Abstract
Background Context
Spinal manipulative therapy (SMT) has shown clinical effectiveness in some patients with musculoskeletal pain.
Purpose
We performed the current experiment to test whether regional pain modulation is to be expected from thoracic SMT.
Study Design/Setting
Randomized experimental design performed in a university pain laboratory.
Outcome Measures
The primary outcome was experimental pain sensitivity in cervical and lumbar innervated area.
Methods
Ninety healthy volunteers were randomly assigned to receive one of three interventions (SMT, exercise or rest) to the upper thoracic spine. Participants completed questionnaires about pain-related affect and expectations regarding each of the interventions. We collected experimental pain sensitivity measures of cervical and lumbar innervated areas before and immediately after randomly assigned intervention. Mixed-model analysis of co-variance was used to test changes in measures of experimental pain sensitivity.
Results
No interactions or intervention (group) effects were noted for pressure or A-delta mediated thermal pain responses. Participants receiving SMT had greater reductions in temporal sensory summation (TSS).
Conclusions
This current study indicates thoracic SMT reduces TSS in healthy subjects. These findings extend our previous work in healthy and clinical subjects by indicating change in the nocioceptive afferent system occurred caudal to the region of SMT application. However, the duration of reduction in TSS is an unknown, and more work needs to be completed in clinical populations for confirm the relevance of these findings.
Spinal manipulation techniques (SMT) are interventions distinguishable from other manual therapies used to manage spinal pain by their characteristic high velocity and low amplitude. SMT are commonly used during the management of disorders in the cervical [1, 2], thoracic [3] and lumbar spines [4]. Proposed models of the mechanisms of SMT [5, 6, 7, 8] suggest that the mechanical stimulus of a manual therapy technique triggers a cascade of neurophysiological effects. Our recent work [9, 10, 11] and that of others [1, 12] suggests these neurophysiological mechanisms result in hypoalgesic responses in patients with musculoskeletal pain and participants in experimental studies of SMT. For example, diminished ratings of pain intensity associated with standardized thermal [9, 10, 11], mechanical [1, 2] and electrical [13] stimuli are observed after SMT.
Our data suggests that the effect of SMT applied to the lumbar spine is strongly associated with a particular aspect of the nociceptive afferent system, temporal sensory summation (TSS) [9, 11]. This finding is of potential importance as TSS is a specific, short lasting aspect of central sensitization of the nervous system. TSS is determined by the administration of consecutive, rapid pulses of standard nociceptive input, all at the same amplitude and frequency. TSS in humans is a reversible phenomenon that occurs rapidly at stimulus rates faster than 0.3–0.5 Hz [14]. TSS is thought to occur predominantly in the dorsal horn of the spinal cord but it also involves areas of the cerebral cortex involved in pain processing and pain behavior.[15] In our prior experimental studies of lumbar SMT involving healthy subjects and subjects with low back pain, immediate reduction of TSS occurred in lumbar innervated regions (lower-extremities) but not in cervical innervated regions (upper-extremities) after SMT [9, 11]. We hypothesized, based on these findings, that SMT directed at the lumbar spine stimulates a neurophysiological response that affects pain modulation in the lumbar, but not the cervical region.
To further test if local pain modulation was to be expected from spinal manipulation we performed the current experiment using a upper thoracic SMT shown to result in short-term reductions in pain and disability for people with neck pain [16]. The objective of the current study was to identify if the immediate pain modulatory effects of upper thoracic SMT were local to areas innervated by the cervical region. We hypothesized that pain modulation would occur for TSS but not other aspects of afferent nociception consistent with our prior studies [9, 17, 18]. We also hypothesized that if the effect of SMT was regionally specific, there would be immediate modulation of pain sensitivity in the upper extremity but not the lower extremity.
