Abstract
Signals generated in distal subcellular compartments of neurons must often travel long distances to the nucleus to trigger changes in gene expression. This retrograde signaling is critical to the development, function and survival of neural circuits, and neurons have evolved multiple mechanisms to transmit signals over long distances. In this review, we briefly summarize the range of mechanisms whereby distally-generated signals are transported to neuronal nuclei. We then focus on the transport of soluble signals from the synapse to the nucleus during neuronal plasticity.
Introduction
Activity-dependent changes in gene expression allow eukaryotic cells to alter their structure and function in response to a range of environmental stimuli. The signal transduction cascades within neurons that couple extracellular stimulation with transcription face a unique set of challenges, since signals received in distal subcellular compartments often travel long distances to reach the nucleus. The transport of distal signals into the nucleus is critical to many processes in neurons. For example, signals generated at distal growth cones trigger transcriptional changes that are essential for neuronal development [1,2]; synaptically generated signals elicit changes in transcription that are required for persistent forms of learning-related synaptic plasticity [3,4]; and signals transported from sites of axonal injury to the nucleus are critical to axonal regeneration [5•,6]. In this review, we briefly summarize the various mechanisms whereby signals are transported to the neuronal cell body and nucleus, and then focus on the active nucleocytoplasmic trafficking of soluble, synaptically generated signals during neuronal plasticity. We conclude by addressing future challenges in the field.
A range of mechanisms mediate retrograde signaling in neurons
Rapid electrochemical signaling
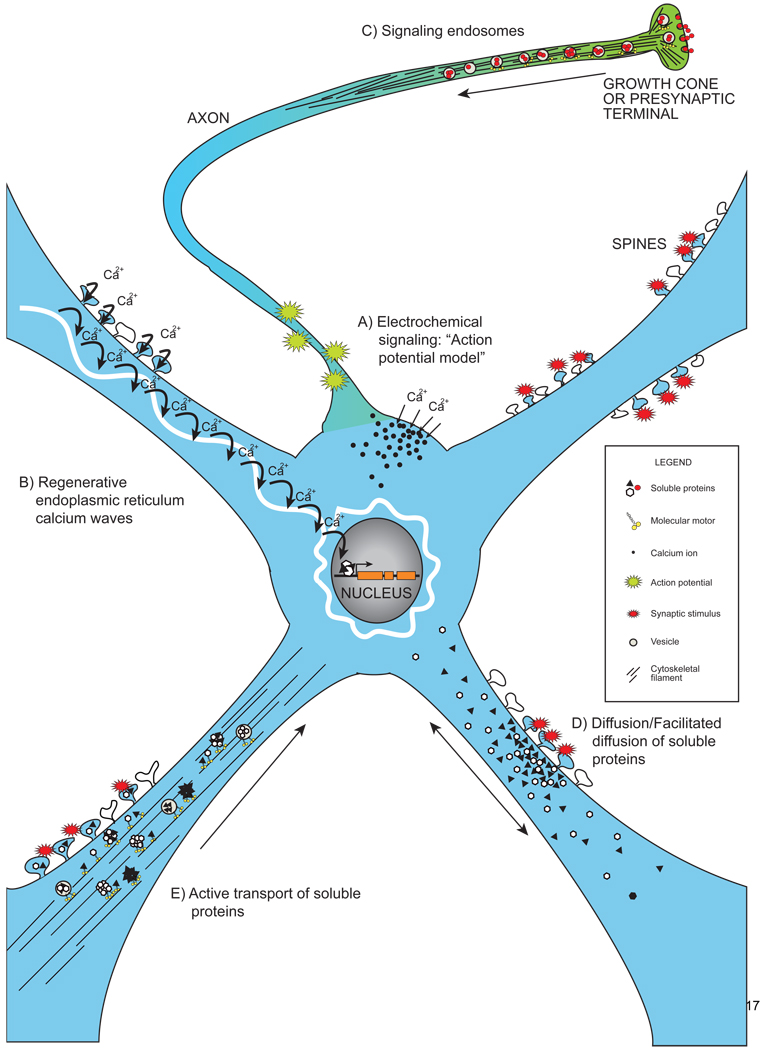
Neurons utilize multiple mechanisms for signaling between distal subcellular compartments and the nucleus. Electrochemical signaling allows for extremely rapid communication to the cell body and nucleus (Fig. 1-A). Local activation of ion channels in distal compartments triggers action potentials that reach the soma within milliseconds, leading to the opening of somatic voltage-gated calcium channels. The influx of calcium at the soma activates calcium-sensitive signaling cascades that in turn activate transcription factors, thereby coupling neuronal activity to changes in gene expression within minutes [7–9].
