Abstract
We recently reported that subantimicrobial-dose doxycycline (SDD) significantly reduced serum bone-resorption biomarkers in subgroups of post-menopausal women. We hypothesize that changes in serum bone biomarkers are associated not only with systemic bone mineral density (BMD) changes, but also with alveolar bone changes over time. One hundred twenty-eight eligible post-menopausal women with periodontitis and systemic osteopenia were randomly assigned to receive SDD or placebo tablets twice daily for two years, adjunctive to periodontal maintenance. Sera were analyzed for bone biomarkers. As expected, two-year changes in a serum bone biomarker were significantly associated with systemic BMD loss at the lumbar spine (osteocalcin, bone-turnover biomarker, p = 0.0002) and femoral neck (osteocalcin p = 0.0025). Two-year changes in serum osteocalcin and serum pyridinoline-crosslink fragment of type I collagen (ICTP; bone-resorption biomarker) were also significantly associated with alveolar bone density loss (p < 0.0001) and alveolar bone height loss (p = 0.0008), respectively. Thus, we have shown that serum bone biomarkers are associated with not only systemic BMD loss, but with alveolar bone loss as well. Clinical Trial Registration Information: Protocol registered at ClinicalTrials.gov, NCT00066027.
Abbreviations: bone mineral density (BMD), bone-specific alkaline phosphatase (BSAP), computer-assisted densitometric image analysis (CADIA), confidence interval (CI), deoxypyridinoline-containing degradation fragment of the C-terminal telopeptide region of type I collagen (CTX), coefficient of variation (CV), dual-energy x-ray absorptiometry (DXA), pyridinoline-crosslink-containing degradation fragment of the C-terminal telopeptide region of type I collagen (ICTP), and subantimicrobial-dose doxycycline (SDD).
Keywords: subantimicrobial-dose doxycycline, periodontitis, osteopenia, serum biomarkers, bone, post-menopausal
Introduction
Our research group recently reported that a two-year subantimicrobial-dose doxycycline (SDD) regimen significantly reduced the serum bone-resorption biomarkers, deoxypyridinoline-crosslink fragment of type I collagen (CTX) and pyridinoline-crosslink fragment of type I collagen (ICTP), in subgroups of post-menopausal women with periodontitis and systemic osteopenia (Golub et al., 2010) enrolled in a double-blind, placebo-controlled randomized clinical trial. In this trial, SDD did not significantly affect serum biomarkers of bone formation (bone-specific alkaline-phosphatase [BSAP]) or bone turnover (osteocalcin). SDD is approved by the United States Food and Drug Administration to treat chronic periodontitis as an adjunct to scaling and root planing. To the best of our knowledge, our clinical trial was the first to examine SDD effects on systemic, as opposed to only local, bone turnover.
Serum bone biomarkers appear to be useful for the assessment of therapeutic responses to anti-osteoporosis therapies (Seibel, 2006), and changes in these biomarkers precede treatment-induced systemic bone mineral density (BMD) changes (Blumsohn et al., 2011). It has been suggested that serum CTX should be monitored as a means of predicting risk of osteonecrosis of the jaw (Marx et al., 2007) in patients taking oral bisphosphonates and requiring oral surgical procedures, although the use of serum CTX in this fashion has not been rigorously evaluated in clinical studies. There is a gap in our understanding of the relationship between systemic bone biomarkers and alveolar bone loss. Therefore, the purpose of this study was to test the hypothesis that serum bone biomarkers, which are known to reflect systemic bone loss, are also associated with alveolar (local) bone loss.
Materials & Methods
Clinical Trial Design
The design of this clinical trial has been described previously (Payne et al., 2007). One hundred twenty-eight eligible women participated, and all signed University of Nebraska Medical Center and Stony Brook University Institutional Review Board-approved consent forms. The trial was double-blind, randomized, and placebo-controlled. Participants were randomized to SDD (n = 64) or placebo (n = 64). They were instructed to take the study drug (SDD [20 mg doxycycline hyclate] or placebo tablets) twice daily for 2 yrs. In addition, they were provided calcium and vitamin D supplements to be taken twice daily (a total of 1200 mg calcium and 400 IU vitamin D daily) for 2 yrs, as the standard of care for post-menopausal women. Participants were instructed to take calcium and vitamin D supplements no sooner than 1 hr after taking the study drug. All participants received periodontal maintenance every 3 to 4 mos throughout the study by their own dental provider and at no cost.
