Abstract
Neural activation in a 12-item probe-recognition task was examined to investigate the contribution of the hippocampus to long-term memory (LTM) retrieval and working memory (WM) retrieval. Results indicated a dissociation between the last item that participants studied and other items of the study list: Compared with all other serial positions, activation was reduced for the item in the most recent position (for which no items intervened between study and test). This finding suggests that this last item was in focal attention at test time, and, therefore, no retrieval operation was required to access it. However, contra the assertion that the hippocampus should selectively support LTM, activation of the medial temporal lobe was observed for all serial positions other than the last position, and activation level could be predicted from the underlying memory strength. Collectively, these findings support single-store accounts that assume there are similar operating principles across WM and LTM representations, and the focus of attention is limited.
Keywords: working memory, short-term memory, long-term memory, memory systems, focal attention, item recognition, functional magnetic resonance imaging (fMRI), medial temporal lobe, hippocampus
Are short- and long-term memories governed by the same principles, and do the retention and retrieval of these two forms of memory draw on the same neural structures? There are several respects in which current approaches to working memory (WM) suggest a negative answer to both questions. A salient assumption is that WM provides a time- or capacity-limited workspace in which a few products of recent cognitive processing can be maintained in a more accessible state than representations in long-term memory (LTM) can, either because these products have residual activation due to recent processing (e.g., Cowan, 1999; Oberauer, 2002), or because they are maintained in specialized stores (e.g., Baddeley & Hitch, 1974).
However, the evidence advanced for a time- or capacity-limited short-term store is indirect and has been criticized on several grounds; such criticism has led to alternative accounts positing that short- and long-term memories are both governed by the same principles and processes (e.g., McElree, 2006; Nairne, 2002; Surprenant & Neath, 2009). Moreover, studies directly measuring access speed in several WM tasks (reviewed in McElree, 2006) have not found that WM representations are accessed with qualitatively different retrieval operations or are accessed faster than representations in LTM. Several studies that have used the speed-accuracy trade-off procedure to conjointly measure the speed and accuracy of accessing recent events have found unequivocal evidence that information that is the focus of processing at test time—that is, information within focal attention—is accessed faster than representations in memory. It is crucial, however, that no differences in retrieval speed have been found for information that is hypothesized to be within the span of WM and information residing in LTM alone.
This pattern is illustrated by results reported by Wickelgren, Corbett, and Dosher (1980), who examined the time-course profiles for recognition of different serial positions (SPs) within lists of 16 sequentially presented items. Accuracy of retrieval decreased monotonically with the decreasing recency of the tested item; this finding indicates that memory strength declines as time or activity intervenes between study of the items and when they are tested. Retrieval speed, however, was constant across all SPs, except for the last position, which was accessed at a speed 50% faster than were all other positions. Because no other item intervened between the most recently studied item and the test, the test probe could be matched directly to the content of focal attention (i.e., information that is being actively processed), where items do not need to be retrieved from memory.
As reviewed in McElree (2006), the pattern reported by Wickelgren et al. (1980) of markedly faster access to the last unit processed and uniformly slower access to all other items has been replicated across a wide range of tasks and manipulations. Collectively, these time-course measures motivate a distinction between focal attention and memory. But these measures are inconsistent with approaches positing an intermediate WM store between focal attention and LTM. One such approach is the three-layer memory model suggested by Oberauer (2001, 2002; see also Cowan, 2005); this model posits that between three to four items remain in an active state (the activated part of LTM) intermediate between focal attention and LTM.
Although direct behavioral measures of retrieval speed do not motivate accounts that assume a time- or capacity-limited short-term store, it is possible that evidence for separate stores might exist in the underlying neural structures. Here, we report a functional magnetic resonance imaging (fMRI) study of item recognition similar in design to the study conducted by Wickelgren et al. (1980); our study examined whether there is neural evidence to support a traditional distinction between WM and LTM. One structure of particular importance in testing this hypothesis is the medial temporal lobe (MTL), a brain region well-known to be critical for encoding and retrieval processes in LTM (see Davachi, 2006, and Mayes, Montaldi, & Migo, 2007, for reviews). Dual-store accounts often predict that the MTL supports LTM operations only. For instance, on the basis of a finding of enhanced MTL activation in the recognition of the first two SPs as compared with the last two SPs in a 12-item study list, Talmi, Grady, Goshen-Gottstein, and Moscovitch (2005) argued for the traditional dual-store account, in which the MTL is involved in retrieval of LTM but not of WM representations.
