Abstract
Neural crest (NC) cells invade the vertebrate embryo in ordered migratory streams, yet it is unclear whether cells communicate to maintain spacing and direction. Here, we examined NC cell communication in detail, using optical highlighting and photobleaching to monitor cell contact dynamics. We observed cytoplasmic transfer between NC cell neighbors through thin cellular bridges. The transfer of molecules between NC cells was bi-directional, not at equal rates, and independent of bridge dynamics. The cytoplasmic transfer was prevalent in recently divided NC cells. Molecular simulations, based on Brownian motion and measured cell volumes, predicted that simple diffusion could not account for observed cytoplasmic transfer rates. Cell tracking revealed that exchange of cytoplasmic material preceded the re-orientation of cells to the direction of migration. Our data suggest a mechanism by which NC cells communicate position information through the formation of cellular bridges that allow exchange of cytoplasmic material through active transport.
Keywords: neural crest, cell communication, avian, embryo, photoconversion, FLIP, KikGR
INTRODUCTION
The highly migratory neural crest (NC) are an excellent in vivo model to study questions of cell communication since NC cell behaviors are accessible to interrogation with light microscopy (Kulesa and Fraser, 2000; Alfandari et al., 2010; Clay and Halloran, 2010; Klymkowsky et al., 2010). NC cells emerge from the dorsal neural tube in a rostral-to-caudal manner and are sculpted onto stereotypical migratory pathways (Tosney, 1982; LeDouarin and Kalcheim, 1999; Kulesa and Gammill, 2010). Exchange of position information between neighboring NC cells would help to explain how cells coordinate with each other to promote their ordered invasion program. Although typical cell tracing methods using fluorescent proteins or lipophilic dyes have provided insights into complex NC cell migratory behaviors (Bhattacharyya et al., 2008), it is still unclear to what extent cell contact influences a NC cell’s trajectory (Erickson et al., 1980; Davis and Trinkaus, 1981; Mayor and Carmona-Fontaine, 2010; Kulesa et al., 2010). Thus, in order to study NC cell communication, there is a clear need to apply advanced optical techniques that allow the observation and measurement of in vivo NC cell contact dynamics.
Cell communication by touch is an important mechanism that several multi-cellular organisms use to maintain growth and functionality (reviewed in Rorth, 2003). For example, in Drosophila border cell migration, a single long cellular extension forms at the initiation of migration from one cell within the border cell cluster (Fulga and Rorth, 2002). The long cellular extension is thought to function as a pathfinder in response to guidance cues (Fulga and Rorth, 2002). Disruption of border cell motility may occur by inhibition or promotion of lead cell activities, suggesting that information from the lead cell is transmitted to the cell cluster to initiate movement along the migratory pathway (Fulga and Rorth, 2002).
The discovery of cell communication mediated by numerous thin membraneous strands running between cells has revealed that cells can transmit signals to other distant cells through a physically connected network (reviewed in Davis and Sowinski, 2008). These physical connections (50–200nm wide), originally described in cultured neural cells by digital interference contrast (DIC) microscopy, were termed tunneling nanotubes (Rustom et al., 2004). Tunneling nanotubes have also been shown to be associated with immune cells (Onfelt et al., 2004; Watkins and Salter, 2005) and can be explored by HIV to spread between cells (Sowinski et al., 2008). In contrast to tunneling nanotubes, we previously observed thin (1–2um wide) short- and long-range cellular protrusions on in vivo migratory NC cells (Teddy and Kulesa et al., 2004). NC cell-to-cell contact often led to follow-the-leader cell migratory behavior, revealed by whole chick embryo confocal time-lapse microscopy (Teddy and Kulesa, 2004). However, it was unclear whether the cell contacts led to an exchange of information between NC cells. This was due to the difficulties to monitor the in vivo dynamics of the NC cellular extensions and cytoplasmic material within the cells.
In this study, we examined the dynamics of cell contact during chick NC cell migration in living embryos with advanced optical techniques. We took advantage of techniques such as targeted photoconversion and fluorescence loss in photobleaching (FLIP) that allowed for in vivo investigation of NC cell communication in living chick embryos. We have previously utilized photoconversion of green fluorescent protein (GFP) and its variants to trace complex NC cell behaviors in embryos (Stark and Kulesa, 2005; 2007) and now applied this knowledge to study cell contact dynamics in more detail. We monitored the movement of fluorescent molecules within migratory NC cells after cell contact and cell division. We characterized the formation of thin (0.5–2um wide) cellular bridges between neighboring NC cells and measured the dynamics of NC cell cytoplasmic exchange and the result on NC cell trajectories. Our approach provides a powerful tool for analyzing the in vivo cellular mechanisms underlying NC cell communication.