The different mechanisms of synapse to nucleus signaling.
Signals generated in distal compartments (axons and dendrites) in neurons are transmitted to the nucleus by multiple mechanisms. Electrochemical signaling (A) and regenerative calcium waves in the endoplasmic reticulum (B) allow for extremely rapid signaling. Signals received at growth cones and axon terminals can be internalized into signaling endosomes that are transported back to the nucleus by molecular motors (C). Soluble proteins can be physically transported from distal sites to the nucleus by passive or facilitated diffusion (D) or by active motor-driven transport (E). All black arrows indicate the net movement of proteins, vesicles or ions in neurons following stimulation. All solid black lines indicate cytoskeletal filaments.
Rapid signaling through regenerative calcium waves in the ER
Another method for rapid, calcium-dependent nuclear signaling in neurons involves regenerative calcium waves propagated along the endoplasmic reticulum (ER; Figure 1-B) [10]). In addition to its functions in the secretory pathway, the ER serves as an internal calcium store that is continuous with the nuclear membrane and extends into distal neuronal axons and dendrites [11,12]. The ER contains two calcium channels on its surface: the inositol triphosphate receptor (InsP3R) and the ryanodine receptor (RYR), both of which are calcium-sensitive calcium channels [13]. Hence, calcium influx via cell surface voltage-gated calcium channels also triggers the release of internal calcium stores from the ER, with the local release of calcium activating neighboring InsP3R and RYR, creating a regenerative calcium wave that propagates toward the nucleus.
Physical translocation of signaling molecules from distal compartments to the nucleus
Yet another mechanism for synapse to nucleus signaling involves the physical transport of signaling molecules from their site of initiation to the nucleus. This type of signaling includes the transport of signaling endosomes (Fig. 1-C) and the diffusion (Fig. 1-D) or active transport (Fig. 1-E) of soluble signaling molecules. The physical translocation of signals from distal sites to the nucleus is slower than electrochemical signaling or regenerative calcium waves in the ER and can also persist over greater lengths of time.
Neurotrophins and signaling endosomes
Compelling evidence supports a role for motor-driven retrograde transport of ligand-bound, activated neurotrophin receptors in axons via signaling endosomes (for reviews, see [14]. Many studies have focused on the binding of trophic factors such as nerve growth factor (NGF) to TrkA tyrosine receptors at axon terminals, a process that regulates neuronal survival and axonal outgrowth [15]. The NGF-bound activated TrkA receptor is endocytosed into a signaling endosome and then trafficked to the cell body via microtubules [14,16–19]. Once the endosome arrives in the soma, the active complex interacts with a range of kinases or second messenger molecules including Ras-MAPK, PI3K, PLCγ and ERK5, which in turn activate transcription factors such as CREB to trigger gene expression in the nucleus [18,20,21]. A recent study reported that signaling endosomes travel not only from distal axons to the cell body, but further into dendrites where they regulate development of the postsynaptic compartment [22••]. It is important to note that while there is a wealth of data to support the Signaling Endosome hypothesis in neurotrophic signaling, there are several alternative models such as the “wave” or the “signaling effector” models to describe the transport of signals from axon terminals [14,20,23].
Transport of soluble proteins from synapse to nucleus
One of the biggest challenges for synapse-to-nucleus trafficking is the distance proteins traverse from distal synapses to the nucleus. It is unlikely that diffusion-mediated signaling is efficient enough to allow distally generated signals to reach the nucleus in adequate concentrations to alter transcription (Fig 1-D). Indeed, using large scale Monte Carlo simulations, Howe argues that fast local signaling via diffusion can occur effectively only at distances of less than 200 nm [24]. Hence, long distance movement of signaling proteins likely occurs via “information packets” that are transported by molecular motors [24]. Kholodenko and colleagues have also demonstrated that simple diffusion alone is insufficient for signal transfer in the MAP kinase cascade [25,26]. In contrast, Wiegert and colleagues recently reported that stimulus-induced ERK1/2 nuclear translocation is largely mediated by facilitated diffusion [27].