METHODS
We recruited 90 volunteer healthy subjects (mean age 22.9 ± 2.7 years, 66 women) using advertisements posted in XXXXX. All participants read and signed a consent form. The consent from and protocol for this study were approved by the Institutional Review Board. Inclusion criteria for this study were: 1) between 18-35 years of age and 2) no neck or upper-extremity complaints within the past sixty days. Exclusion criteria for this study were: 1) identification of any medical signs and/or symptoms suggesting systemic dysfunction, 2) history of whiplash-type injury within 60 days of initial examination, 3) previous spinal or upper-extremity surgical interventions, 4) regular use of analgesic or anti-inflammatory drugs (within past 48 hours).
Measures
Demographics
Subjects completed a demographic survey that included information about their education, prior pain experiences and prior experience with the interventions used in this study.
Psychological Questionnaires
We chose to measure these psychological factors given the reported influences of these constructs on pain intensity in experimental models of thermally induced pain [19]. Any measure different between groups post-randomization was to be included in subsequent analysis as a covariate.
The Fear of Pain Questionnaire (FPQ) is a 30-item, 5-point rating scale developed to measure fear about specific situations that may cause pain. We use the total score for the FPQ-III, as we were most interested in measuring subjects’ general fear of pain [20].
Tampa Scale of Kinesiophobia – General Population (TSK-G) – The TSK-G utilizes a 12-item, 4-point Likert scale with higher scores indicating elevated levels of fear of movement/(re)injury [21].
The Anxiety Sensitivity Index (ASI) uses a 16-item, 4-point rating scale to assess anxiety sensitivity, which is the perception of whether experiencing symptoms of anxiety causes harm. The ASI has been validated in community samples [22] and demonstrated factor invariance across different sex and age groups [23].
Pain Catastrophizing Scale (PCS) - The PCS utilizes a 13-item, 5-point Likert scale with higher scores indicating elevated levels of Catastrophizing [24].
Expectations for reductions in induced pain
Subjects completed a 1-item, 3-point Likert scale survey about their expectation for reductions in pain associated with the thermal stimuli. Subjects rated if they would experience more, the same, or less pain during testing of thermal pain sensitivity.
Outcome measures
Experimental Pain Sensitivity
We used mechanical and thermal stimuli to determine experimental pain sensitivity in cervical and lumbar innervated areas. Pain sensitivity was measured before, and immediately after, the application of any intervention.
Mechanical Stimuli
Pressure pain threshold (PPT) was assessed using a pressure algometer (Pain Diagnostics & Treatment, Great Neck, NY). The tip of the algometer is equipped with a rubber foot-plate of 1-cm diameter. During testing, pressure was gradually applied until the subject reported that the sensation changed from pressure to pain. At that point the force application was stopped and the applied force was recorded in kg-force. This test was performed at the dorsal web-space between: 1) the first and second fingers, and 2) the first and second toes.
Thermal Stimuli
All thermal stimuli were delivered to the skin of subjects using a computer-controlled Medoc Neurosensory Analyzer (TSA-2001, Ramat Yishai, Israel). Stimulation sites were varied to prevent carryover effects due to local sensitization. We had both a male and female examiner present during testing to account for sex and/or gender influence on pain reporting [25].
Before each testing session, subjects underwent a practice session. During this session subjects experienced the entire range of temperatures to which they were to be exposed. Subjects practiced using the rating scale to rate the intensity of pain experienced in response to each stimulus. In order to standardize the scaling instructions, a standardized instructional set was used for all subjects during every exposure to the thermal stimuli. The scaling instructions were repeated for every set of ratings within each session [26].
First pain
Thermal stimuli of 5 seconds duration were applied to the volar forearm and to the posterior surface of the upper calf below the popliteal fossa, with the subject in a sitting position. The subjects experienced a sequence of four thermal pulses that included 45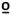 C, 47
C, 47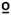 C, 49
C, 49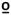 C, or 51°C presented randomly.
C, or 51°C presented randomly.
Subjects were cued to provide a rating of any pain experienced immediately after the peak of each thermal pulse using a numeric rating scale (NRS) anchored at 0 (no pain sensation at all) and 100 (worst pain imaginable). These response ratings were categorized as first pain responses [12].