However, given the robust behavioral findings indicating that the last-encoded item is likely to be active in focal attention at test time, the reduced MTL activation that Talmi et al. (2005) noted for the last two SPs more plausibly reflects the reduced need for retrieval when items are maintained in focal attention, rather than a traditional distinction between WM and LTM. Indeed, we previously found dissociations in the MTL (specifically, in the hippocampus) between the last SP and all other SPs in five-item study lists, in both item-recognition and judgment-of-recency tasks (Öztekin, McElree, Staresina, & Davachi, 2008; see also Nee & Jonides, 2008, for a replication of this dissociation with three-item lists in item recognition). Hence, MTL dissociations converge with behavioral measures of memory access to motivate a distinction between focal attention and passive memory representations only.
Unfortunately, extant fMRI studies have not provided optimal tests of potential differences in retrieval across representations hypothesized to be in focal attention, WM, and LTM: The study conducted by Talmi et al. (2005) sampled SPs that likely resided in either focal attention or LTM, and the studies conducted by us (Öztekin, McElree, et al., 2008) and by Nee and Jonides (2008) sampled SPs either in focal attention or hypothesized to be in WM (e.g., Cowan, 1999; Oberauer, 2002). Here, we examined critical SPs in 12-item lists to evaluate potential differences among all three hypothesized states, and to test for a neural dissociation between representations hypothesized to be in WM and representations hypothesized to be in LTM. The design of this study provided an optimal test of approaches, such as Oberauer’s (2001, 2002) three-layer memory model, which posits three distinct representational states: (a) the current focus of attention, which is a readily accessible representation limited to only one item; (b) the activated part of LTM, which is a set of representations limited to three or four items that are in an active state but need to be retrieved from WM; and (c) passive memory representations, which need to be retrieved from LTM.
Given the results of the study by Talmi et al. (2005), we would expect to see markedly distinct MTL activation for the retrieval of representations in LTM and for the retrieval of representations in either focal attention or in an active state. Alternatively, if there is no distinction between representations in LTM and representations in WM (as suggested in Öztekin, McElree, et al., 2008), MTL activation should be evident in the retrieval of all items other than those in focal attention.
Method
Participants
Twenty healthy right-handed adults (10 females and 10 males; age = 19–36 years) participated in the study.
Design and procedure
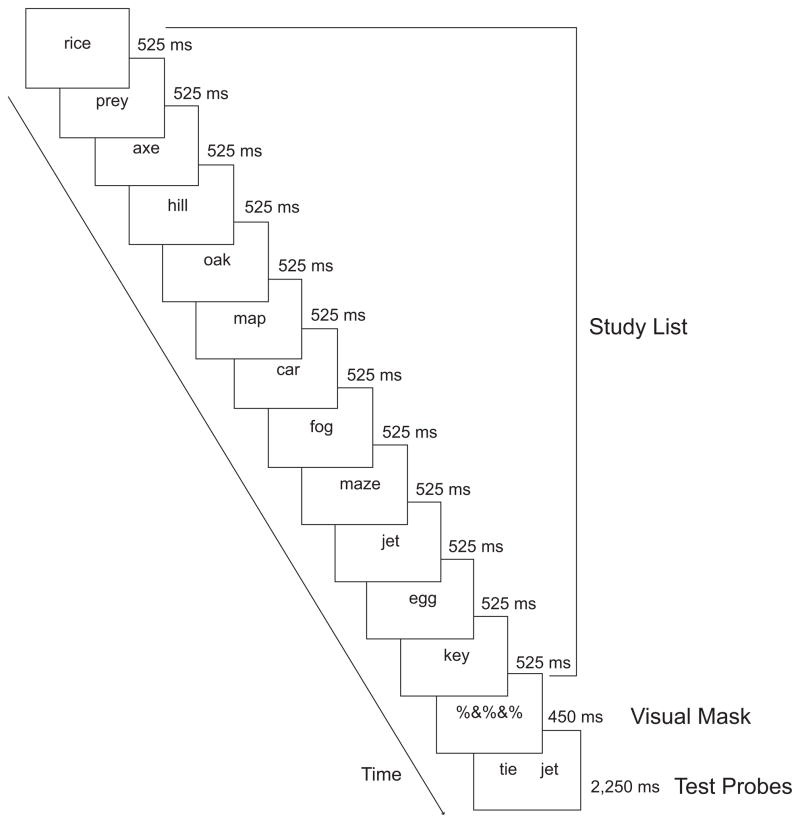
Stimuli consisted of 338 one-syllable words obtained from the Medical Research Council psycholinguistics database (Wilson, 1986). Study lists contained 12 words randomly selected from the word pool. Each trial began with a sequential presentation of words on the 12-word study list for 525 ms each (Fig. 1). Following a brief (450 ms) visual mask, two test probes were presented for a total of 2,250 ms. One probe was a word from the study list, and the other was a new word. Participants indicated which word was from the study list. The order of test probes was determined randomly, and each order appeared equally often. There were 40 trials for SPs 1 and 12, and 20 trials each for SPs 2 through 11. The experiment consisted of five runs containing 56 trials each. The intertrial interval consisted of a fixation point presented for 4,500 ms.