Analysis of motor-driven nuclear import requires consideration of neuronal polarity. Vertebrate central nervous system neurons are polarized into axons and dendrites, while many invertebrate neurons elaborate unpolarized processes, or neurites, which contain both pre- and post-synaptic elements. Microtubules in axons are uniformly aligned with minus-ends directed toward the cell body, and thus, the majority of signals that originate from the axons, growth cones or axon terminals engage the motor protein dynein for the retrograde movement of the signaling endosomes toward the nucleus [17,28–30]. In contrast, dendritic microtubules are of mixed polarity, although recent studies have suggested that dendritic microtubules may be polarized in an age-dependent and cell type specific manner [31,32]. It is not known whether synaptic cargoes engage only one class of motor proteins for dendritic transport, constantly “hopping” between microtubules of the same polarity, or whether they engage both plus-end (kinesin) and minus-end (dynein) directed motor proteins with regulation of these motor proteins yielding a net movement toward the cell body [33]. There is also evidence of crosstalk between microtubules and actin-driven anterograde transport [34•,35•,36], although this type of molecular motor crosstalk has not been well-examined during retrograde trafficking.
Importin-mediated nuclear transport from synapse to nucleus
In the early 1990s, Ambron and colleagues microinjected rhodamine-labeled human serum albumin coupled to the nuclear localization signal (NLS) of SV40 large T antigen into the distal growth cones of cultured Aplysia californica sensory neurons and found that the NLS was necessary and sufficient for translocation of the microinjected protein into the nucleus [37]. This study implicated a role for the classical nuclear import pathway (via binding of the importin α/β1 heterodimeric complex to NLS-bearing cargo, [38]) in long-distance retrograde transport from distal sites to the soma.
Importin-mediated nuclear transport from sites of axonal injury to the nucleus in mammalian neurons has also been shown to occur during injury-induced regeneration. Hanz et al. [29] localized importin α and β1 to the axoplasm of sciatic nerve and cultured dorsal root ganglion neurons. They found that axonal injury triggered the local translation of importin β1 mRNA in axons, and that the newly synthesized importin β1 formed a transport complex with importin α and NLS-containing cargo proteins in axons to mediate nuclear import of injury-induced signals. Later studies by these authors identified phosphorylated ERK1/2 as an injury-induced, retrogradely transported cargo of importin β1 [39]. The nuclear import of ERK1/2 by importins is thought to initiate a transcriptional program that underlies injury-induced axonal regeneration. More recently, the Fainzilber lab used a combination of phosphoproteomics and microarray analysis to identify the axonal signaling networks and transcriptional factors that are recruited by nerve crush injury [5•].
Our laboratory defined a role for importin-mediated active nuclear import in mediating synapse-to-nucleus trafficking during long-term plasticity. We demonstrated that importins localize to distal synapses and accumulate in the nucleus in response to plasticity-inducing stimuli in both rodent hippocampal neurons and cultured Aplysia sensory-motor neurons [40]. In Aplysia, we further demonstrated that the active nuclear import pathway is required for long-term, but not short-term forms of plasticity. More recently, we proposed a mechanism whereby activity regulates the synaptic localization of importins in hippocampal neurons. We showed that importin α binds to an NLS encoded by exon 21 in the alternatively spliced NMDA receptor subunit NR1-1a. This interaction was regulated by activity, such that stimuli that induced transcription dependent long-term potentiation of hippocampal synapses triggered protein kinase C phosphorylation of serine residues within and flanking the NLS, disrupting the binding of importin α. The released importin α was then free to bind soluble cargoes and transport them to the nucleus [41•].
Studies in Drosophila melanogaster have revealed a role for importin-mediated nuclear import during development of the nervous system. Importin α3 has been shown to be required for transport of dSmad2 from the growth cone to the nucleus, with null mutations in importin α3 showing defects in the axonal tiling of photoreceptors [42]. More recently, Schwarz and colleagues reported that importin α2, together with importin β11, relays Wnt signals from synapse to nucleus [43••]. Specifically, they demonstrated that binding of the ligand wingless to the postsynaptic Drosophila Frizzled-2 (dFz2) receptor leads to proteolytic cleavage of Frz2, with the carboxy-terminal cleavage product binding to importin α2 and importin β11, followed by translocation to the nucleus. This synapse-to-nucleus transport also requires the PDZ protein dGRIP [44]. The subsequent transcriptional activation by Frz2 promotes the elaboration of the postsynaptic plasma membrane [43••]. Additional genetic studies from the Schwarz lab revealed roles for importin α2 in determining active zone density and axonal projections [43••,45].
