICH (Figures 3 and 4). Its excellent negative predictive value may allow us to exclude the presence of a high-flow BVM in patients with ICH and equivocal conventional MRI findings sparing them from invasive DSA evaluation, whereas patients with AVS on SWI could undergo DSA more urgently to further characterize the high-flow BVM.
Figure 3.
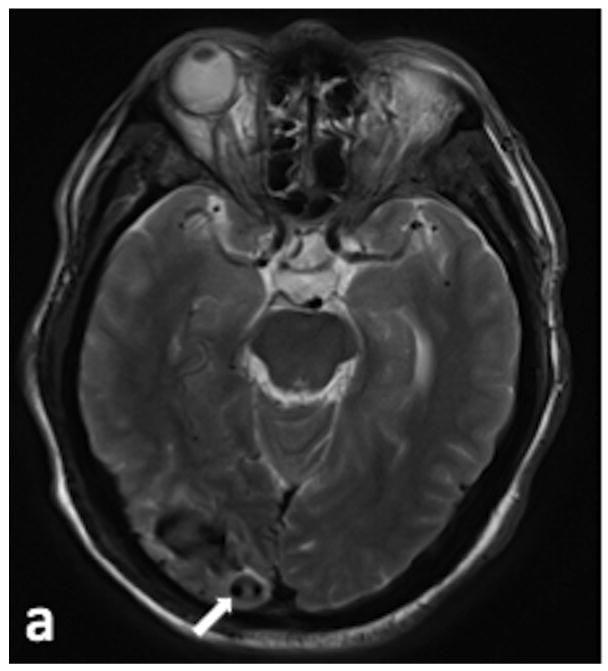
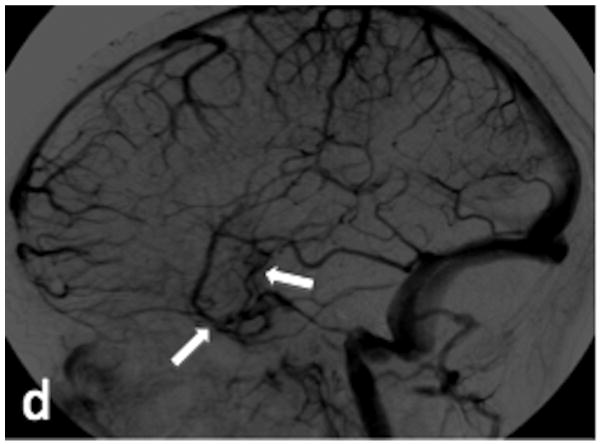
48 y/o female presents for evaluation of a right occipital ICH. (a) Axial T2-weighted image demonstrates enlarged vascular structures adjacent to the ICH (arrow). (b) Axial SWI image demonstrates hyperintensity within the enlarged vascular structures (arrow), indicating AVS. (c) Sagittal SWI reconstruction redemonstrates the signal hyperintensity within the vascular structures (arrows). (d) Arterial phase of a right external carotid angiogram in the lateral projection demonstrates a type III DAVF draining into the right transverse sinus (arrows).
Figure 4.
14 year-old male presents for evaluation of a left frontal ICH. (a) Axial contrast-enhanced T1-weighted image demonstrates an enlarged vascular structure adjacent to the ICH (arrow). (b) Axial SWI image demonstrates no signal hyperintensity within the enlarged vascular structure (arrow), indicating absent AVS. (c) Sagittal SWI reconstruction redemonstrates the lack of signal hyperintensity within the vascular structure (arrow). (d) Venous phase of a left common carotid angiogram in the lateral projection demonstrates that the enlarged vascular structure represents a DVA (arrows).
SWI was also reliable in excluding residual AVS in patients with previously-treated AVMs (Figure 5). In these patients, SWI may represent an accurate non-invasive alternative technique to repeated follow-up DSA studies for evaluating post-treatment changes.
Figure 5.