This procedure was performed twice and the average of both trials was used in subsequent analyses. The interstimulus interval was at least 60 seconds to avoid carryover effects from one stimulus to another, to prevent changes in receptor responses and to prevent tissue changes. Temperature levels were monitored by a contactor-contained thermistor, and returned to a preset baseline of 35°C by active cooling at a rate of 10°C.sec−1 [27, 28].
Temporal sensory summation
A train of 10 heat pulses peaking at 50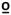 C was applied to the glabrous skin of the hand (thenar eminence) and foot (medial longitudinal arch). Temperature levels were monitored by a contactor-contained thermistor, and returned to a preset baseline by active cooling at a rate of 10
C was applied to the glabrous skin of the hand (thenar eminence) and foot (medial longitudinal arch). Temperature levels were monitored by a contactor-contained thermistor, and returned to a preset baseline by active cooling at a rate of 10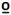 C.sec−1. The participants were asked to rate the magnitude of their second pain sensation following each heat pulse using the same 101 point NRS. This increase in the delayed or second pain intensity rating that occurs from early to later inputs is referred to as TSS. We used a simple slope measure (the rating of pulse 1 subtracted from the rating of pulse 5) in our analyses.
C.sec−1. The participants were asked to rate the magnitude of their second pain sensation following each heat pulse using the same 101 point NRS. This increase in the delayed or second pain intensity rating that occurs from early to later inputs is referred to as TSS. We used a simple slope measure (the rating of pulse 1 subtracted from the rating of pulse 5) in our analyses.
Interventions
Participants were randomly assigned to one of three intervention groups: 1) High velocity, low amplitude intervention (SMT), 2) specific cervical exercise (CE) or, 3) control group.
The SMT group received an intervention used for patients with cervical disorders and has been shown to be effective in reducing pain and self-report of disability [16]. This technique is hypothesized to affect the lower cervical and upper thoracic region [29]. The technique was performed with the subject in the supine position. The subject clasped their hands behind their neck and brought their elbows together in front of their chest. One hand of the physical therapist was placed on the upper thoracic region and the other on the subjects’ elbows. The force application was directed through the subjects’ elbows. The technique was applied two times in concordance with clinical literature related to the technique. In the event that a cavitation (audible-pop) occurred during the intervention, it was documented. This particular procedure was chosen to replicate the procedure used in previous studies of clinical efficacy and effectiveness.
The CE group performed a common exercise used for patients with cervical disorders [30] that has been reported to be associated with immediate effects on complaints of cervical pain with movement. This exercise has also been associated with immediate hypoalgesic treatment effects in patients experiencing cervical pain [31]. Subjects were instructed on the performance of an active exercise to be performed in the supine position. “The movement involves flexion of the cranium on the cervical spine while ensuring the back of the head remains in contact with the supporting surface” [31]. The subject was asked to perform three sets of ten repetitions with a five second hold at a sub-maximal (<25%) effort.
The control group rested quietly in supine for five minutes.
Data Analysis
Demographic variables were compared among groups using one way analyses of variance (ANOVA) with t-tests for post-hoc comparisons of group means from continuous parametric and Mann-Whitney U and Chi-squared for non-parametric data. Additionally, the frequencies of cavitation noted by the patient or researcher were calculated.
We used three-way mixed model ANOVAs to examine changes in each of the primary outcome variables for this study. Those outcomes were the ratings of pain at 47°C and 49°C, TSS, and the force measured at the pressure pain threshold. For first pain responses, data for 45°C and 51°C were not analyzed because these represented sub-threshold and tolerance values respectively, for a majority of patients in our previous studies [9, 10, 11].
Each model contained the between subjects factor group (SMT, CE, control) and there were two within subject factors: time (pre, post) and body region (upper extremity, lower extremity). Confounding variables were defined as those variables different among groups post-randomization and associated with the outcomes. Any confounding variables identified were to be included as covariates.
Significant interactions were followed using a two-way or one way ANOVA as indicated. Pair-wise comparisons were made using t-tests, and Type 1 error was maintained at 5% using Bonferroni corrections. All analysis was performed using SPSS 17 (SPSS Inc, Chicago, IL).