Inclusion and exclusion criteria have been published (Payne et al., 2007). Briefly, participants were between 45 and 70 yrs of age at telephone screening, post-menopausal, osteopenic at the lumbar spine or femoral neck (defined as a T-score of -1.0 to -2.5 inclusive on dual-energy x-ray absorptiometry [DXA] scan), and were not receiving any medications that would influence bone remodeling (e.g., hormone replacement therapy or bisphosphonates). All participants had a history of generalized moderate to advanced chronic periodontitis (defined as at least 2 sites, on different posterior teeth, with probing depths and clinical attachment loss ≥ 5 mm with bleeding on probing), were in good general health, and did not have osteoporosis at either the lumbar spine or femoral neck, as measured by DXA (see below).
Measurement of Serum Bone Biomarkers
Non-fasting blood samples were collected from each individual at baseline, one-year, and two-year visits. Serum was obtained by standard techniques and then frozen at −80°C until analysis. The intra-assay coefficient of variation (CV) for this study is reported below for each biomarker. Serum CTX (Nordic Bioscience Diagnostics, Herlev, Denmark; intra-assay CV = 10.2%), BSAP (Quidel Corp., San Diego, CA, USA; intra-assay CV = 7.0%), and osteocalcin (Nordic Bioscience Diagnostics; intra-assay CV = 3.3%) were determined by enzyme immunoassay, as previously described (Golub et al., 2010). Serum ICTP (Immunodiagnostic Systems, Fountain Hills, AZ, USA; intra-assay CV = 7.5%) was determined by radioimmunoassay (Golub et al., 2010).
Measurement of Alveolar Bone Density and Height
Four posterior bite-wing radiographs (maxillary and mandibular right and left) were taken at baseline, one-year, and two-year visits. Images were exposed by the extended geometry method (Payneet al., 1999) introduced by Jeffcoat et al. (1987). Alveolar bone density was measured by computer-assisted densitometric image analysis (CADIA) at crestal and subcrestal areas of interest (Payneet al., 2007). Alveolar bone height measurements were made by the method described by Hausmann et al. (1992); linear measurements were made between a fixed reference point (cemento-enamel junction or restoration margin) and the alveolar crest. Alveolar bone measurements were made at the Longitudinal Radiographic Assessment Facility in San Antonio, TX, by a single masked examiner (PVN).
Measurement of Systemic Bone Mineral Density
Annual BMD scans of the lumbar spine and femoral neck were taken by DXA (Hologic 4500, Waltham, MA, USA), as previously described (Payne et al. 2007).
Statistical Analyses
Two-year changes in serum-biomarker measures from baseline were modeled as categorical variables, where categories were defined by the observed tertiles (tertiles of lowest, intermediate, and highest). Linear regression models were fit to investigate the association between serum-biomarker (independent factor) changes and concurrent changes in alveolar and systemic bone (dependent factor). Generalized estimating equations methodology was used to fit the models for the alveolar bone outcomes with a working exchangeable correlation structure to account for correlation among tooth-site-level measures on the same participant. We performed tests for linear trends to assess mean changes in bone among the ordered biomarker change categories. Models were adjusted for baseline bone measures, randomized treatment (SDD/placebo), baseline smoking status (current smoker/not), center (Nebraska/ Stony Brook), baseline age, and assay batch (all samples for a given participant were analyzed in the same batch by one person [Dr. Hsi-ming Lee], blinded to treatment assignment, and batches were well balanced by treatment group). Fitted models included interaction terms to determine whether the association between biomarker levels and bone changes differed between the randomized treatment groups or time-points (one-year vs. two-year change). Data were analyzed for the SDD and placebo groups combined, because no significant interactions with treatment were found. Two-year changes are reported because associations were evident long-term (2 yrs), but not at 1 yr, when significant modification by time was found, and were consistent with aggregated time-point analyses (1 and 2 yrs combined), when significant modification by time was not found (see Appendix). Based on a Bonferroni adjustment for 8 multiple comparisons (4 biomarkers with 2 pair-wise comparisons each), a 2-sided 0.006 alpha level defined statistical significance.
Results
One hundred sixteen individuals completed the one-year visit and provided serum (SDD n = 55, placebo n = 61), while 113 individuals completed the two-year visit and provided serum (SDD,n = 51; placebo, n = 62). Serum was not drawn at the one-year visit for one placebo participant who provided a two-year sample, and four SDD participants withdrew from the study between the one- and two-year visits. In total, 117 individuals provided serum samples at either the one-year or two-year visit and were included in the analyses of the combined time-points. The Fig. shows the flow of participants through randomization, follow-up, and analysis. Participants were primarily white, non-Hispanic or Latino, more than 5 years post-menopausal, never or former smokers, and were 58.3 yrs old (standard deviation 5.7) on average (Table 1).