RESULTS
Migratory neural crest cells exchanged cytoplasmic material through thin cellular bridges
To investigate NC cell communication in detail, we applied optical imaging techniques that allowed for interrogation of intracellular dynamics during cell contact. First, we used photoconversion of a dual-colored fluorescent reporter, KikGR, to selectively highlight a portion of a NC cell that was identified in contact with a neighboring cell in a living chick embryo (Fig. 1A,C,G,I). KikGR is a photoconvertible fluorescent protein that undergoes a color change from green to red when excited with 405nm laser light (Tsutsui et al., 2005; Stark and Kulesa, 2007). Second, we monitored the movement of photoconverted fluorescent molecules and measured fluorescence intensity profiles in neighboring NC cells, as we continued to photoactivate the region of interest (Fig. 1B,D-F,H,J-L). We also monitored the fluorescence in non-neighboring NC cells as a control. We found that we were not excessively photobleaching the tissue outside of the region of interest (Fig 1D-F,J-L; compare fluorescence intensity in non-photoconverted cells). Regions of interest were selected by locating two neighboring NC cells, judged as discrete cells by their nuclear H2B-mRFP label (Fig. 1C,I; red-colored nuclei).
Figure 1. Photoconversion of KikGR-labeled NC cells revealed thin cellular bridges between neighbors and transfer of cytoplasmic material.
Diagram of typical NC cells before (A and G) and after (B and H) photoconversion of KikGR (405nm excitation) in a subregion (box) of an individual cell. Lead and trailing NC cells were identified by position from the neural tube (in vitro, a lead cell was more distal from the neural tube midline; in vivo, a lead cell was more distal from the neural tube midline). (B) Cellular bridges between neighboring NC cells were measured to determine the average length and width of the bridge. (C-F) Selected images from a typical confocal time-lapse imaging session of individual NC cells (identified by separate nuclear cell label) in contact that displayed no cytoplasmic transfer between cells after photoconversion of KikGR (subregion marked by box). These images are from an in vitro experiment but similar results for both in vitro and in vivo were found. The images show the movement of photoconverted molecules (red) to the point of contact (arrowhead) between the cells. (I-L) Selected images from a typical confocal time-lapse imaging session of individual NC cells (identified by nuclear label) in contact that displayed cytoplasmic transfer. The images show the movement of photoconverted molecules (red) into a neighboring NC cell through a cellular bridge (arrow and arrowhead). These images are from an in vivo experiment but cytoplasmic transfer can also be seen in recently divided cells in vitro. Each NC cell nucleus is approximately 10um. See Supplemental Movies for more examples.
We discovered two interesting results. In a small number of in vivo NC cell contacts analyzed (~13%; n=7/54), we discovered that photoconverted fluorescent molecules moved from one NC cell into a neighboring NC cell through a thin (0.5–2um wide) cellular bridge (Fig. 1I-L; Movie 2). In contrast, we also found that photoconverted fluorescent molecules traveled to a midpoint in the cellular extensions (~87%, n=47/54; no cytoplasmic transfer), identifying the point of contact between the two neighbors (Fig. 1C-F; Movie 1). When we quantified the distance between neighboring NC cells in contact (measured between individual cell nuclei), we found the average length of a cellular bridge (n=54) was approximately 36.2um and average width was 2.3um at the midpoint between cells (Fig. 1B).