Together, these studies describe roles for importin-mediated retrograde nuclear transport in axonal injury and regeneration, synaptic plasticity, axon guidance and synapse formation.
Synaptically localized signaling proteins that undergo activity-dependent nuclear import
In this section, we review specific synaptically localized proteins that undergo retrograde transport into the nucleus following distinct types of stimuli. Many of these proteins are transcription factors. As such, their nuclear import directly couples synaptic activity with changes in transcription.
cAMP Responsive Element Binding protein -2 (CREB2/ATF4)
We recently showed that the transcriptional repressor CREB2 (also known as ATF4) translocates from distal neuronal processes to the nucleus during long-term depression (LTD) of cultured rodent hippocampal neurons and FMRF-amide induced LTD of Aplysia californica sensory motor synapses [46]. Biochemical studies revealed that ATF4/CREB2 binds specifically to importins α1 and α6. Consistent with importin-mediated transport, retrograde trafficking was blocked by saturating concentrations of NLS peptides.
Nuclear factor kappa of light chain enhancer of activated B-cells (NF–κB)
The NF–κB complex consists of a transcriptionally active dimer that is anchored in the cytoplasm through its interaction with an inhibitory subunit [47]. In neurons, the complex most commonly contains a p65 and p50 transcriptionally active dimer and the IκBα inhibitory subunit [48], and is found at synapses [49–51]. Several labs have demonstrated that transcriptionally active p65 subunits fused to eGFP translocate into the nucleus in an NLS-dependent manner upon glutamatergic stimulation [50,52]. This nuclear translocation of active p65 requires association with microtubules via the dynein/dynactin molecular motor protein complex [53,54]. An intriguing report by Marcora et al. suggested that NF–κB translocate from activated synapse to the nucleus by associating with wildtype (but not mutant) huntington protein via association with importin α2 [51].
ApCAM Associated Protein (CAMAP)
Long-term synaptic strengthening of Aplysia sensory-motor synapses is accompanied by new synaptic growth, which has been shown to involve the internalization and degradation of the Aplysia cell adhesion molecule ApCAM [55]. A yeast two-hybrid screen for ApCAM-interacting proteins identified a novel transcriptional coactivator of CRE-driven gene expression, CAMAP [56]. Local synaptic stimulation triggered CAMAP phosphorylation by PKA, dissociation from the cytoplasmic tail of ApCAM and translocation of CAMAP into the sensory neuron nucleus. In the nucleus, CAMAP bound CREB1a and promoted transcription of specific CRE-driven genes [56].
Amyloid Precursor Protein Intracellular Domain Associated Protein-1 (AIDA-1)
Mass spectrometric analysis of PSD fractions from rat brain led identified AIDA-1 as a synaptically localized protein [57]. Subsequent analysis of AIDA-1 revealed that it binds PSD95 and that NMDA receptor activation drives proteolysis and subsequent nuclear translocation of AIDA-1 into the nucleus [58]. The proteolytically cleaved N-terminus of AIDA-1 contains a functional NLS that mediates its transport into the nucleus [58,59]. In the nucleus, AIDA1 accumulates in nucleoli, sites of ribosomal RNA transcription and assembly [58,60]. Functional studies suggest that the synapse-to-nucleus transport of AIDA-1 regulates nucleolar number and global protein synthesis during persistent synaptic stimulation [58].
Jacob
Jacob was identified as a binding partner of the neuronal calcium sensor caldendrin that undergoes regulated synapse-to-nucleus trafficking. NMDA receptor activation and calcium influx was found to trigger dissociation of Jacob from caldendrin, unmasking of an NLS, subsequent binding to importin α1, and nuclear import [61•]. Unlike AIDA-1, the nuclear translocation of Jacob requires activation of extrasynaptic NR2B-containing NMDA receptors, activation of which are thought to trigger the cell death pathway by activating a CREB “shutoff” mechanism [9]. Subsequent studies by Kindler et al. revealed that NMDA receptor-induced and calpain-mediated proteolysis of the myristoylated N-terminal fragment of Jacob is required for nuclear translocation [62].
Abelson Interacting Protein-1 (Abi-1)
Abi-1 is a substrate for the non-receptor tyrosine kinase c-Abl and is a binding partner for the postsynaptic density protein ProSAP2/Shank3. In developing neurons, Abi-1 is responsible for regulating dendritic outgrowth and determining the morphology and number of synaptic contacts [63,64]. In mature neurons, Abi-1 is found in dendrites, and a brief application of NMDA in both cultured neurons and acute rodent brain slices resulted in the reversible, stimulus-dependent, translocation of Abi-1 from dendrites into the nucleus [63,64]. Importantly, this nuclear translocation is dependent on an active microtubule network and does not require new protein synthesis. Abi-1 was also shown to associate with the Myc/Max complex of transcription factors that enhance E-box regulated gene transcription [64].