Figure.
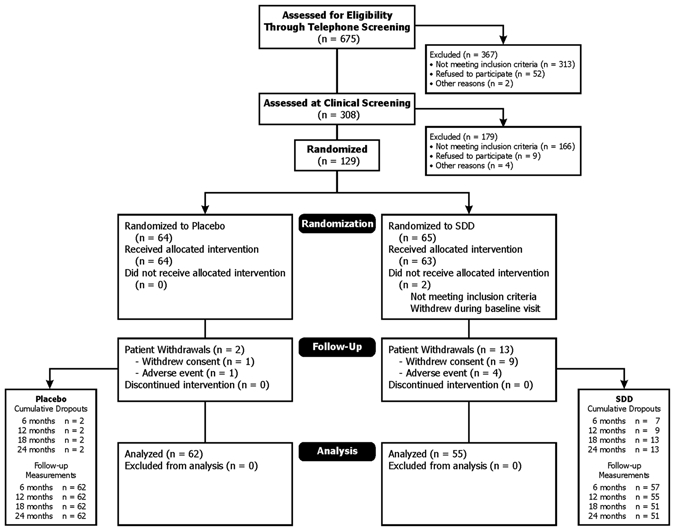
Consolidated Standards of Reporting Trials flow diagram. This Fig. shows the flow of participants through randomization, follow-up, and analysis. This diagram was previously published (Payne et al., 2007) and is reproduced with permission from Wiley-Blackwell.
Table 1.
Baseline Demographic and Clinical Characteristics (n = 117)
| Characteristic | Mean (SD) or Count (%) |
|---|---|
| Age (yrs) | 58.3 (5.7) |
| Ethnicity | |
| Hispanic or Latino | 5 (4%) |
| Not Hispanic or Latino | 112 (96%) |
| Race | |
| Asian | 3 (3%) |
| African American | 2 (2%) |
| White | 112 (96%) |
| Years post-menopausal | |
| 5 or fewer | 44 (38%) |
| More than 5 | 73 (62%) |
| Smoking status | |
| Current smoker | 24 (21%) |
| Former smoker | 38 (32%) |
| Never smoker | 55 (47%) |
| Number of teeth | 25.8 (2.6) |
| Lumbar spine | |
| Bone mineral density (g/cm2) | 0.9 (0.08) |
| T-score | –1.2 (0.7) |
| Femoral neck | |
| Bone mineral density (g/cm2) | 0.7 (0.07) |
| T-score | –1.3 (0.6) |
| Alveolar bone height (mm) | 3.2 (1.4) |
| Manual probing depth (mm) | 3.8 (1.2) |
Serum osteocalcin changes in the highest tertile were significantly associated with alveolar bone density loss at 2 yrs compared with osteocalcin changes in the lowest tertile (p < 0.0001) (Table 2). Likewise, serum osteocalcin changes in the highest tertile were significantly associated with lumbar spine BMD loss (p = 0.0002) and femoral neck BMD loss (p = 0.0025). No significant association was noted between changes in serum osteocalcin and alveolar bone height changes, based on a test of linear trend (p = 0.47).
Table 2.
Association between Two-year Changes from Baseline in Osteocalcin and Concurrent Alveolar Bone Density (computer-assisted densitometric image analysis [CADIA]), Alveolar Bone Height, Lumbar Spine Bone Mineral Density (BMD), and Femoral Neck Bone Mineral Density (BMD) Changes (n = 113)
| Bone Measure | Biomarker Change Category | Difference in MeanBone Changes | 95% CI** | p-value |
|---|---|---|---|---|
| CADIA changes(CADIA units) | Lowest tertile (< −6.5 ng/mL) | 0 (reference) | ||
| Intermediate tertile (−6.5 to −0.5 ng/mL) | −1.38 | −2.95 to 0.19 | 0.086 | |
| Highest tertile (> −0.5 ng/mL) | −3.56 | −5.10 to −2.03 | < 0.0001 | |
| Alveolar bone height changes (mm)* | Lowest tertile (< -6.5 ng/mL) | 0 (reference) | ||
| Intermediate tertile (−6.5 to −0.5 ng/mL) | 0.019 | −0.022 to 0.059 | 0.37 | |
| Highest tertile (> −0.5 ng/mL) | 0.016 | −0.027 to 0.059 | 0.47 | |
| Lumbar spine BMD% changes | Lowest tertile (< −6.5 ng/mL) | 0 (reference) | ||
| Intermediate tertile (−6.5 to −0.5 ng/mL) | −0.35% | −2.07% to 1.38% | 0.69 | |
| Highest tertile (> −0.5 ng/mL) | −3.13% | −4.80% to −1.46% | 0.0002 | |
| Femoral neck BMD% changes | Lowest tertile (< −6.5 ng/mL) | 0 (reference) | ||
| Intermediate tertile (−6.5 to −0.5 ng/mL) | −1.78% | −3.84% to 0.28% | 0.091 | |
| Highest tertile (> −0.5 ng/mL) | −2.82% | −4.65% to −0.99% | 0.0025 |
Alveolar bone height change was calculated as two-year alveolar bone height (mm) minus baseline alveolar bone height (mm). A positive difference reflects alveolar bone height loss.