Fig. 1.
Sample sequence for an experimental trial. Participants studied a list of 12 words (presented sequentially for 525 ms each). The list was followed by a brief visual mask (450 ms). Then, two test probes (one word from the study list and one new word) were presented (for 2,250 ms total), and participants indicated the word that was from the study list.
fMRI protocol
A 3-T scanner acquired functional and anatomical images. Forty coronal slices (3 mm × 3 mm × 3 mm with a 0.6-mm interslice gap) were obtained (repetition time = 2.25 s; echo time = 30 ms; flip angle = 90°). Following the functional runs, T1-weighted high-resolution anatomical images (magnetization-prepared rapid-acquisition gradient echo, MP-RAGE) were obtained for anatomical localization.
Image processing
Image processing and data analysis were performed using Statistical Parametric Mapping Version 2 (SPM2; Functional Imaging Laboratory, 2003) software. Preprocessing of images consisted of correcting slice-acquisition timing across slices, realigning the images to the first volume in each fMRI run to correct for head movement, normalizing functional and anatomical images to a standard template echo-planar image provided by SPM2, and smoothing images with a 6-mm full-width, half-maximum isotropic Gaussian kernel.
fMRI data analysis
Data analysis was conducted using the general linear model in SPM2. To evaluate changes in neural activation across SPs, we sorted correct trials (i.e., trials in which participants accurately indicated the word that appeared in the study list) by conditions and modeled from the probe onset with a canonical hemodynamic response function and its temporal derivative (incorrect trials were also included in the model, but were excluded from analysis). Response times (RTs) were entered into this model as one regressor to ensure that the results of voxel-wise contrasts were not confounded with time-on-task differences.
A second model was constructed to evaluate the regions that exhibited neural activation correlated with accuracy. Correct and incorrect trials were modeled from the probe onset with a canonical hemodynamic response function and its temporal derivative (RTs were not entered into this model). In both models, data across runs were concatenated and modeled as one session with mean signal and scanner drift entered as covariates. For each participant, contrasts were derived using a subject-specific fixed-effects model. Then, contrast images from participants were carried forward to a second-level random-effects analysis. Regions consisting of at least 5 contiguous voxels1 that exceeded an uncorrected threshold of p < .001 were considered significant.
Regions of interest that emerged from functional contrasts were further analyzed using the Marseille boîte à région d’intérêt (MarsBaR 0.41; Brett, Anton, Valabregue, & Poline, 2002) region-of-interest toolbox for SPM. Statistical comparisons across conditions were conducted on the average of the peak time point (i.e., point of maximum percent signal change, PSC) and the two adjacent time points (peak time point ±1 repetition time) to account for potential differences in time to peak across conditions.
Results
Behavioral data
Following Oberauer’s (2001, 2002) three-layer memory model, we grouped SPs 1 through 8 as the passive set, SPs 9 through 11 as the active set, and SP 12 as the focus of attention. As expected, RT for the focus of attention was faster than RT for the active set, F(1, 19) = 73.139, p < .001, η2 = .794, and the active set exhibited faster RTs than the passive set did, F(1, 19) = 62.503, p < .001, η2 = .767 (Figs. 2a and 2c). A similar pattern emerged in accuracy: The focus of attention was more accurate than the active set, F(1, 19) = 26.832, p < .001, η2 = .585, and the active set was more accurate than the passive set, F(1, 19) = 58.017, p < .001, η2 = .753 (Figs. 2b and 2d). This pattern of accuracy and RT is consistent with previous investigations’ findings, which have motivated this three-layer memory structure (e.g., Oberauer, 2002).
Fig. 2.
Participants’ response times (RTs) and accuracy (d′) as a function of serial position (SP) of the word from the study list. The graphs in (a) and (b) show results across SPs; in (c) and (d), the results are grouped for three SP categories: passive set (SPs 1–8), active set (SPs 9–11), and the focus of attention (SP 12). Error bars indicate 95% confidence intervals.