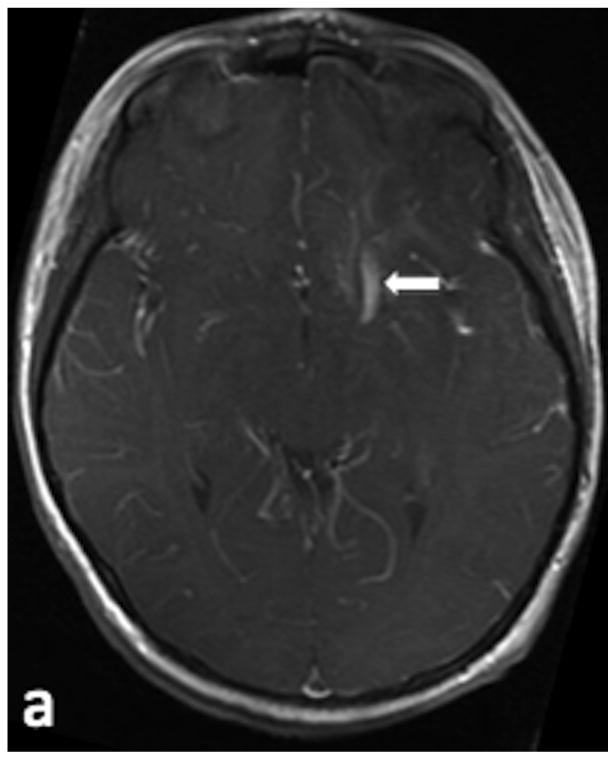
20 year-old male presents for evaluation of a previously-treated left temporo-parietal AVM. (a) Axial SWI image demonstrates hyperintensity within a slightly enlarged vascular structure adjacent to the previously-treated AVM (arrow), which indicates AVS. (b) Sagittal SWI reconstruction redemonstrates hyperintensity within the enlarged vascular structure (white arrow) adjacent to the AVM (arrowhead). (c) Arterial phase of a left common carotid angiogram in the lateral projection demonstrates the residual AVM nidus (arrowhead) and a corresponding early draining vein (arrow).
Of note, there was a false-positive SWI study in a patient with a DVA and ICH secondary to a cavernoma. We speculate that this may have resulted from imaging when the patient was breathing supplemental oxygen. We have found that when patients on supplemental oxygen are imaged, all the major venous structures demonstrate hyperintense signal, due to lower deoxyhemoglobin than usual (online Figure a).
There are several limitations to our study. First, our study was retrospective in nature. A prospective study evaluating the accuracy of SWI for the detection of AVS in BVMs in a large number of patients is needed. Second, our study does not elucidate the degree of AVS that is required before SWI signal hyperintensity can be identified in a venous structure draining a BVM, which may be particularly crucial in the follow-up of patients with treated AVMs/AVFs with small residual AVS. Third, the exact contribution of oxygenation versus time-of-flight effects to the abnormal venous SWI signal hyperintensity in the setting of AVS is also unclear and requires further study. However, we did find that, even in the presence of signal hyperintensity within venous structures on routine time-of-flight images, if there was no signal hyperintensity within these structures on the SWI sequence, then there was no AVS (online figure). A prospective comparative study between time-of-flight and SWI for the evaluation of AVS may be useful in this regard.
Conclusion
The presence of SWI signal hyperintensity within the venous structures draining a BVM is an accurate indicator of AVS from an underlying high-flow vascular malformation. Although a larger prospective study is needed, our results suggest that when available, SWI should be included in the MRI protocol for suspected BVM, and that attention should be paid to hyperintense signal in venous structures as it is highly correlated with AVS.
Supplementary Material
Acknowledgments
Funding: Dr. Benzinger received support from the Bracco/American Roentgen Ray Society (ARRS) Scholar Award, the National Multiple Sclerosis Society (NMSS) RG 4190A2/1, and the NIH R01 1RO1NS066905-01 and NIH/NIA AG003991-27 for this research.
Footnotes
Conflicts of Interest: Dr. Benzinger has previously served on the Siemens speakers' bureau where she received travel support. No conflicts of interest are identified pertaining to this research project for any of the authors.