RESULTS
Group differences
Demographic variables are summarized in Table 1. The groups were not different in key demographic (sex, age, race, and education) and most psychological (anxiety, fear, kinesiophobia) variables. There were post-randomization group differences in pain catastrophizing with the control group reporting elevated levels of catastrophizing in comparison to the group receiving exercise (p=0.029). However, catastrophizing was not associated with any of the outcome measures used in this study. See Table 2. Likewise, while post-randomization differences were noted in the expectation for hypoalgesia (chi-square=10.8, p=0.028). Residual analysis indicated that this effect was driven by more participants in the control group expecting to worsen after intervention (residual=2.09). There were no differences in any of the outcomes among the categories of expectation. Within the group receiving SMT, nine participants experienced cavitation with each application of SMT (four total), and three experienced no cavitation at all. The median number of cavitations per participant was two.
Table 1.
Demographic Variables, and Baseline Measures of Pain Sensitivity, and Psychological Variables
| SMT | Exercise | Control | p-value | |
|---|---|---|---|---|
| Demographic factors | ||||
| Age (years) | 23.03 ± 2.44 | 23.03 ± 2.95 | 22.47 ± 2.21 | 0.61 |
| Sex (number female) | 19 | 22 | 25 | 0.22 |
| Race | 0.53 | |||
| Caucasian | 23 | 23 | 24 | |
| African American | 2 | 0 | 2 | |
| Asian | 4 | 3 | 2 | |
| Native Hawaiian or Pacific Islander | 0 | 2 | 0 | |
| Other | 1 | 2 | 2 | |
| Education (years) | 16.5 ± 2.06 | 16.05 ± 1.5 | 15.95 ± 1.17 | 0.38 |
| Episodes of neck pain in life? | 1.33 ± 1.63 | 1.87 ± 5.03 | 2.38 ± 4.35 | 0.60 |
| Prior treatment with manipulation | 10 | 11 | 8 | 0.70 |
| Psychological factors | ||||
| Anxiety (ASI) | 15.37 ± 6.42 | 15.03 ± 6.38 | 18.47 ± 8.7 | 0.14 |
| Fear of pain (FPQ-9) | 12.93 ± 4.39 | 14.2 ± 3.89 | 14.03 ± 3.59 | 0.14 |
| Catastrophizing (PCS) | 15.83 ± 9.77 | 13.63 ± 8.87 | 19.6 ± 7.38 | 0.03 |
| Kinesiophobia (TSK-G) | 19.7 ± 4 | 19.63 ± 4.93 | 20.9 ± 4.34 | 0.46 |
| Pre-intervention expectation | 0.03 | |||
| Less pain | 8 | 3 | 5 | |
| Same pain | 19 | 23 | 14 | |
| More pain | 3 | 4 | 11 |
SMT – high velocity low amplitude technique
ASI – Anxiety State Index (0-64)
FPQ-9 – Fear of pain questionnaire (0-36)
PCS – Pain Catastrophizing Scale (0-52)
TSK-G – Tampa Scale of Kinesiophobia – General (11-44)
Pre-intervention expectations were measured by asking subjects to indicate what that they expected to experience during the thermal testing after the intervention.
Table 2.
Associations among psychological measures and change in pain sensitivity.
| ASI | FPQ-9 | PCS | TSK-G | ||
|---|---|---|---|---|---|
| Upper extremity |
47 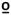 C C |
−0.025 | 0.073 | −0.091 | 0.048 |
49 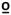 C C |
0.039 | −0.090 | −0.031 | 0.115 | |
| PPT | 0.200 | 0.162 | 0.088 | 0.132 | |
| TSS | −0.180 | 0.019 | −0.061 | −0.074 | |
| Lower extremity |
47 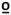 C C |
0.177 | 0.104 | 0.128 | −0.112 |
49 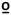 C C |
0.060 | 0.036 | 0.066 | −0.031 | |
| PPT | 0.011 | 0.110 | 0.003 | −0.004 | |
| TSS | −0.021 | 0.188 | 0.110 | 0.068 |
ASI – Anxiety Sensitivity Index (0-64)
FPQ-9 – fear of pain questionnaire (0-36)
PCS – Pain catastrophizing scale (0-52)
TSK-G – Tampa scale of kinesiophobia – General (11-44)
Experimental Pain Sensitivity
Mechanical Stimuli: Pressure Pain Threshold
For PPT, no interaction effects were noted to be significant (all p>0.27). There were no significant effects for group; however, a main effect for limb (F1,85=62.3, p<0.001, partial η2 = 0.41) was significant. On average, the lower extremity threshold values were higher than the upper extremity values regardless of the type of intervention received or testing time. A time effect was also noted (F1,85=9.6, p=0.009, partial η2 = 0.10) in which all groups increased in PPT from pre to post-intervention.