Neuroimaging data
To contrast regions that support retrieval of items from memory with regions that support information in focal attention, we compared blood-oxygenation-level-dependent (BOLD) activation for SPs 1 through 11 (items that need to be retrieved from memory) with BOLD activation for SP 12 (in which the test probe can be directly matched to focal attention). Regions that showed reliable activation in these contrasts are presented in Table 1.
Table 1.
Regions of Interest Exhibiting Reliable Activations
| Contrast and area | Coordinates
|
Z score | Number of voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| (Passive set and active set) > focus of attention | |||||
| Right hippocampus | 36 | −36 | −6 | 3.75 | 7 |
| Cingulate cortex | −15 | −24 | 27 | 3.35 | 6 |
| Cingulate cortex | 21 | −30 | 27 | 3.93 | 17 |
| Passive set > focus of attention | |||||
| Hippocampus | 36 | −36 | −6 | 3.61 | 6 |
| Cingulate cortex | −15 | −24 | 27 | 3.53 | 7 |
| Cingulate cortex | 21 | −30 | 27 | 4.24 | 22 |
| Cingulate cortex | 12 | 3 | 30 | 4.19 | 5 |
| Active set > focus of attention | |||||
| Left hippocampus | −27 | −33 | −3 | 4.50 | 6 |
| Right hippocampus | 36 | −36 | −3 | 3.78 | 7 |
| Focus of attention > (passive set and active set) | |||||
| Supramarginal gyrus (BA 40) | −54 | −51 | 42 | 4.88 | 199 |
| Superior/middle temporal gyrus | 48 | −51 | 33 | 4.79 | 431 |
| Superior frontal gyrus (BA 6) | 18 | 27 | 57 | 4.52 | 82 |
| Superior frontal gyrus (BA 6) | 15 | 54 | 39 | 4.01 | 12 |
| Superior frontal gyrus (BA 6) | 24 | 57 | 12 | 3.94 | 7 |
| Middle frontal gyrus | 51 | 21 | 33 | 4.24 | 14 |
| Middle temporal gyrus | −66 | −30 | −12 | 4.51 | 111 |
| Middle temporal gyrus | 63 | −30 | −12 | 4.14 | 96 |
| Medial frontal gyrus | −3 | 42 | 33 | 3.96 | 26 |
| Precuneus (BA 7) | 0 | −33 | 45 | 3.74 | 13 |
| Active set > passive set | |||||
| Supramarginal gyrus (BA 40) | −42 | −57 | 27 | 3.82 | 12 |
Note: We compared brain-region activation for retrieval of items in 12 serial positions (SPs): SPs 1 through 8 were the passive set, SPs 9 through 11 were the active set, and SP 12 was the focus of attention. No reliable activations were found for passive set > active set. BA = Brodmann’s area.
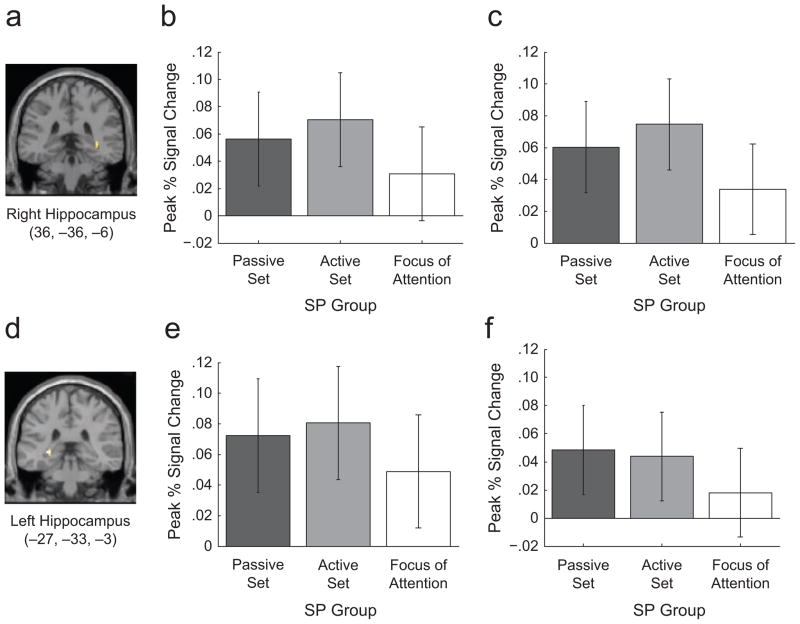
Consistent with our previous findings (Öztekin, McElree, et al., 2008), the results of this analysis revealed enhanced activation in the right hippocampus (Fig. 3a) for SPs 1 through 11 compared with SP 12 (Fig. 3b). When SPs 1 through 11 were divided into the active set and the passive set, pair-wise comparisons of the peak PSC revealed that activation in the right hippocampus was significantly reduced for the focus of attention compared with the active set, F(1, 19) = 6.175, p < .022, η2 = .245, and was marginally less for the focus of attention compared with the passive set, F(1, 19) = 3.482, p < .078, η2 = .155. Additionally, assessment of regions showing greater neural activation for the active set compared with the focus of attention revealed the left hippocampus (Fig. 3d), which also exhibited reduced activation for the focus of attention—the active set versus the focus of attention: F(1, 19) = 9.256, p < .007, η2 = .328; the passive set versus the focus of attention: F(1, 19) = 2.931, p < .10, η2 = .134 (Fig. 3e). Following the procedures of Talmi et al. (2005), we further compared neural activation for the first two SPs with neural activation for the last two SPs. This comparison indicated that the right hippocampus was at the .005 threshold. Hence, our data suggest that the findings of Talmi et al. may indeed reflect a dissociation between focal attention and memory representations, rather than a dissociation between LTM and STM.