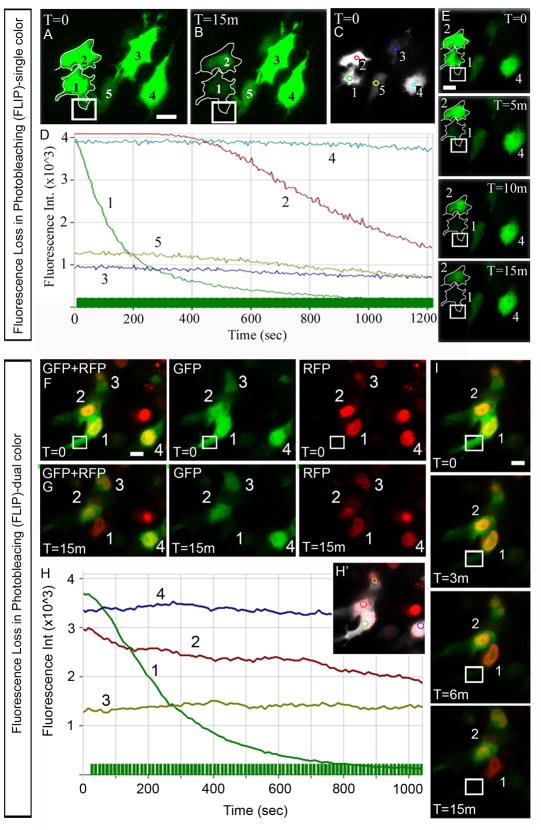
Fluorescence loss in photobleaching (FLIP) confirmed the movement of fluorescent molecules between neighboring, migratory NC cells
To investigate NC cell communication with a second optical imaging technique, we photobleached a region of interest within a migratory NC cell and monitored the loss of fluorescent molecules within neighboring NC cells (Fig. 2). We performed the experiment on in vivo NC cells labeled with either GFP alone (Fig. 2A-E; movie 3) or with a combination of GFP and H2B-mRFP (Fig. 2F-I; movie 4). By labeling the cell body and nucleus, we could confirm contact between distinct, individual neighboring NC cells (Fig. 2F). In GFP-only labeled NC cells, cellular protrusions from the same cell could be confused as separate, neighboring NC cells (Fig. 2A). In both labeling strategies, we discovered a transfer of fluorescent molecules from one NC cell into the neighboring NC cell (Fig. 2D, E, H, I). This appeared as a loss in fluorescence in the NC cell neighboring the photobleached NC cell (Fig. 2D, E, H, I). That is, during successive photobleaching iterations, the fluorescence within the photobleached region of interest decreased exponentially (Fig. 2D, E, H, I). Within the subregion of the neighboring NC cell, the fluorescence intensity did not initially decrease, but over time decreased approximately linearly for the duration of the successive photobleaching iterations (Fig. 2D, H). In other non-local neighbors (>30um away), the mild loss of fluorescence intensity was due to photobleaching of the entire field of view, collected after each iteration of local photobleaching within the NC cell (Fig. 2D; sub-regions 3,4,5; Fig. 2H, sub-regions 3,4). Our FLIP experiments were conducted as an alternative method to photoactivation for validation of cytoplasmic transfer, therefore detailed analysis of the cellular bridge or transfer rates were not conducted for these experiments but were assumed similar since the experimental design was identical except for the fluorescent proteins used.
Figure 2. Fluorescence loss in photobleaching (FLIP) experiments confirm cytoplasmic transfer between neighboring NC cells.
(A,B) The projection of a confocal z-stack of images shows 5 GFP-labeled NC cells in vivo before (A) and after (B, 15m=minutes) photobleaching of the subregion (box). (C) The fluorescence intensity was measured in one z-plane for each of the 5 cells in the subregions indicated by colored circles and (D) plotted as a function of time. (E) Selected sequence of single plan confocal images from the FLIP experiment in A-D showing the loss of fluorescence in a photobleached NC cell (labeled by 1) and its nearest neighbor (labeled by 2). (F,G) The projection of a confocal z-stack of images shows 4 dual color (GFP and mRFP) labeled NC cells in vivo before (F) and after (G, 15m=minutes) photobleaching of the subregion (box). Each row (F and G) shows the same projected z-stack with GFP+RFP, GFP, and RFP images separately to confirm the individual NC cells. (H-H′) The fluorescence intensity was measured in one z-plane for each of the 4 cells (H′) in the subregions indicated by colored circles and (H) plotted as a function of time. (I) Selected sequence of images from the FLIP experiment in F-H′ showing the loss of fluorescence in a photobleached NC cell (labeled by 1) and its nearest neighbor (labeled by 2). The scalebars in A,E,F,I are 10um.