Thermal Stimuli: First Pain
No three-way or two-way interactions were evident for ratings of first pain at 47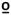 C. However, main effects were identified for time (F1,85=41.8, p<0.001, partial η2 = 0.32) in which the average rating of 47
C. However, main effects were identified for time (F1,85=41.8, p<0.001, partial η2 = 0.32) in which the average rating of 47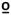 C was lower at the post-test. Additionally, a limb effect was identified (F1,85=8.2, p=0.005, partial η2 = 0.41) whereby the ratings of 47
C was lower at the post-test. Additionally, a limb effect was identified (F1,85=8.2, p=0.005, partial η2 = 0.41) whereby the ratings of 47 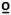 C were higher in the upper extremity than the lower extremity (p=0.005). Similar effects for time (F1,85=21.1, p<0.001, partial η2 = 0.20) were noted for the ratings at 49
C were higher in the upper extremity than the lower extremity (p=0.005). Similar effects for time (F1,85=21.1, p<0.001, partial η2 = 0.20) were noted for the ratings at 49 C. No limb effects were noted. The figure shows the changes in rating of first pain over time.
C. No limb effects were noted. The figure shows the changes in rating of first pain over time.
Figure 1.
This figure shows the group by time interaction for the measure of temporal sensory summation. The asterisk indicates that TSS was significantly decreased after intervention for the SMT group. Neither of the other groups was different after intervention.
Thermal Stimuli: Temporal Sensory Sensation
No three way interactions were noted (F2,85=0.07, p=0. 935, partial η2 = 0.012). However, a two-way interaction occurred between group × time (F2,85=6.9, p=0.010, partial η2 = 0.07). Here the SMT group experienced a significant reduction in TSS after the intervention (p=0.003) to have a significantly lower TSS than both CE (p=0.005) and control (p=0.014) groups. There were no other effects noted.
DISCUSSION
The primary finding of this study was that changes in TSS occurred immediately after SMT to the upper thoracic spine, supporting our first hypothesis. This effect of upper thoracic SMT is consistent with our previous work in the lumbar spine [5, 9, 11] and provides additional evidence that immediate inhibition of TSS may result following SMT.
Demonstrating a reduction in TSS has potentially meaningful implications. Patients with chronic pain conditions often demonstrate increased rates and magnitudes of TSS compared to pain free individuals [32, 33, 34]. TSS is a behavioral measure of wind-up in humans. Windup is activity dependent modulation of dorsal horn activity[35] characterized by a progressive increase in output from dorsal horn neurons in response to repeated unchanging low-frequency nociceptor stimuli [35, 36]. Windup is a short term and reversible event related to central sensitization that is independent of transcription phenomena that characterize long lasting facilitation and changes in central sensitization [37]. However, repeated exposure to increased nociceptor activity resulting from windup can cause facilitated transmission in dorsal horn neurons and long-lasting changes in synaptic properties. This in turn could drive long-lasting changes in dorsal horn and CNS excitability resulting in reduced thresholds to future episodes of nociception. We speculate that an intervention that reduces TSS may inhibit or reduce the potential for central sensitization in maintaining musculoskeletal pain. Additionally, interventions that inhibit or reduce TSS may prevent long-term facilitation from occurring preventing the progression to central sensitization and persistent pain states. This explanation is speculative as the current study did not include subjects with musculoskeletal pain.