CI, confidence interval.
Serum ICTP changes in the highest tertile were significantly associated with alveolar bone height loss at 2 yrs (p = 0.0008) (Table 3), while no significant association was noted with changes in alveolar bone density (p = 0.30), lumbar spine BMD (p = 0.71), or femoral neck BMD (p = 0.68), based on a test of linear trend.
Table 3.
Association between Two-year Changes from Baseline in Pyridinoline-crosslink Fragment of Type I Collagen (ICTP) and Concurrent Alveolar Bone Density (computer-assisted densitometric image analysis [CADIA]), Alveolar Bone Height, Lumbar Spine Bone Mineral Density (BMD), and Femoral Neck Bone Mineral Density (BMD) Changes (n = 113)
| Bone Measure | Biomarker Change Category | Difference in MeanBone Changes | 95% CI** | p-value |
|---|---|---|---|---|
| CADIA changes (CADIA units) | Lowest tertile (< −0.3 ng/mL) | 0 (reference) | ||
| Intermediate tertile (−0.3 to 0.3 ng/mL) | −1.20 | −3.17 to 0.78 | 0.23 | |
| Highest tertile (> 0.3 ng/mL) | −0.76 | −2.19 to 0.68 | 0.30 | |
| Alveolar bone height changes (mm)* | Lowest tertile (< −0.3 ng/mL) | 0 (reference) | ||
| Intermediate tertile (−0.3 to 0.3 ng/mL) | 0.030 | −0.0078 to 0.067 | 0.12 | |
| Highest tertile (> 0.3 ng/mL) | 0.060 | 0.025 to 0.095 | 0.0008 | |
| Lumbar spine BMD % changes | Lowest tertile (< −0.3 ng/mL) | 0 (reference) | ||
| Intermediate tertile (−0.3 to 0.3 ng/mL) | 0.98% | −0.83% to 2.79% | 0.29 | |
| Highest tertile (> 0.3 ng/mL) | 0.33% | −1.40% to 2.05% | 0.71 | |
| Femoral neck BMD % changes | Lowest tertile (< −0.3 ng/mL) | 0 (reference) | ||
| Intermediate tertile (−0.3 to 0.3 ng/mL) | 1.21% | −0.79% to 3.22% | 0.24 | |
| Highest tertile (> 0.3 ng/mL) | 0.41% | −1.58% to 2.40% | 0.68 |
Alveolar bone height change was calculated as two-year alveolar bone height (mm) minus baseline alveolar bone height (mm). A positive difference reflects alveolar bone height loss.
CI, confidence interval.
No significant association was noted between changes in CTX and changes in alveolar bone density (p = 0.095), alveolar bone height (p = 0.12), lumbar spine BMD (p = 0.011), or femoral neck BMD (p = 0.41), based on a test of linear trend. Similarly, changes in BSAP were not significantly associated with changes in alveolar bone density (p = 0.055), alveolar bone height (p = 0.52), lumbar spine BMD (p = 0.012), or femoral neck BMD (p = 0.67), based on a test of linear trend.