References
- 1.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YCN. Susceptibility-weighted imaging: Technical Aspects and Clinical Applications, Part 1. Am J Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes SRS, Haacke EM. Susceptibility-weighted imaging: Clinical Angiographic Applications. Magn Reson Imaging Clin N Am. 2009;17:47–61. doi: 10.1016/j.mric.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmeister C, Stapf C, Hartmann A, et al. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke. 2000;31:1307–1310. doi: 10.1161/01.str.31.6.1307. [DOI] [PubMed] [Google Scholar]
- 4.Mast H, Mohr JP, Osipov A, Pile-Spellman J, Marshall RS, Lazar RM, Stein BM, Young WL. “Steal” is an unestablished mechanism for the clinical presentation of cerebral arteriovenous malformations. Stroke. 1995;26:1215–1220. doi: 10.1161/01.str.26.7.1215. [DOI] [PubMed] [Google Scholar]
- 5.Carter LP, Gumerlock MK. Steal and cerebral arteriovenous malformations. Stroke. 1995;26:2371–2372. [PubMed] [Google Scholar]
- 6.Miyasaka Y, Yada K, Ohwada T, Kitahara T. An analysis of the venous drainage system as a factor in hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:239–243. doi: 10.3171/jns.1992.76.2.0239. [DOI] [PubMed] [Google Scholar]
- 7.Osipov A, Koennecke HC, Hartmann A, Young WL, Pile-Spellman J, Hacein-Bey L, Mohr JP, Mast H. Seizures in cerebral arteriovenous malformations: type, clinical course, and medical management. Interventional Neuroradiol. 1997;3:37–41. doi: 10.1177/159101999700300104. [DOI] [PubMed] [Google Scholar]
- 8.Turjman F, Massoud TF, Sayre JW, Vinuela F, Guglielmi G, Duckwiler G. Epilepsy associated with cerebral arteriovenous malformations: a multivariate analysis of angioarchitectural characteristics. Am J Neuroradiol. 1995;16:345–350. 9. [PMC free article] [PubMed] [Google Scholar]
- 9.The Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812–1818. doi: 10.1056/NEJM199906103402307. [DOI] [PubMed] [Google Scholar]
- 10.Ogilvy CS, Stieg PE, Awad I, Brown RD, Kondziolka D, Rossenwasser R, Young WL, Hademenos G. Recommendation for the management of intracranial arteriovenous malformations: a statement for health-care professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32:1458–1471. doi: 10.1161/01.str.32.6.1458. [DOI] [PubMed] [Google Scholar]
- 11.Castel JP, Kantor G. Postoperative morbidity and mortality after microsurgical exclusion of cerebral arteriovenous malformations. Current data and analysis of recent literature. Neurochirurgie. 2000;47:369–383. [PubMed] [Google Scholar]
- 12.Nataf F, Merienne L, Schlienger M, Lefkopoulos D, Meder JF, Touboul E, Merland JJ, Devaux B, Turak B, Page P, Roux FX. Cerebral arteriovenous malformations treated by radiosurgery: a series of 705 cases. Neurochirurgie. 2001;47:268–82. [PubMed] [Google Scholar]
- 13.Frizzel RT, Fisher WS. Cure, morbidity, and mortality associated with embolization of cerebral arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery. 1995;37:1031–1040. doi: 10.1227/00006123-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Jahan R, Murayama Y, Gobin YP, Duckwiler GR, Vinters HV, Viñuela F. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48:984–997. doi: 10.1097/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Garner TB, Del Curling O, Kelly DL, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg. 1991;75:715–722. doi: 10.3171/jns.1991.75.5.0715. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin MR, Kondziolka D, Flickinger JC, Lunsford S, Lunsford LD. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998;43:195–200. doi: 10.1097/00006123-199808000-00001. discussion 200-201. [DOI] [PubMed] [Google Scholar]
- 17.Abdulrauf SI, Kaynar MY, Awad IA. A comparison of the clinical profile of cavernous malformations with and without associated venous malformations. Neurosurgery. 1999;44:41–46. doi: 10.1097/00006123-199901000-00020. discussion 46-47. [DOI] [PubMed] [Google Scholar]
- 18.Töpper R, Jürgens E, Reul J, Thron A. Clinical significance of intracranial developmental venous anomalies. J Neurol Neurosurg Psychiatry. 1999;67:234–238. doi: 10.1136/jnnp.67.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kivelev J, Niemelä M, Kivisaari R, Hernesniemi J. Intraventricular cerebral cavernomas: a series of 12 patients and review of the literature. J Neurosurg. 2010;112:140–9. doi: 10.3171/2009.3.JNS081693. [DOI] [PubMed] [Google Scholar]