Our previous lumbar SMT studies have indicated that TSS is reduced in both patients with LBP [5], and healthy subjects [9, 10, 11]. In those studies, reduction of TSS was noted in the lower extremity after application of SMT to the lumbar spine potentially indicating a local or regional treatment effect for SMT. Boal and Gillette [6] suggest that SMT interventions activate A-delta fibers, initiating long-term depression of any local facilitation or potentiation that has occurred in the dorsal horn at or around the level at which SMT is applied. Such a finding may explain the observations of reduced TSS in dermatomes associated with the region in which SMT is targeted. Consequently, we had hypothesized for this current study that if the effect of SMT was local, or regionally specific, there would be immediate reduction in the upper extremity after SMT but not the lower extremity.
This hypothesis was not supported by our data as there were reductions in TSS that occurred in both the upper and lower extremity. Converging evidence from experimental studies of nociception in animal models suggests that the observed phenomenon (changes in TSS distal to the spinal level of application) could be mediated by propriospinal neurons projecting from the lower cervical cord to the lumbar spine. For example, cervical propriospinal neurons mediate inhibition of neurons in the [38], cats [39, 40] and primates [41]. Also, and more specifically, Sandulker et al [42] concluded that propriospinal neurons from the cervical and thoracic cord modulate thermally-evoked noxious responses of lumbar dorsal horn neurons. Furthermore, activation of capsacin sensitive vanilloid receptors in cervical muscles of cats increases the neuronal activity of the cervical and lumbar dorsal horns [39, 40]. This activation in the lumbar dorsal horn occurs bilaterally with the highest concentration of activity in lamina I and V [39, 40] representing areas with the largest concentration of nociceptive neurons receiving terminations from c-fiber afferents, [43] which are hypothesized to be the primary inputs for TSS [27, 44, 45]. The implications for this caudal effect on TSS remain unclear.
More recently, Haavic-Taylor and Murphy [46] reported that cervical SMT produces changes in cortical sensory evoked potentials. These authors hypothesized that cervical SMT alters afferent information from the cervical spine which in turn alters the manner in which the somatosensory cortex responds to subsequent afferent input. Changes in cortical response to afferent input could likely contribute to altered TSS we observed immediately following SMT. What is not clear is whether cortical responses to SMT would differ in region or magnitude based on the spinal region targeted.
Limitations
Our conclusions based on the results of the current study must be tempered by the fact that we tested the response to SMT in healthy subjects and consequently, we are unable to comment about effects in patients with neck pain. Previously we have studied lumbar SMT and our approach has been to examine nervous system responses in experiments in which we were able to control many of the aspects of the encounter such as participant expectation, diurnal fluctuations in physiology, room temperature and experimental setup. Studies in healthy participants provide information about the performance of the nervous system in a baseline condition. Our approach also involves eventual test of the interventions in a clinical model – a situation in which there is far more variability. Determining that similar effects were identified in highly controlled laboratory conditions and the more variable clinical setting allows us to conclude that the response is robust given our observations of across multiple studies involving lumbar SMT [9, 10, 18].
In the current study we proposed that the effect of SMT in the cervical spine would be consistent with the lumbar spine; that is, the mechanisms would likely be the same. If we had performed the clinical study first, and not found an effect, we would be unable to comment whether the negative finding was related to differences in cervical anatomy/physiology or response to the intervention. Therefore, we have done this study in healthy participants first. The logical extension of our findings will be to repeat this study in individuals with upper thoracic and cervical pain.
Additional limitations are that we are unable to describe the duration of these effects on TSS. Future work will address the assessment of caudal/distal changes bilaterally, and evaluate changes in TSS over a longer time period.