The above examples represent a subset of the synaptically localized proteins that are known to translocate into the nucleus following specific stimuli. Additional examples include transmembrane proteins that undergo Regulated Intramembrane Proteolysis (RIP) followed by nuclear import, such as Amyloid Precursor Protein (APP) and erbB4. APP is found in both axons and dendrites and undergoes γ-secretase cleavage to generate a cytoplasmic fragment (AICD) that forms a potent transcription complex with Fe65 and Tip60 [65–67]. ErbB4 is a receptor tyrosine kinase that is cleaved upon neuregulin-induced stimulation to produce the NLS-bearing intracellular fragment E4ICD, which subsequently binds to the signaling protein TAB2 and corepressor N-CoR for nuclear translocation [68,69]. Another transmembrane protein that is proteolytically cleaved in the C-terminal is the L-type voltage-gated calcium channel Cav1.2. The cleaved fragment, CCAT (calcium channel associated transcription regulator) is regulated by intracellular calcium and translocates into the nucleus where it binds the nuclear protein p54(nrb)/NonO to activate transcription [70].
Future challenges
At present, the kinetics of soluble protein transport is not well-characterized. It is not known if soluble proteins are transported individually, in a signaling complex, or how they engage the molecular motor proteins for active transport. Some technical challenges, such as visualizing the long distance transport of cargo proteins from distal synapses to the nucleus, can be surmounted through the use of photoconvertible fluorescent fusion proteins in conjunction with time-lapse imaging [71]. Choosing a microscope and an image capture system with high spatial and temporal resolution is critical since nucleocytoplasmic shuttling is rapid. Newly developed superresolution microscopy techniques provide unprecedented resolution, and advances in instrumentation promise to decrease acquisition time [72]. Equally important is the design of software capable of identifying, tracking and quantifying the movement of soluble proteins, which often exists as a rapidly moving “wave.”
Synaptic inputs received by neurons are discrete and localized, and thus it is important that stimuli be delivered to small regions such as dendritic branches, groups of, or even individual synapses rather than to the entire cell. Many parameters of long-range protein transport in dendrites, such as the kinetics and directionality of movement, may be altered if stimuli are not compartmentalized. Methods for local, synaptic stimulation include photolysis of caged compounds and local perfusion of stimuli. Recent developments in microfluidic technology provide a means of culturing neurons in compartmentalized chambers in which soma and distal processes can be stimulated with great spatial resolution [73•].
Fundamental questions about the function of synapse-to-nucleus signals remain unanswered: What types of stimuli trigger the translocation of synaptically localized signals to the nucleus, and what changes in gene expression and neuronal function do such signals induce? Is there a threshold number of stimulated synapses that is required for effective synapse-to-nucleus signaling? What types of plasticity (e.g. Hebbian versus homeostatic) recruit synapse to nucleus signaling? And finally, can the cell keep track of individual synapses that have been stimulated to send a signal to the nucleus, such that any downstream transcriptional changes function to specifically alter the efficacy of those synapses? Future experiments taking advantage of technical advances in local stimulation and live-cell microscopy hold the promise of providing answers to these questions.
Acknowledgements
The authors would like to thank Carrie Heusner for inspiring Figure 1, and Victoria Ho, Peter Lin, Klara Olofsdotter Otis and Besim Uzgil for comments on the manuscript. Funding is provided by 2010 NARSAD Young Investigator Award (THC) and NIH grant R01MH077022 (KCM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sanchez-Camacho C, Bovolenta P. Emerging mechanisms in morphogen-mediated axon guidance. Bioessays. 2009;31:1013–1025. doi: 10.1002/bies.200900063. [DOI] [PubMed] [Google Scholar]
- 2.Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5. Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY, Rishal I, et al. Signaling to transcription networks in the neuronal retrograde injury response. Sci Signal. 2010;3:ra53. doi: 10.1126/scisignal.2000952. The authors perform phosphoproteomic analysis of sciatic nerve following crush injury and microarray analysis of transcripts in the cell body of sensory neurons following nerve lesion, and cross-correlate the two data sets to uncover signaling pathways that are triggered by injury in neurons.