Dividing neural crest cells displayed cytoplasmic transfer of material through a cellular bridge that persisted between progeny
To study the dynamics of NC cell contact and movement of fluorescent molecules between dividing NC cells, we combined our photoconversion technique with longer confocal time-lapse imaging to capture the persistence of the NC cell contacts (Fig. 3; movies 5 and 6). To do this, we followed migratory NC cells labeled with photoconvertible KikGR, monitored the NC cell rounding before division, then photoconverted a sub-region of interest within one of the NC cells (Fig. 3). Premigratory NC cells were co-labeled with photoconvertible KikGR and H2B-mRFP to uniquely identify NC cell neighbors. Interestingly, we found that in nearly all (81% in vitro, 90% in vivo; n=26) cell divisions, NC cells exchanged photoconverted fluorescent molecules, both in vitro (Fig. 3A-D′; movie 5) and in vivo (Fig. 3E-H′; movie 6). We first performed the in vitro experiment in migratory NC cells leaving from an explanted neural tube (Fig. 3A-D′). This was to confirm that individual cellular processes could be identified as belonging to a particular individual cell and not wrapped around a neighbor that would incorrectly identify NC cell-to-cell cytoplasmic transfer (Fig. 3A-D′). We found that in a typical experiment, in one progeny (photoconverted, donor cell) of the dividing NC cell pair, there was a sharp increase in the red/green fluorescence intensity ratio that correlated to the photoconversion initiation (compare Fig. 3A, A′ with B, B′; Fig. 3I). This was followed by a steady decline in the level of red/green fluorescence intensity in that NC cell (photoconverted, donor cell) progeny (Fig. 3B-D′, Fig. 3I). In the non-photoconverted NC cell progeny (receiver cell), we monitored the level of red/green fluorescence intensity and found a steady increase over the duration of approximately 1 hour (Fig. 3A-D′). The dynamics of the in vitro NC cell division, photoconversion of a progeny, and tracking of photoconverted fluorescence clearly showed a transfer of cytoplasmic material between the NC cell progeny over time (Fig. 3D, D′; movie 5).
Figure 3. Quantification of cytoplasmic transfer of photoconverted KikGR between recently divided NC cells.
(A-D) Selected images from a typical confocal time-lapse imaging session of a recently divided NC cell in vitro. The region of interest is shown (box) in the photoconverted (donor) NC cell and the other NC cell progeny is identified by the nuclear H2B-mRFP label (red). (A′-D′) Line intensity profiles (constructed along a line through both NC cells) shows the fluorescence intensity of the unconverted KikGR (green) and photoconverted KikGR (red). (A′) The line intensity profile of H2B-mRFP (red) is shown at time=0 before photoconversion of KikGR to confirm two individual NC cells. (B′-D′) Photoconverted KikGR (red) intensity decreased in the donor cell and increased in the neighboring NC cell (receiver). (E-H and E′-H′) Similar analysis and line intensity profiles for the in vivo experiment. (I) A typical example of the dynamic changes in fluorescence intensity ratio of photoconverted KikGR (red) to non-photoconverted KikGR (green) for a donor and receiver NC cell over time. The sum of the fluorescence intensity over the whole area of the cell was measured for both channels and the intensity ratio was normalized to the maximum ratio for each curve. (J,K) The red/green intensity ratio for n=15 NC cell pairs each for in vitro (J) and in vivo (K) experiments over time. The curves are centered in time at the point where donor and receiver ratios are equal and shifted such that the ratio is equal to 0. Bold lines are the average for the experiments. (L) The major and minor axes of recently divided NC cells measured over time, as pairs of dividing NC cells. (M) The center of mass of recently divided NC cells tracked over time and the distance in microns between each NC cell progeny. The distance between NC cell progeny was averaged from n=15 pairs of cells both in vitro (red circles) and in vivo (green squares). As a control, the distance between n=6 pairs of migratory NC cells in vitro (brown circles) and in vivo (dark green squares) that simply bumped into each other is shown. The scalebars in A and E are 10um.