Conclusions
This current study confirms that reduction in TSS may be an expected neurophysiological response to SMT for healthy subjects. This is an intriguing pre-clinical finding because interventions that reduce TSS have the potential of limiting the development of central sensitization of pain. This study extends previous work by identifying that reduction in TSS occurred caudal to the region of application of upper thoracic SMT. We suggest that subsequent studies of upper-thoracic SMT should include patient populations and collecting neurophysiological data from multiple areas innervated by regions caudal to application.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fernandez-de-las-Penas C, Perez-de-Heredia M, Brea-Rivero M, et al. Immediate effects on pressure pain threshold following a single cervical spine manipulation in healthy subjects. J Orthop Sports Phys Ther. 2007;37:325–9. doi: 10.2519/jospt.2007.2542. [DOI] [PubMed] [Google Scholar]
- 2.Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain. 1996;68:69–74. doi: 10.1016/S0304-3959(96)03221-6. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JA, Childs JD, McRae M, et al. Immediate effects of thoracic manipulation in patients with neck pain: a randomized clinical trial. Man Ther. 2005;10:127–35. doi: 10.1016/j.math.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141:920–8. doi: 10.7326/0003-4819-141-12-200412210-00008. [DOI] [PubMed] [Google Scholar]
- 5.Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–8. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boal RW, Gillette RG. Central neuronal plasticity, low back pain and spinal manipulative therapy. J Manipulative Physiol Ther. 2004;27:314–26. doi: 10.1016/j.jmpt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Gillette RG. A speculative argument for the co-activation of diverse somatic receptor populations by forceful chiropractic adjustments. Man Med. 1987;3:1–14. [Google Scholar]
- 8.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;2:357–71. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 9.Bialosky JE, Bishop MD, Robinson ME, et al. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord. 2008;9:19. doi: 10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bialosky JE, Bishop MD, Robinson ME, et al. The Immediate Effects of Spinal Manipulative Therapy on Thermal Pain Sensitivity in Participants with Low Back Pain: A Randomized. Controlled Trial Phys Ther. 2009:89. doi: 10.2522/ptj.20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George SZ, Bishop MD, Bialosky JE, et al. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskel Disord. 2006;7:68. doi: 10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–40. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- 13.Terrett AC, Vernon H. Manipulation and pain tolerance. A controlled study of the effect of spinal manipulation on paraspinal cutaneous pain tolerance levels. Am J Phys Med. 1984;63:217–225. [PubMed] [Google Scholar]
- 14.Nielsen J, Arendt-Nielsen L. The importance of stimulus configuration for temporal summation of first and second pain to repeated heat stimuli. Eur J Pain. 1998;2:329–341. doi: 10.1016/s1090-3801(98)90031-3. [DOI] [PubMed] [Google Scholar]
- 15.Staud R, Craggs JG, Robinson ME, et al. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–42. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleland JA, Glynn P, Whitman JM, et al. Short-term effects of thrust versus nonthrust mobilization/manipulation directed at the thoracic spine in patients with neck pain: a randomized clinical trial. Phys Ther. 2007;87:431–40. doi: 10.2522/ptj.20060217. [DOI] [PubMed] [Google Scholar]
- 17.Bialosky JE, Bishop MD, Robinson ME, et al. Spinal Manipulative Therapy Has an Immediate Effect on Thermal Pain Sensitivity in People With Low Back Pain: A Randomized Controlled Trial. Phys Ther. 2009 doi: 10.2522/ptj.20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George SZ, Bishop MD, Bialosky JE, et al. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006;7:68. doi: 10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson ME, Bialosky JE, Bishop MD, et al. Supra-threshold scaling, temporal summation, and after-sensation: relationships to each other and anxiety/fear. J Pain Res. 2010;3:25–32. doi: 10.2147/jpr.s9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil DW, Rainwater AJ., 3rd Development of the Fear of Pain Questionnaire--III. J Behav Med. 1998;21:389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 21.Woby SR, Roach NK, Urmston M, et al. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117:137–44. doi: 10.1016/j.pain.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt NB, Joiner T,E. Structure of the Anxiety Sensitivity Index psychometrics and factor structure in a community sample. J Anxiety Disord. 2002;16:33–49. doi: 10.1016/s0887-6185(01)00087-1. [DOI] [PubMed] [Google Scholar]