Discussion
In this study, we have shown that changes in serum bone biomarkers over time are associated not only with systemic bone density loss, but also with loss of alveolar bone density and alveolar bone height, in post-menopausal women with periodontitis and systemic osteopenia. Menopause is associated with a substantial increase in bone turnover, accompanied by increases in both bone formation and resorption and reflected by increases in serum bone formation and resorption biomarkers (Kushidaet al., 1995; Midtby et al., 2001). However, the rate of bone resorption exceeds the rate of bone formation, resulting in a net loss of bone mass (Manolagas, 2000). Serum osteocalcin was significantly associated with alveolar bone density loss at 2 yrs, while serum ICTP was significantly associated with alveolar bone height loss at 2 yrs. It is presumed that alveolar bone density loss precedes alveolar bone height loss (Ortman et al., 1982), and we have shown that alveolar bone density loss over a two-year period is more common than alveolar bone height loss (12% of posterior sites manifesting alveolar bone density loss vs. 8% manifesting alveolar bone height loss) (Payne et al., 2007). It is possible that serum osteocalcin is a more sensitive biomarker of alveolar bone loss, since it was associated with alveolar bone density loss rather than alveolar bone height loss. In contrast, ICTP was associated with more pronounced bone loss, manifesting as loss of alveolar bone height. Gingival crevicular fluid (a serum exudate) ICTP has been shown to be related to active bone destruction in the Beagle dog (Giannobile et al., 1995). Since the serum bone biomarkers measure different processes (formation vs. resorption), some are collagen breakdown products (ICTP and CTX), and others are products of different cells (e.g., osteocalcin is synthesized by osteoblasts but may also be released during bone resorption; BSAP is a specific product of osteoblasts but may cross-react with the liver isoenzyme), dissimilar findings with different serum biomarkers are expected (Seibel, 2005).
In this study, data were pooled from participants who received SDD and placebo, because there was no significant evidence that SDD modified the association between serum biomarker levels and bone loss. In our clinical trial, based on intent-to-treat analyses, SDD did not significantly alter alveolar or systemic bone loss (Payne et al., 2007) and did not significantly alter serum bone biomarkers, although, in subgroups, SDD significantly reduced serum ICTP and CTX (Golub et al., 2010), and SDD significantly reduced the loss of both alveolar bone density and bone height (Payne et al., 2007). However, the lack of intent-to-treat effect may explain why treatment did not modify the association between the serum biomarker levels and bone changes.
We have shown for the first time that serum bone biomarkers are associated with alveolar bone loss. Future studies should further examine the relationship between serum bone biomarkers and alveolar bone loss in larger patient populations, preferably osteoporotic rather than osteopenic individuals. These studies also should utilize fasting serum samples at the same time of day for each visit. The knowledge acquired can potentially lead to a better understanding of systemic influences on the periodontium and use of systemic (serum) biomarkers to determine risk of progressive alveolar bone loss. With further research, prediction of overall susceptibility to alveolar bone loss on an individual basis with a simple blood test could potentially provide valuable information to a practitioner who, at this time, still cannot identify susceptible patients prior to their manifesting periodontitis progression.
Examples of therapeutic approaches to treat both oral and systemic bone loss include SDD, as discussed earlier, and teriparatide, which has been shown, in a recent clinical trial, to be associated with greater resolution of alveolar bone defects at 6 mos and 1 yr (Bashutski et al., 2010). In this trial, at 6 wks, individuals in the teriparatide group had a statistically significant transient increase in serum alkaline phosphatase levels relative to placebo, which preceded statistically significant resolution of osseous defects beginning at 6 mos. Other novel pro-anabolic and/or anti-catabolic treatment strategies to simultaneously treat both oral and systemic bone loss include the chemically modified tetracyclines (Golub et al., 1999) and newer non-tetracyclines (poly-enolic/phenolic zinc-binding molecules) designed to incorporate a putative active site on tetracycline and chemically modified tetracycline compounds (i.e., the β-diketone, metal ion [calcium and zinc]-binding site at carbon-11 and carbon-12), which are now being tested (Payne and Golub, 2011). By 2020, approximately one in every two women (41 million women) aged 50 and over is projected to have osteoporosis or osteopenia of the hip (Population Division, US Census Bureau, 2008; US DHHS, 2004), and greater than 60% of women over age 50 have at least 3 mm of attachment loss (Albandar et al., 1999). Since post-menopausal osteoporotic women with periodontitis represent a large patient cohort, and the morbidity associated with both systemic and oral bone loss is significant, future therapeutic studies should target this patient population and also evaluate serum bone biomarkers.