To determine whether NC cell-to-cell transfer of cytoplasmic material occurred in vivo, we repeated these experiments (Fig. 3E-H; movie 6). Using confocal time-lapse microscopy, NC cells were followed by visual observation and cell divisions were identified by cell behaviors that included the slowing of NC cell speed and rounding up of cells (movie 6). One progeny of the dividing NC cells was then photoconverted (Fig. 3E-H; movie 6). Similarly, we found that photoconverted fluorescent molecules transferred between neighboring NC cell progeny shortly after in vivo cell division (Fig. 3E-H). Line intensity profiles revealed that the increase in red fluorescence in the non-photoconverted NC cell progeny increased over time (Fig. 3E′-H′). In a typical experiment, the level of green fluorescence in the photoconverted NC cell rapidly decreased after photoconversion and remained at low levels in the photoconverted NC cell progeny (Fig. 3E-H′; movie 6). In comparison between in vitro and in vivo events, we found that fluorescence intensity changes of photoconverted fluorescent molecules between donor and receiver NC cell progeny were similar (compare Fig. 3J (in vitro) and 3K (in vivo)). In the in vivo scenario, there appeared to be a more gradual decrease in red/green fluorescence intensity from the photoconverted NC cell progeny (compare Fig. 3K (donor cell) and Fig. 3J (donor cell)). Measurements also showed a rapid increase in red/green fluorescence intensity in the non-photoconverted NC cell progeny (Fig. 3J, K (receiver cell)).
Dividing NC cells oriented their major and minor axes to the cellular bridge between cells and tended to remain short-term neighbors in vivo during re-acquisition of directed migration
To explore possible effects of NC cell-to-cell cytoplasmic transfer, we monitored the shape and position of recently divided NC cells that shared a cytoplasmic bridge. The length of the major and minor axes of a pair of dividing NC cells was measured at the time of photoconversion and over time (Fig 3L). A polarized versus non-polarized NC cell was judged by a larger difference between its major and minor axes. This indicated that the NC cell had a directed orientation. When we compared NC cell progeny that had recently divided, we found that each NC cell was approximately round with little orientation (Fig. 3L); the major and minor axes were approximately equal. As the NC cell progeny moved apart to reveal a cellular bridge between cells, both cells acquired polarity, indicated by the lengthening of the major axis (Fig. 3L). The dividing NC cell’s minor axis remained approximately similar (Fig. 3L). This pattern was repeated in dividing NC cells that showed cytoplasmic transfer.
We also tracked the positions of recently divided NC cells and measured the distance between the two progeny as the cells resumed their migratory behavior in vitro or in vivo (Fig. 3M). As a control, NC cells that simply bumped into each other were also tracked and measured (Fig. 3M). We found that in vivo dividing NC cells tended to stay neighbors (Fig. 3M; light green colored line), in a similar manner to non-dividing cells over the course of 1 hr after division. Cells that bumped into each other in vitro tended to move apart (Fig. 3M; brown colored line), as expected of cells migrating randomly that were not directed towards a particular target.
Cytoplasmic transfer between neural crest cells was bi-directional, but with unequal rates of exchange
To examine the transfer rates of cytoplasm between NC cells that formed cellular bridges, we measured the fluorescence intensity of the non-converted KikGR (green) and converted KikGR (red) (Fig. 4A). Specifically, we measured the quantity Red in Cell 1 = Red1 / (Red1 + Red2) (Fig. 4A). Displaying the data in this way enabled easier interpretation of the transfer rates of each fluorophore between two NC cells (Fig. 4A). We assumed that any changes in fluorescence intensity from photobleaching or NC cells that moved out of focus were identical in each cell. In a representative experiment, we found that the transfer of photoconverted KikGR from the donor cell into the receiver cell was rapid (Fig. 4A). The non-converted KikGR also transferred between the NC cells at a much slower rate (Fig. 4A). We assumed that the act of photoconverting the fluorescent protein did not have any major conformational change that would cause the protein to aggregate or bind to elements inside the cell. Also, the KikGR was not pre-attached to any cytoskeletal machinery, so the unequal rates of transfer of the KikGR represented an unequal rate of cytoplasmic exchange between the two NC cells. We found that in a majority of our experiments (67%; n=20/30), the rates of cytoplasmic transfer were unequal (Fig. 4A, Table 1). (Two data sets are not included in the table that had unequal rates of transfer where a leader or follower could not be determined.) That is, one NC cell lost cytoplasm faster than it gained from the other NC cell. In contrast, in 33% (n=10/30) of our experiments, the rates of transfer of the cytoplasm from the donor NC cell to the receiver NC cell and vice versa were approximately the same.
Figure 4. Model simulations of NC cell cytoplasmic transfer dynamics.