- 23.Dehon C, Weems CF, Stickle TR, et al. A cross-sectional evaluation of the factorial invariance of anxiety sensitivity in adolescents and young adults. Behav Res Ther. 2005;43:799–781. doi: 10.1016/j.brat.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995 [Google Scholar]
- 25.Robinson ME, Wise EA, Gagnon C, et al. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Rosier EM, M.J. I, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98:205–216. doi: 10.1016/s0304-3959(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 27.Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–75. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 28.Wright A, Graven-Nielsen T, Davies II, et al. Temporal summation of pain from skin, muscle and joint following nociceptive ultrasonic stimulation in humans. Exp Brain Res. 2002;144:475–82. doi: 10.1007/s00221-002-1062-4. [DOI] [PubMed] [Google Scholar]
- 29.Ross JK, Bereznick DE, McGill SM. Determining cavitation location during lumbar and thoracic spinal manipulation: is spinal manipulation accurate and specific? Spine. 2004;29:1452–1457. doi: 10.1097/01.brs.0000129024.95630.57. [DOI] [PubMed] [Google Scholar]
- 30.Falla D, Jull G, Dall’Alba P, et al. An electromyographic analysis of the deep cervical flexor muscles in performance of craniocervical flexion. Phys Ther. 2003;83:899–906. [PubMed] [Google Scholar]
- 31.O’Leary S, Falla D, Hodges PW, et al. Specific therapeutic exercise of the neck induces immediate local hypoalgesia. J Pain. 2007;8:832–839. doi: 10.1016/j.jpain.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Price DD, Staud R, Robinson ME, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 33.Staud R, Cannon RC, Mauderli AP, et al. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 34.Maixner W, Fillingim R, Sigurdsson A, et al. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 35.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 37.Ji R-R, Kohno T, Moore KA, et al. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Miller KE, Douglas VD, Richards AB, et al. Propriospinal neurons in the C1-C2 spinal segments project to the L5-S1 segments of the rat spinal cord. Brain Res Bull. 1998;47:43–47. doi: 10.1016/s0361-9230(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 39.Kalezic I, Pilyavskii AI, Maisky VA, et al. Distinctive pattern of c-fos expression in the feline cervico-lumbar spinal cord after stimulation of vanilloid receptors in dorsal neck muscles. Neurosci Lett. 2004;364:94–97. doi: 10.1016/j.neulet.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Pilyavskii AI, Maznychenko AV, Maisky VA, et al. Capsaicin-induced effects on c-fos expression and NADPH-diaphorase activity in the feline spinal cord. Eur J Pharmacol. 2005;521:70–78. doi: 10.1016/j.ejphar.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Hobbs SF, Oh UT, Chandler MJ, et al. Evidence that C1 and C2 propriospinal neurons mediate the inhibitory effects of viscerosomatic spinal afferent input on primate spinothalamic tract neurons. J Neurophysiol. 1992;67:852–860. doi: 10.1152/jn.1992.67.4.852. [DOI] [PubMed] [Google Scholar]
- 42.Sandkühler J, Stelzer B, Fu QG. Characteristics of propriospinal modulation of nociceptive lumbar spinal dorsal horn neurons in the cat. Neuroscience. 1993;54:957–967. doi: 10.1016/0306-4522(93)90587-6. [DOI] [PubMed] [Google Scholar]
- 43.Calvino B, Grilo GM. Central pain control. Joint Bone Spine. 2006;73:10–16. doi: 10.1016/j.jbspin.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Price DD, Bennett GJ, Rafii A. Psychophysical observations on patients with neuropathic pain relieved by a sympathetic block. Pain. 1989;36:273–88. doi: 10.1016/0304-3959(89)90086-9. [DOI] [PubMed] [Google Scholar]
- 45.Staud R, Robinson ME, Vierck CJ, Jr., et al. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–22. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 46.Haavik-Taylor H, Murphy B. Cervical spine manipulation alters sensorimotor integration: a somatosensory evoked potential study. Clin Neurophysiol. 2007;118:391–402. doi: 10.1016/j.clinph.2006.09.014. [DOI] [PubMed] [Google Scholar]