- 6.Rishal I, Fainzilber M. Retrograde signaling in axonal regeneration. Exp Neurol. 2010;223:5–10. doi: 10.1016/j.expneurol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Adams JP, Dudek SM. Late-phase long-term potentiation: getting to the nucleus. Nat Rev Neurosci. 2005;6:737–743. doi: 10.1038/nrn1749. [DOI] [PubMed] [Google Scholar]
- 8.Bengtson CP, Freitag HE, Weislogel JM, Bading H. Nuclear Calcium Sensors Reveal that Repetition of Trains of Synaptic Stimuli Boosts Nuclear Calcium Signaling in CA1 Pyramidal Neurons. Biophys J. 2010;99:4066–4077. doi: 10.1016/j.bpj.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 11.Hanus C, Ehlers MD. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic. 2008;9:1437–1445. doi: 10.1111/j.1600-0854.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renvoise B, Blackstone C. Emerging themes of ER organization in the development and maintenance of axons. Curr Opin Neurobiol. 2010;20:531–537. doi: 10.1016/j.conb.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 14.Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics. 2009;72:46–55. doi: 10.1016/j.jprot.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe CL, Mobley WC. Signaling endosome hypothesis: A cellular mechanism for long distance communication. J Neurobiol. 2004;58:207–216. doi: 10.1002/neu.10323. [DOI] [PubMed] [Google Scholar]
- 16.Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Ramirez A, Cui B, Ding J, Delcroix JD, Valletta JS, Liu JJ, Yang Y, Chu S, Mobley WC. A functional dynein-microtubule network is required for NGF signaling through the Rap1/MAPK pathway. Traffic. 2007;8:1503–1520. doi: 10.1111/j.1600-0854.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- 18.Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, Osakada Y, Vrljic M, Chen L, Mudrakola HV, Cui B. Single-molecule imaging of NGF axonal transport in microfluidic devices. Lab Chip. 2010;10:2566–2573. doi: 10.1039/c003385e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008;18:270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 22. Sharma N, Deppmann CD, Harrington AW, St Hillaire C, Chen ZY, Lee FS, Ginty DD. Long-distance control of synapse assembly by target-derived NGF. Neuron. 2010;67:422–434. doi: 10.1016/j.neuron.2010.07.018. Assembly of pre and post-synaptic compartments requires target-derived NGF in sympathetic neurons. NGF-TrkA signaling endosomes are retrogradely transported from distal axons to cell bodies and dendrites, where they induce formation of post-synaptic densities in a MEK/MAPK and PI3K-dependent manner.
- 23.Campenot RB. NGF uptake and retrograde signaling mechanisms in sympathetic neurons in compartmented cultures. Results Probl Cell Differ. 2009;48:141–158. doi: 10.1007/400_2009_7. [DOI] [PubMed] [Google Scholar]
- 24.Howe CL. Modeling the signaling endosome hypothesis: why a drive to the nucleus is better than a (random) walk. Theor Biol Med Model. 2005;2:43. doi: 10.1186/1742-4682-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kholodenko BN. MAP kinase cascade signaling and endocytic trafficking: a marriage of convenience? Trends Cell Biol. 2002;12:173–177. doi: 10.1016/s0962-8924(02)02251-1. [DOI] [PubMed] [Google Scholar]
- 26.Kholodenko BN. Four-dimensional organization of protein kinase signaling cascades: the roles of diffusion, endocytosis and molecular motors. J Exp Biol. 2003;206:2073–2082. doi: 10.1242/jeb.00298. [DOI] [PubMed] [Google Scholar]
- 27.Wiegert JS, Bengtson CP, Bading H. Diffusion and not active transport underlies and limits ERK1/2 synapse-to-nucleus signaling in hippocampal neurons. J Biol Chem. 2007;282:29621–29633. doi: 10.1074/jbc.M701448200. [DOI] [PubMed] [Google Scholar]
- 28.Ha J, Lo KW, Myers KR, Carr TM, Humsi MK, Rasoul BA, Segal RA, Pfister KK. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J Cell Biol. 2008;181:1027–1039. doi: 10.1083/jcb.200803150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 30.Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- 31.Kwan AC, Dombeck DA, Webb WW. Polarized microtubule arrays in apical dendrites and axons. Proc Natl Acad Sci U S A. 2008;105:11370–11375. doi: 10.1073/pnas.0805199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone MC, Roegiers F, Rolls MM. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol Biol Cell. 2008;19:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welte MA. Bidirectional transport: matchmaking for motors. Curr Biol. 2010;20:R410–R413. doi: 10.1016/j.cub.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 34. Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. The authors employ an inducible trafficking assay in hippocampal neurons to show that dynein drives the bidirectional transport of cargo vesicles into dendrites in a microtubule-dependent manner.