(A) Fluorescence intensities over time of the photoconverted KikGR-labeled NC cell (donor) and non-photoconverted neighboring NC cell (receiver). The fluorescence intensity ratio was calculated by the fluorescence intensity in one channel in each cell divided by the total fluorescence intensity in that channel for both cells (Ratio Red 1 = Red1/(Red1 + Red2)). (B) Length to width ratio of cellular bridges connecting two NC cells over time. The length and width of the cellular bridges were roughly fit to a polynomial and the equation was used in model simulations. (D) A schematic of the model simulations. Each NC cell pair was represented by a rectangular box and the cellular bridge (open tube connecting the boxes). Red and green particles were initially distributed separately and randomly in each simulated NC cell and then allowed to move in random directions at each time step with reflective boundary conditions. Over time, the particles mixed and the shape of the cellular bridge changed according to our experimental data. Each simulation was run for the same amount of time as the experimental data. The positions of simulated particles are shown at three time points (zero, 250 and 500 seconds). Particles were allowed to move a random distance based on a given diffusion constant and reflected off the edges of the cell. (C) Model simulations were run using different diffusion constants, D, and the resulting fluorescence ratios from the simulations were compared to the experimental data.
Table 1.
Biological data of neural crest cell cytoplasm transfer dynamics
| In Vivo | In Vitro | Description of cytoplasm transfer dynamics after photoactivation |
|---|---|---|
| 3/14 (21%) | 7/16 (44%) | Lead cell loses cytoplasm faster |
| 5/14 (36%) | 3/16 (19%) | Follower cell loses cytoplasm faster |
| 4/14 (28%) | 6/16 (38%) | Equal rates of transfer |
In order to better understand the unequal rate of cytoplasmic transfer between NC cells that formed a cellular bridge, we separately photoconverted NC cells further from the neural tube (lead) and NC cells closer to the neural tube (follower) both in vivo and in neural tube explants. Interestingly, we found a difference in the direction of cytoplasmic transfer between the in vitro and in vivo data (Table 1). In vivo, the follower cell transferred cytoplasm faster into the lead cell (Table 1), suggesting communication from follower to lead cell. In vitro, the direction of more rapid cytoplasmic transfer was reversed (Table 1).
Cytoplasmic transfer between neural crest cells occurred faster than random diffusion
To assess the kinetics of the cytoplasmic transfer between two NC cells that formed a cellular bridge, we developed Brownian motion simulations to determine the diffusion constant of the KikGR (Fig. 4; movie 7). NC cells were simulated as rectangular volumes approximately the size of the actual NC cells in each experiment (measured from confocal images) (Fig. 4D). The simulated NC cells were attached by a bridge through which photoconverted and non-photoconverted KikGR molecules passed (Fig. 4D). The length and width of the cellular bridge in the simulations was approximated by the actual data from experiments, a typical example of which is shown in Fig. 4B. That is, we found that the NC cellular bridges changed shape over time (Fig. 4B). The NC cellular bridges were dynamic and both increased or decreased in length over time and both slightly widened or narrowed at the midpoint between cell bodies (Fig. 4B,D).
In model simulations, we found that the shape and size of the tether connecting the NC cells could dramatically change the rate of transfer between the cells (a typical simulation is shown in Fig. 4; movie 7). Thus, it was necessary to initially fit a rough curve to the tether dimensions and incorporate that into the simulations. In the time-lapse data, the measured length and width of the cellular bridge did not have any correlation with the speed at which the fluorescent molecules moved between the cells for in vivo or in vitro data. That is, for relatively small volumes, we can find both fast and slow diffusion of cytoplasmic material in the time-lapse data. (data not shown) Each simulation began with 5000 randomly positioned photoconverted KikGR molecules in one NC cell and 5000 non-photoconverted KikGR molecules in the second NC cell. Over time, the particles diffused across the cellular bridge in both directions and came to an equilibrium (example simulation in Fig. 4; movie 7). Each simulation was run under the cell and bridge geometries determined from the time-lapse data with various diffusion constants, D, until the rate of KikGR transfer from the simulation best fit the rate of fluorescence loss or gain from the time-lapse data for each experiment for both in vitro and in vivo data (one example shown in Fig. 4C). The best fit D is then compared to a theoretical value of a freely diffusing KikGR in the cytoplasm of the cell. We determined KikGR to have a theoretical diffusion coefficient of approximately 3um^2/sec by using the diffusion coefficient of EGFP in HeLa cells of 5um^2/sec (unpublished data, Jay Unruh personal communication), and a volume 4 times as large, since KikGR is a tetramer. Our simulations revealed that in 71% of the experiments in vivo, the transfer of cytoplasm from at least one of the NC cells had a diffusion constant larger than our theoretical value (Table 2). In only 29% of the experiments, the cytoplasm was transferred faster than free diffusion in vitro (Table 2). This revealed that there existed a reasonable value of the diffusion coefficient, D, of KikGR that closely reproduced our experimental data. This result suggested that the cytoplasm was actively transported into the other NC cell and this was observed more often in vivo than in vitro.