Supplementary Material
Acknowledgments
The project was supported by Grant Numbers R01DE012872 (Dr. Jeffrey Payne, PI and Dr. Lorne Golub, Co-PI) and R03DE019805 (Dr. Julie Stoner, PI) from the National Institute of Dental and Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health. Dr. Golub is listed as an inventor on patents on the test medication in this trial, and those have been fully assigned to his institution, Stony Brook University. He is also a consultant to Galderma Research and Development (Lausanne, Switzerland), which has licensed a series of tetracycline patents from the State University of New York. No other conflicts of interest exist with the other authors.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Albandar JM, Brunelle JA, Kingman A. (1999). Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol 70:13-29; erratum in J Periodontol 70:351, 1999 [DOI] [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. (2010). Teriparatide and osseous regeneration in the oral cavity.N Engl J Med 363:2396-2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumsohn A, Marin F, Nickelsen T, Brixen K, Sigurdsson G, Gonzalez de la Vera J, et al. (2011). Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporos Int [Epub ahead of print, October 12, 2010] (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC. (1995). Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis. A pilot study in beagle dogs. J Clin Periodontol 22:903-910 [DOI] [PubMed] [Google Scholar]
- Golub LM, Ramamurthy NS, Llavaneras A, Ryan ME, Lee HM, Liu Y, et al. (1999). A chemically modified nonantimicrobial tetracycline (CMT-8) inhibits gingival matrix metalloproteinases, periodontal breakdown, and extra-oral bone loss in ovariectomized rats. Ann NY Acad Sci 878:290-310 [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Stoner JA, Reinhardt RA, Sorsa T, Goren AD, et al. (2010). Doxycycline effects on serum bone biomarkers in post-menopausal women. J Dent Res 89:644-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann E, Allen K, Carpio L, Christersson LA, Clerehugh V. (1992). Computerized methodology for detection of alveolar crestal bone loss from serial intraoral radiographs. J Periodontol 63:657-662 [DOI] [PubMed] [Google Scholar]
- Jeffcoat MK, Reddy MS, Webber RL, Williams RC, Ruttimann U. (1987). Extra oral control of geometry for digital subtraction radiography.J Periodontal Res 22:396-402 [DOI] [PubMed] [Google Scholar]
- Kushida K, Takahashi M, Kawana K, Inoue T. (1995). Comparison of markers for bone formation and resorption in premenopausal and postmenopausal subjects, and osteoporosis patients. J Clin Endocrinol Metab 80:2447-2450 [DOI] [PubMed] [Google Scholar]
- Manolagas SC. (2000). Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115-137 [DOI] [PubMed] [Google Scholar]
- Marx RE, Cillo JE, Jr, Ulloa JJ. (2007). Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention and treatment. J Oral Maxillofac Surg 65:2397-2410 [DOI] [PubMed] [Google Scholar]
- Midtby M, Magnus JH, Joakimsen RM. (2001). The Tromsø Study: a population-based study on the variation in bone formation markers with age, gender, anthropometry and season in both men and women. Osteoporos Int 12:835-843 [DOI] [PubMed] [Google Scholar]
- Ortman LF, McHenry K, Hausmann E. (1982). Relationship between alveolar bone measured by 125I absorptiometry with analysis of standardized radiographs: 2. Bjorn technique. J Periodontol 53:311-314 [DOI] [PubMed] [Google Scholar]
- Payne JB, Golub LM. (2011). Using tetracyclines to treat osteoporotic/osteopenic bone loss: from the basic science laboratory to the clinic. Pharmacol Res 63:121-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. (1999). Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 10:34-40 [DOI] [PubMed] [Google Scholar]
- Payne JB, Stoner JA, Nummikoski PV, Reinhardt RA, Goren AD, Wolff MS, et al. (2007). Subantimicrobial dose doxycycline effects on alveolar bone loss in post-menopausal women. J Clin Periodontol 34:776-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Division, US Census Bureau (2008). Table 2. Projections of the Population by Selected Age Groups and Sex for the United States: 2010 to 2050 (NP2008-T2), Release Date: August 14, 2008. URL available at: http://www.census.gov/population/www/projections/files/nation/summary/np2008-t2.xls (accessed January 21, 2011).
- Seibel MJ. (2005). Biochemical markers of bone turnover. Part I: biochemistry and variability. Clin Biochem Rev 26:97-122 [PMC free article] [PubMed] [Google Scholar]
- Seibel MJ. (2006). Biochemical markers of bone turnover. Part II: clinical applications in the management of osteoporosis. Clin Biochem Rev 27:123-138 [PMC free article] [PubMed] [Google Scholar]
- US DHHS (2004). Bone health and osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General. URL available at:http://www.surgeongeneral.gov/library/bonehealth/content.html (accessed 01/21/ 2011). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