- 35. Lewis TL, Jr, Mao T, Svoboda K, Arnold DB. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat Neurosci. 2009;12:568–576. doi: 10.1038/nn.2318. The authors show that actin filaments are important in localizing proteins to dendrites and that myosin Va is necessary and sufficient for dendritic targeting of cargo proteins in cultured cortical neurons.
- 36.Schlager MA, Hoogenraad CC. Basic mechanisms for recognition and transport of synaptic cargos. Mol Brain. 2009;2:25. doi: 10.1186/1756-6606-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambron RT, Schmied R, Huang CC, Smedman M. A signal sequence mediates the retrograde transport of proteins from the axon periphery to the cell body and then into the nucleus. J Neurosci. 1992;12:2813–2818. doi: 10.1523/JNEUROSCI.12-07-02813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- 39.Perlson E, Michaelevski I, Kowalsman N, Ben-Yaakov K, Shaked M, Seger R, Eisenstein M, Fainzilber M. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J Mol Biol. 2006;364:938–944. doi: 10.1016/j.jmb.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 40.Thompson KR, Otis KO, Chen DY, Zhao Y, O'Dell TJ, Martin KC. Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway. Neuron. 2004;44:997–1009. doi: 10.1016/j.neuron.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 41. Jeffrey RA, Ch'ng TH, O'Dell TJ, Martin KC. Activity-dependent anchoring of importin alpha at the synapse involves regulated binding to the cytoplasmic tail of the NR1-1a subunit of the NMDA receptor. J Neurosci. 2009;29:15613–15620. doi: 10.1523/JNEUROSCI.3314-09.2009. This study shows that importin α interacts with the NLS encoded in exon 21 in the alternatively spliced NMDA receptor subunit NR1-1a. Synaptic activity associated with long-term plasticity results in disruption and subsequent release of the importin α interaction with NMDAR to bind cargo.
- 42.Ting CY, Herman T, Yonekura S, Gao S, Wang J, Serpe M, O'Connor MB, Zipursky SL, Lee CH. Tiling of r7 axons in the Drosophila visual system is mediated both by transduction of an activin signal to the nucleus and by mutual repulsion. Neuron. 2007;56:793–806. doi: 10.1016/j.neuron.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mosca TJ, Schwarz TL. The nuclear import of Frizzled2-C by Importins-beta11 and alpha2 promotes postsynaptic development. Nat Neurosci. 2010;13:935–943. doi: 10.1038/nn.2593. The authors demonstrate that upon cleavage of dFrz2, the C-terminal fragment binds to importin α2 and β11 for nuclear translocation and subsequent activation of transcription that promotes elaboration of the post-synaptic plasma membrane.
- 44.Ataman B, Ashley J, Gorczyca D, Gorczyca M, Mathew D, Wichmann C, Sigrist SJ, Budnik V. Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc Natl Acad Sci U S A. 2006;103:7841–7846. doi: 10.1073/pnas.0600387103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosca TJ, Schwarz TL. Drosophila Importin-alpha2 Is Involved in Synapse, Axon and Muscle Development. PLoS ONE. 2010;5:e15223. doi: 10.1371/journal.pone.0015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai KO, Zhao Y, Ch'ng TH, Martin KC. Importin-mediated retrograde transport of CREB2 from distal processes to the nucleus in neurons. Proc Natl Acad Sci U S A. 2008;105:17175–17180. doi: 10.1073/pnas.0803906105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1:a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 49.Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech Dev. 1993;43:135–147. doi: 10.1016/0925-4773(93)90031-r. [DOI] [PubMed] [Google Scholar]
- 50.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 51.Marcora E, Kennedy MB. The Huntington's disease mutation impairs Huntingtin's role in the transport of NF-kappaB from the synapse to the nucleus. Hum Mol Genet. 2010;19:4373–4384. doi: 10.1093/hmg/ddq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wellmann H, Kaltschmidt B, Kaltschmidt C. Retrograde transport of transcription factor NF-kappa B in living neurons. J Biol Chem. 2001;276:11821–11829. doi: 10.1074/jbc.M009253200. [DOI] [PubMed] [Google Scholar]