Table 2.
Simulation results of neural crest cell cytoplasm transfer dynamics
| In Vivo | In Vitro | Cytoplasm transfer |
|---|---|---|
| 5/7 (71%) | 2/7 (29%) | Transfer (either direction) faster than random diffusion |
| 2/7 (29%) | 4/7 (57%) | Transfer (both directions) slower than random diffusion |
| 0 (0%) | 1/7 (14%) | Transfer (both directions) approximately equal random diffusion |
DISCUSSION
We used the chick neural crest as a model system and optical highlighting techniques to study cell communication in the developing embryo. The use of the photoconvertible fluorescent protein KikGR and fluorescence loss in photobleaching provided a more precise and less damaging way to examine the diffusion of large molecules within migratory NC cells. Here, our results demonstrate a novel form of intercellular signaling between migratory NC cells that consisted of several aspects. First, we found that a small number of migratory NC cells formed thin cellular connections between neighboring cells that we termed cellular bridges. Second, NC cells that formed cellular bridges exchanged cytoplasmic material. Third, the transfer of cytoplasmic material between NC cells was bi-directional, but at unequal rates. Fourth, the cytoplasmic transfer between NC cells occurred faster than random diffusion.
Migratory NC cells formed sustained cellular bridges between cells suggesting a unique physical form for NC cell-to-cell communication. NC cellular bridges were thin (0.5–2um wide) and approximately 36um in length measured between cell nuclei, on average (Fig. 1). The NC cellular bridges were dynamic and formed de novo between some migratory NC cells (Fig. 1) and between nearly all dividing NC cells (Fig. 3). The size of NC cellular bridges were larger than previously reported long cytoplasmic extensions, such as Drosophila cytonemes (0.2um in diameter; Ramirez-Weber and Kornberg, 1999) or tunneling nanotubes (50–200nm) observed on cultured PC12 (Rustom et al., 2004) and dendritic cells (Watkins and Salter, 2005). NC cellular bridges persisted for over 1hr during NC cell migration and division (see movies 1–6) and were typically unique to a pair of neighboring NC cells. That is, we did not observe a physically connected network of cellular bridges between multiple migratory NC cells, as reported within dendritic cells (Watkins and Salter, 2005). Thus, NC cell-cell contact dynamics and cell division included the potential formation of thin cellular bridges that persisted over the short term between neighboring migratory NC cells.
NC cells exchanged cytoplasmic material (Figs. 1–3; movies 1–6), suggesting that intercellular signals were transmitted in the form of small molecules (photoconvertible KikGR is a tetrameric fluorescent protein that is ~103kDa in size) between NC cell neighbors. Our observation that only some migratory NC cells that formed de novo cellular bridges transferred cytoplasmic material supported the hypothesis that NC cells migrate as a loosely connected subpopulation of cells, rather than as a collective group within a physically connected network. In contrast to tunneling nanotubes (50–200nm wide), NC cellular bridges were wide enough to facilitate the selective transfer of fluorescent molecules as well as smaller membrane vesicles and organelles. We did not explore the architecture of the NC cellular bridges or trace the cytoplasmic material in more detail. Thus, it was unknown as to the nature of the cytoplasmic material transferred between NC cells, however future experiments that trace molecular dynamics in more detail or use electron microscopy could help to shed light on this question.
The NC cellular bridges that appeared in late telophase were lengthy extensions of the cellular membrane that typically forms between two daughter cells (Eggert et al., 2006). The sustained formation of NC cellular bridges as NC cell progeny began to change shape (re-extend lamellipodia and filopodia) and re-acquire directed migration hinted that a mechanism(s) actively sustained the midbody. In time-lapse observations, the NC cellular bridges appeared to break by tension when NC cells moved further apart (movies 5–6), rather than by midwifery or intervention by another cell as shown by Nagasaki and Uyeda, 2008. Thus, it would be interesting to see whether NC cellular bridges could be sustained or prematurely severed by manipulating molecules that typically sustain the midbody (Eggert et al., 2006; Saurin et al., 2008).