Fig. 3.
Blood-oxygenation-level-dependent activation in both hippocampal regions, as identified from the voxel-wise contrasts. A coronal slice indicating activation in the right hippocampal region is shown in (a). Peak percentage signal change in the right hippocampus across the three serial-position (SP) groups (passive set: SPs 1–8; active set: SPs 9–11; focus of attention: SP 12) is shown in (b). Peak percentage signal change in the right hippocampus when we redefined the active set on the basis of individual participants’ behavioral data is shown in (c). A coronal slice indicating activation in the left hippocampus is shown in (d). Peak percentage signal change in the left hippocampus across the three SP groups is shown in (e), and peak percentage signal change in the left hippocampus when we redefined the active set on the basis of individual participants’ behavioral data is shown in (f). Error bars indicate 95% confidence intervals.
Accuracy analysis
The reported regions were additionally subjected to an analysis that assessed whether the observed BOLD activation predicted memory success. Both left and right hippocampal regions exhibited activation correlated with task accuracy: Activation was enhanced for correct trials compared with incorrect trials—right hippocampus: t(19) = 2.45, p < .024; left hippocampus: t(19) = 3.12, p < .006.
Hippocampal activation and quality of the retrieved memory representation
As it is well established that the hippocampus is important for memory retrieval, the reduced BOLD response for SP 12 aligns with the findings of behavioral studies indicating that the last item studied often resides in focal attention and therefore does not need to be retrieved. We found significantly enhanced hippocampal activation for both active-set items and passive-set items relative to SP 12, with a larger BOLD response for the active set. Enhanced activation for active-set items is at odds with the claim that the MTL is not involved in the retrieval of items from WM. It is also inconsistent with a weaker variant of this hypothesis, in which MTL activation, although not completely absent when items are retrieved from WM, is believed to be less for items within WM than for items in LTM alone. We conducted additional analyses that indicated that the observed activation patterns in the hippocampus rather reflect differences in the probability of successful retrieval.
It is well known that neural activation in the hippocampus during encoding predicts later successful LTM performance (e.g., Davachi, Mitchell, & Wagner, 2003), and activation in the hippocampus is enhanced during successful LTM retrieval (e.g., Dobbins, Rice, Wagner, & Schacter, 2003; Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000). Recently, we have also shown that activation in the hippocampus correlates with WM-retrieval success (Öztekin, Curtis, & McElree, 2008; Öztekin, McElree, et al., 2008). Accordingly, we found that hippocampal activation was enhanced during correct compared with incorrect trials; this finding indicates this region’s contribution to successful recognition-memory performance in our task. A related question is whether hippocampal activation further differs depending on the underlying quality (strength) of the memory representation for successfully retrieved memory representations (e.g., see Squire, Wixted, & Clark, 2007). To the degree that successful retrieval is a function of the quality (strength, distinctiveness, or analogous constructs) of the memory representation, hippocampal activation should vary with the recency of the probe; for example, activation should decrease as the probe is drawn from less recent SPs. If this is the case, it would provide a principled account for all observed activation patterns.