- 20.Pryor J, Setton A, Berenstein A. Venous anomalies and associated lesions. Neurosurg Clin N Am. 1999;10:519–525. [PubMed] [Google Scholar]
- 21.Crivelli G, Dario A, Cerati M, Dorizzi A. Third ventricle cavernoma associated with venous angioma. Case report and review of the literature. J Neurol Neurosurg Psychiatry. 2002;19:1–11. [PubMed] [Google Scholar]
- 22.Seiz M, Brockmann MA, Schneider UC, Woitzik J, Scharf J. Combination of supratentorial vrnous anomaly and infratentorial developmental venous anomalies mimicking AV-malformation: a case report. Zentralbl Neurochir. 2007;68:217–219. doi: 10.1055/s-2007-985854. [DOI] [PubMed] [Google Scholar]
- 23.Komiyama M, Yamanaka K, Iwai Y, Yasui T. Venous angiomas with arteriovenous shunts: report of three cases and review of the literature. Neurosurgery. 1999;44:1328–1334. doi: 10.1097/00006123-199906000-00100. discussion 1334-1335. [DOI] [PubMed] [Google Scholar]
- 24.Boukobza M, Enjolras O, Guichard JP, Gelbert F, Herbreteau D, Reizine D, Merland JJ. Cerebral developmental venous anomalies associated with head and neck venous malformations. Am J Neuroradiol. 1996;17:987–994. [PMC free article] [PubMed] [Google Scholar]
- 25.Aksoy FG, Gomori JM, Tuchner Z. Association of intracerebral venous angioma and true arteriovenous malformation: a rare, distinct entity. Neuroradiology. 2000;42:455–457. doi: 10.1007/s002340000307. [DOI] [PubMed] [Google Scholar]
- 26.Lee KE, Choi CG, Choi JW, Choi BS, Lee DH, Kim SJ, Kwon do H. Detection of residual brain arteriovenous malformations after radiosurgery: diagnostic accuracy of contrast-enhanced three-dimensional time of flight MR angiography at 3.0 tesla. Korean J Radiol. 2009;10:333–9. doi: 10.3348/kjr.2009.10.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unlu E, Temizoz O, Albayram S, Genchellac H, Hamamcioglu MK, Kurt I, Demir MK. Contrast-enhanced MR 3D angiography in the assessment of brain AVMs. Eur J Radiol. 2006;60:367–78. doi: 10.1016/j.ejrad.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Hadizadeh DR, von Falkenhausen M, Gieseke J, Meyer B, Urbach H, Hoogeveen R, Schild HH, Willinek WA. Cerebral arteriovenous malformation: Spetzler-Martin classification at subsecond-temporal-resolution four-dimensional MR angiography compared with that at DSA. Radiology. 2008;246:205–213. doi: 10.1148/radiol.2453061684. [DOI] [PubMed] [Google Scholar]
- 29.Eddleman CS, Jeong HJ, Hurley MC, Zuehlsdorff S, Dabus G, Getch CG, Batjer HH, Bendok BR, Carroll TJ. 4D radial acquisition contrast-enhanced MR angiography and intracranial arteriovenous malformations: quickly approaching digital subtraction angiography. Stroke. 2009;40:2749–53. doi: 10.1161/STROKEAHA.108.546663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Maki JH, Prince MR. 3D contrast-enhanced MR angiography. J Magn Reson Imaging. 2007;25:13–25. doi: 10.1002/jmri.20767. [DOI] [PubMed] [Google Scholar]
- 31.Cashen TA, Carr JC, Shin W, Walker MT, Futterer SF, Shaibani A, McCarthy RM, Carroll TJ. Intracranial time-resolved contrast-enhanced MR angiography at 3T. Am J Neuroradiol. 2006;27:822–829. [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto M, Ide M, Jimbo M, Takakura K, Lindquist C, Steiner L. Neuroimaging studies of postobliteration nidus changes in cerebral arteriovenous malformations treated by gamma knife radiosurgery. Surg Neurol. 1996;45:110–122. doi: 10.1016/s0090-3019(96)80003-6. [DOI] [PubMed] [Google Scholar]
- 33.Du YP, Jin Z. Simultaneous acquisition of MR angiography and venography (MRAV) Magn Reson Med. 2008;59:954–958. doi: 10.1002/mrm.21581. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 35.Fujima N, Kudo K, Terae S, Hida K, Ishizaka K, Zaitsu Y, Asano T, Yoshida D, Tha KK, Haacke EM, Sasaki M, Shirato H. Spinal Arteriovenous Malformation: Evaluation of Change in Venous Oxygenation with Susceptibility-Weighted MR Imaging after Treatment. Radiology. 2010;254:891–9. doi: 10.1148/radiol.09090286. [DOI] [PubMed] [Google Scholar]
- 36.Tsui YK, Tsai FY, Hasso AN, Greensite F, Nguyen BV. Susceptibility-weighted imaging for differential diagnosis of cerebral vascular pathology: a pictorial review. J Neurol Sci. 2009;287:7–16. doi: 10.1016/j.jns.2009.08.064. [DOI] [PubMed] [Google Scholar]
- 37.Saini J, Thomas B, Bodhey NK, Periakaruppan A, Babulal JM. Susceptibility-weighted imaging in cranial dural arteriovenous fistulas. Am J Neuroradiol. 2009;30:E6. doi: 10.3174/ajnr.A1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.