- 53.Mikenberg I, Widera D, Kaus A, Kaltschmidt B, Kaltschmidt C. Transcription factor NF-kappaB is transported to the nucleus via cytoplasmic dynein/dynactin motor complex in hippocampal neurons. PLoS ONE. 2007;2:e589. doi: 10.1371/journal.pone.0000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shrum CK, Defrancisco D, Meffert MK. Stimulated nuclear translocation of NF-kappaB and shuttling differentially depend on dynein and the dynactin complex. Proc Natl Acad Sci U S A. 2009;106:2647–2652. doi: 10.1073/pnas.0806677106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayford M, Barzilai A, Keller F, Schacher S, Kandel ER. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992;256:638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- 56.Lee SH, Lim CS, Park H, Lee JA, Han JH, Kim H, Cheang YH, Lee YS, Ko HG, Jang DH, et al. Nuclear translocation of CAM-associated protein activates transcription for long-term facilitation in Aplysia. Cell. 2007;129:801–812. doi: 10.1016/j.cell.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 57.Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3:857–871. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- 58.Jordan BA, Fernholz BD, Khatri L, Ziff EB. Activity-dependent AIDA-1 nuclear signaling regulates nucleolar numbers and protein synthesis in neurons. Nat Neurosci. 2007;10:427–435. doi: 10.1038/nn1867. [DOI] [PubMed] [Google Scholar]
- 59.Kurabi A, Brener S, Mobli M, Kwan JJ, Donaldson LW. A nuclear localization signal at the SAM-SAM domain interface of AIDA-1 suggests a requirement for domain uncoupling prior to nuclear import. J Mol Biol. 2009;392:1168–1177. doi: 10.1016/j.jmb.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Jacob AL, Jordan BA, Weinberg RJ. Organization of amyloid-beta protein precursor intracellular domain-associated protein-1 in the rat brain. J Comp Neurol. 2010;518:3221–3236. doi: 10.1002/cne.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dieterich DC, Karpova A, Mikhaylova M, Zdobnova I, Konig I, Landwehr M, Kreutz M, Smalla KH, Richter K, Landgraf P, et al. Caldendrin-Jacob: a protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol. 2008;6:e34. doi: 10.1371/journal.pbio.0060034. The authors report that Jacob translocates from synapse to nucleus following stimulation of extrasynaptic NR2B-containing NMDA receptors (which triggers the cell death pathway). Jacob engages Importin α1 as an adaptor for import into the nucleus.
- 62.Kindler S, Dieterich DC, Schutt J, Sahin J, Karpova A, Mikhaylova M, Schob C, Gundelfinger ED, Kreienkamp HJ, Kreutz MR. Dendritic mRNA targeting of Jacob and N-methyl-d-aspartate-induced nuclear translocation after calpain-mediated proteolysis. J Biol Chem. 2009;284:25431–25440. doi: 10.1074/jbc.M109.022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito H, Morishita R, Shinoda T, Iwamoto I, Sudo K, Okamoto K, Nagata K. Dysbindin-1, WAVE2 and Abi-1 form a complex that regulates dendritic spine formation. Mol Psychiatry. 2010;15:976–986. doi: 10.1038/mp.2010.69. [DOI] [PubMed] [Google Scholar]
- 64.Proepper C, Johannsen S, Liebau S, Dahl J, Vaida B, Bockmann J, Kreutz MR, Gundelfinger ED, Boeckers TM. Abelson interacting protein 1 (Abi-1) is essential for dendrite morphogenesis and synapse formation. Embo J. 2007;26:1397–1409. doi: 10.1038/sj.emboj.7601569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 67.Cao X, Sudhof TC. Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation. J Biol Chem. 2004;279:24601–24611. doi: 10.1074/jbc.M402248200. [DOI] [PubMed] [Google Scholar]
- 68.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 70.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 2010;90:1103–1163. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 72.Patterson G, Davidson M, Manley S, Lippincott-Schwartz J. Superresolution imaging using single-molecule localization. Annu Rev Phys Chem. 2010;61:345–367. doi: 10.1146/annurev.physchem.012809.103444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron. 2010;66:57–68. doi: 10.1016/j.neuron.2010.03.022. The authors designed a novel microfluidic device with a local perfusion channel that transects neuronal processes. This allows users to manipulate specific dendritic and axonal segments separately from cell bodies.