In order to investigate whether the quality of the memory representations predicts BOLD activation in the hippocampus across SPs, and whether the focus of attention exhibits privileged access, we fit the peak PSC in the reported hippocampal regions with a model that scaled the corresponding accuracy for each condition:
| (1) |
In Equation 1, PSC_p is the predicted PSC for condition i, d is the observed accuracy for condition i, and k is a constant that scales d. In a second model, an additional parameter was used to account for the probability of the representation being in focal attention for SP 12:
| (2) |
In Equation 2, pfoc is the probability of the representation being in focal attention. For SPs 1 through 11, the k constant scales the corresponding d′ to estimate PSC as in Equation 1. For SP 12, PSC_p is estimated by scaling d′ by (1 − pfoc). That is, activation in the hippocampus is predicted only for the proportion of times when the last item is not maintained in focal attention; this is consistent with the notion that a retrieval operation is not required when an item is in focal attention.
The two models were evaluated with an adjusted R2 statistic2, which reflects the proportion of variance accounted for by a model, adjusted by the number of free parameters. Overall, Equation 2 resulted in higher adjusted R2 measures compared with Equation 1. The resultant R2 measures for the two regions were .969 to .99 and .349 to .652 for Equation 2 and Equation 1, respectively (Fig. 4). Consistent with R2 measures, the estimates of BOLD activation presented in Figure 4 indicate that the data are predicted better with the model that takes into account the probability of the representation being in focal attention (Equation 2). Specifically, when the probability of the representation being in focal attention is not considered (Equation 1), the fit overestimates the last SP (see McElree & Dosher, 1989, for a related finding, in which memory strength is used to predict RT SP functions), whereas Equation 2 can capture this difference in the data (Fig. 4a vs. 4c and Fig. 4e vs. 4g). In both hippocampal regions, the difference between R2 measures across the two model fits were statistically significant at p < .01. Hence, BOLD activation in the hippocampus seems to reflect the quality of the memory representation, with the exception of the most recently studied item, for which no retrieval operation is engendered when the item is maintained in focal attention.
Fig. 4.
Estimates of blood-oxygenation-level-dependent activation in both hippocampal regions (reported in Fig. 3), based on corresponding accuracy across conditions. Results for the right hippocampus are shown in (a) through (d). The observed and the fitted peak percentage signal change for the three serial-position (SP) groups (passive set: SPs 1–8; active set: SPs 9–11; focus of attention: SP 12) is shown in (a). The observed and the fitted peak percentage signal change across SPs is shown in (b). Bars indicate the data; the line indicates the fit with Equation 2. The observed and the fitted peak percentage signal change for the three SP groups when redefined on the basis of individual participants’ behavioral data is shown in (c). The observed and the fitted peak percentage signal change across SPs when redefined on the basis of individual participants’ behavioral data is shown in (d). The bars indicate the data; the line indicates the fit with Equation 1. Corresponding data for activation in the left hippocampus are shown in (e) through (h).
Individual differences in memory performance
To ensure that the reported differences in hippocampal activation were not confounded with individual differences in WM, we conducted an analysis in which we defined the passive set and the active set from each participant’s behavioral data and reassessed neural-activation patterns in the hippocampus. For each participant, the beginning of the active set was defined as the SP in which accuracy started to consistently rise after the drop from a primacy effect. This approach resulted in a range of two to six items in the active set across participants (M = 3.3). Accordingly, the passive set was defined as the SPs that came before the active set, and the focus of attention was fixed to SP 12. We then examined neural activation in the reported hippocampal regions across the three states. Consistent with our earlier results, these analyses indicated reduced activation for the focus of attention compared with both the active set—right hippocampus: t(19) = −10.04, p < .009; left hippocampus: t(19) = −3.02, p < .09—and the passive set—right hippocampus: t(19) = −8.04, p < . 015; left hippocampus: t(19) = −11.46, p < .001 (Figs. 3c and 3f). Hence, neural-activation patterns are the same when individual differences in memory performance are also taken into account.
Discussion
Overall, our results are inconsistent with the traditional view that posits that there are distinct stores for WM and LTM. We used a probe-recognition task with 12-item study lists to assess potential differences in neural activation for recognition judgments of items hypothesized to be in focal attention, within the span of WM, or in LTM. Our primary focus was on the hippocampus, as this region has been unequivocally linked to memory retrieval and is predicted by dual-store models to support LTM retrieval only (e.g., Talmi et al., 2005). We used a brief (450 ms) interval between study and test to minimize rehearsal and maintenance operations; this procedure allowed us to isolate retrieval-specific differences in neural activation.
Focal attention
Our first goal was to examine dissociations for recognition judgments of items in focal attention and items that needed to be retrieved from memory. Our data indicated that hippocampal activation was enhanced for the passive and active sets (SPs 1–11) compared with the last-studied item (SP 12), which had a high probability of being maintained in focal attention at test time, thereby circumventing the need for retrieval. This finding replicates the results of our previous study (Öztekin, McElree, et al., 2008), and it further demonstrates that the same pattern holds when the task requires the retention of information that exceeds the hypothesized span of WM. It is important to note that, when viewed in light of behavioral evidence demonstrating privileged access for the last item studied (McElree, 2006; McElree & Dosher, 1989; Öztekin & McElree, 2007; Wickelgren et al., 1980), our findings indicate that previously reported dissociations in the MTL across early and recent items in item recognition (e.g., Talmi et al., 2005) likely reflect a dichotomy between focal attention and memory representations rather than a classical distinction between LTM and WM representations.
Memory retrieval
Our second goal was to determine whether there was neural evidence supporting a further distinction between WM and LTM. When SPs were concatenated into representations hypothesized to be within WM span—namely, SPs 9 through 11; this range corresponds to Oberauer’s (2001, 2002) active set—and representations hypothesized to be outside WM span—namely, SPs 1 through 8; this range corresponds to Oberauer’s (2001, 2002) passive set—MTL activation was found to be present in both and, indeed, was greater in the former than in the latter. Moreover, the same pattern held when individual differences in memory performance were taken into account. These findings demonstrate that the MTL is involved in retrieval of short-term information, contra predictions of dual-store accounts (Talmi et al., 2005). Strikingly, the observed pattern is opposite to what would be naturally predicted from the classical distinction between LTM and WM; in the traditional view, representations in WM are argued to be more accessible (active) than those in LTM. Rather than suggesting different representational states or different retrieval operations, hippocampal activation across SPs appears to be related to the probability of successfully retrieving an item from memory; success varies as a function of recency. Hence, the data appear better explained by a single-store account, in which hippocampal activation is modulated by underlying memory strength.
Our fMRI findings and the corresponding time-course findings (reviewed in McElree, 2006) could be viewed as contrasting with research on visual short-term memory with the change-detection paradigm (e.g., Luck & Vogel, 1997; Vogel & Machizawa, 2004; Vogel, Woodman, & Luck, 2001; Xu & Chun, 2006), which has been interpreted as evidence for a three- to four-item WM capacity. The discrepancies are likely due to three salient procedural differences between the two lines of research. First, the research on visual short-term memory using the change-detection paradigm relies on accuracy measures alone and estimate capacity with a single-parameter equation, whereas research investigating the full time course relies on direct measures of accessibility. Second, change-detection tasks use simultaneously presented multiobject visual displays, whereas our research has used sequentially presented lists of items. The former affords greater potential for grouping operations and for the immediate coding of relational information, whereas the latter requires the retention of individual items over time and across new study items. Third, the two lines of research may assess different notions of “capacity.”
We believe that research on multiobject visual displays measures the upper limit on encoding of concurrently presented elements, whereas our research uses measures of accessibility to determine the state of distinctly encoded episodic events across time or intervening events (see McElree & Dosher, 2001). Finally, given these differences, particularly the third, it is not surprising that the supporting neural research focused on different brain regions: The previous research has focused on the parietal cortex (e.g., Xu & Chun, 2006), whereas we have focused on the MTL, a region known to be critical for encoding and retrieving memories (see also Nee & Jonides, 2008; Öztekin, McElree, et al., 2008).
It is clear that additional research is needed to fully reconcile the two lines of research, but discrepancies may be less significant than they might first appear. McElree (1998) found that the privileged access for the last item found here and in other studies (McElree, 2006) extends to the last three items if they form an easily coded chunk (e.g., three instances of a semantic category); this finding suggests that the contents of focal attention should be defined in terms of an encoding epoch. From this perspective, our findings call into question the notion of a WM store for maintaining information outside of focal attention over time, but our findings are not inconsistent with the idea that three to four elements of a display could be encoded into a single event within focal attention (see also Jonides et al., 2008).
In conclusion, our findings indicate that hippocampal activation correlates with successful item recognition and is further modulated by the quality of the memory representation both within and outside of the hypothesized span of WM. These findings stand in contrast to dual-store accounts, which assert that the hippocampus should selectively support access to LTM representations. The observed pattern, in which activation in the hippocampus systematically tracks underlying memory strength across SPs (with the exception of the last-studied item, which resides in focal attention), aligns more directly with accounts that assume there is both a common store and similar operating principles across WM and LTM representations (e.g., Crowder, 1993; Nairne, 2002; Surprenant & Neath, 2009; Wickelgren, 1973).
Acknowledgments
We thank Nick Ketz for assistance in data collection.
Funding
This research was supported by the New York University Center for Brain Imaging and by grants from the National Science Foundation to B.M. (BCS-0236732) and the National Institutes of Health to L.D. (MH-074692) and B.M. (HD-056200).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
To determine the appropriate cluster-size threshold, we conducted a simulation on a bilateral structural hippocampal region-of-interest mask using AlphaSim software (Ward, 2010). This simulation indicated that 4 voxels was the appropriate cluster-size threshold.
References
- Baddeley AD, Hitch G. Working memory. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Marseille boîte à région d’intérêt (MarsBaR; Version 0.41) [Computer software] 2002 Retrieved May 21, 2010, from http://marsbar.sourceforge.net/
- Cowan N. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. Cambridge, England: Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- Cowan N. Working memory capacity: Essays in cognitive psychology. New York: Psychology Press; 2005. [Google Scholar]
- Crowder RG. Short-term memory: Where do we stand? Memory & Cognition. 1993;21:142–145. doi: 10.3758/bf03202725. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences, USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuoropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Functional Imaging Laboratory. Statistical Parametric Mapping (Version 2) [Computer software] 2003 Retrieved May 21, 2010, from http://www.fil.ion.ucl.ac.uk/spm/software/spm2/
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annual Review of Psychology. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;3:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McElree B. Attended and non-attended states in working memory: Accessing categorized structures. Journal of Memory and Language. 1998;38:225–252. [Google Scholar]
- McElree B. Accessing recent events. In: Ross BH, editor. The psychology of learning and motivation. Vol. 46. San Diego, CA: Academic Press; 2006. pp. 155–200. [Google Scholar]
- McElree B, Dosher BA. Serial position and set size in short-term memory: Time course of recognition. Journal of Experimental Psychology: General. 1989;18:346–373. [Google Scholar]
- McElree B, Dosher BA. The focus of attention across space and time. Behavioral & Brain Sciences. 2001;24:129–130. [Google Scholar]
- Nairne JS. Remembering over the short-term: The case against the standard model. Annual Review of Psychology. 2002;53:53–81. doi: 10.1146/annurev.psych.53.100901.135131. [DOI] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Neural correlates of access to short-term memory. Proceedings of the National Academy of Sciences, USA. 2008;105:14228–14233. doi: 10.1073/pnas.0802081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer K. Removing irrelevant information from working memory: An age-comparative study with the modified Sternberg task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:948–957. [PubMed] [Google Scholar]
- Oberauer K. Access to information in working memory: Exploring the focus of attention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:411–421. [PubMed] [Google Scholar]
- Öztekin I, Curtis C, McElree B. The medial temporal lobe and the left inferior prefrontal cortex jointly support interference resolution in verbal working memory. Journal of Cognitive Neuroscience. 2008;21:1967–1979. doi: 10.1162/jocn.2008.21146. [DOI] [PubMed] [Google Scholar]
- Öztekin I, McElree B. Proactive interference slows recognition by eliminating fast assessments of familiarity. Journal of Memory and Language. 2007;57:126–149. [Google Scholar]
- Öztekin I, McElree B, Staresina B, Davachi L. Working memory retrieval: Contributions of left prefrontal cortex, left posterior parietal cortex and hippocampus. Journal of Cognitive Neuroscience. 2008;21:581–593. doi: 10.1162/jocn.2008.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant AM, Neath I. The 9 lives of short-term memory. In: Thorn A, Page M, editors. Interactions between short-term and long-term memory in the verbal domain. Hove, England: Psychology Press; 2009. pp. 16–43. [Google Scholar]
- Talmi D, Grady CL, Goshen-Gottstein Y, Moscovitch M. Neuroimaging the serial position curve: A test of single-store versus dual-store models. Psychological Science. 2005;16:716–723. doi: 10.1111/j.1467-9280.2005.01601.x. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Ward BD. AlphaSim [Computer software] 2010 Retrieved May 21, 2010, from http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html.
- Wickelgren WA. The long and the short of memory. Psychological Bulletin. 1973;80:425–438. [Google Scholar]
- Wickelgren WA, Corbett AT, Dosher BA. Priming and retrieval from short-term memory: A speed accuracy tradeoff analysis. Journal of Verbal Learning and Verbal Behavior. 1980;19:387–404. [Google Scholar]
- Wilson M. Medical Research Council psycholinguistics database [Database] 1986 Retrieved May 21, 2010, from http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm.
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]