Cytoplasmic transfer between dividing NC cells was bi-directional, but at unequal rates. These results indicated that NC intercellular signals traveled between NC cells and there was a bias to the communication direction. It was not clear whether the transfer of cytoplasmic material between dividing NC cells represented an exchange of spatial information. We were unable to track whether photoconverted KikGR fluorescent molecules traveled into the neighboring NC cell nucleus during cytokinesis; an event that would suggest exchange of position information. There is evidence that the tetrameric complexes of photoconvertible KikGR (~103kDa) can enter newly assembled nuclei during a ~20min period after the completion of cytokinesis in cultured HeLa cells (Shimozono et al., 2009). We had some evidence that NC cell progeny re-oriented their major axes in the direction of the cellular bridge and remained short-term migratory neighbors more so than NC cells that randomly bumped into each other (Fig. 3L,M). Future experiments could use laser ablation to cut the cellular bridge between migratory NC cells and study the changes to cell orientation and migratory behavior in more detail.
We calculated the level of red fluorescence (photoconverted KikGR) in the donor NC cell decreased linearly (Fig. 4A) and red fluorescence increased in the receiver cell (Fig. 4A). We also observed the level of green fluorescence (non-photoconverted KikGR) decreased in the receiver cell and increased in the donor cell eventhough the green fluorescence should decrease as almost all the KikGR fluorescent molecules are photoconverted (Fig. 4A). It was unclear why there was a bias to the direction of intercellular signaling between dividing NC cells. One possibility is that the movement of photoconvertible KikGR molecules was inhibited in the lead NC progeny, more distally positioned in the migratory stream with respect to the target direction. Differences in protein mobility between pioneer versus follower growth cones has been shown in vivo in zebrafish embryos (Kulkarni et al., 2007). Using multiphoton fluorescence recovery after photobleaching (FRAP) and pharmacological perturbations of the cytoskeleton, Kulkarni and colleagues were able to show that a more complex actin network existed within the pioneer growth cone that inhibited molecular diffusion (Kulkarni et al., 2007). Slower loss of photoconverted KikGR from the lead NC cell progeny would correlate with a re-acquisition of directed migration by the lead NC cell progeny. Quantitative measurements showed that in vivo the follower NC cell lost cytoplasm faster than the lead NC cell (5/14; 36%) more often (Table 1). However, FRAP experiments within each NC cell progeny during the separation of cells and re-acqusition of directed migration would help to test this hypothesis.
Cytoplasmic transfer between NC cells occurred faster than random diffusion, suggesting that active transport moved molecules between neighboring NC cells. Our model simulations that varied the diffusion coefficient, D, of cytoplasmic material, revealed that cytoplasm was actively transported through NC cellular bridges (Fig. 4; movie 7). It was not clear whether the active transport of material through the NC cellular bridges was microtubule based or some other form of transport. However, the shape of the cellular bridges had no correlation with the speed at which molecules passed between cells, further supporting our conclusion that molecules actively transported between NC cells. Future experiments that disrupt microtubule transport within the cellular bridges, but do not inhibit NC cell motility or cell division will help to better understand the kinetics of the cytoplasmic transfer.
To our knowledge, these data represent the first report of in vivo transmission of cytoplasmic material through cellular bridges in the migratory neural crest. This appears to represent a novel mechanism that is independent of gap junctions. Based upon the rate at which de novo cellular bridges formed and the dynamics of photoconvertible KikGR fluorescent molecules exchanged between cells, an interesting possibility is that NC cells communicate spatial information during migration. Whether the movement of cytoplasmic material between NC cells is a form of intercellular signaling is not clear. The movement of exchanged cytoplasmic material could serve as a mechanism for amplifying local guidance signals, helping NC cells navigate, and ultimately controlling NC targeting. Further experiments will have the challenge to dissect the function of proteins exchanged between NC cells and understanding how this affects a NC cell’s choice of direction. The approach shown here is an exciting step forward in our ability to directly observe and measure communication in cells in living embryos.
METHODS
Embryo Preparation
Fertilized white leghorn chicken eggs (supplied by Centurion Poultry Inc., Lexington, GA) were incubated at 38°C in a humidified incubator until the appropriate stages. Eggs were rinsed with 70% ethanol and 3 ml of albumin was removed before windowing the eggshell. A solution of 10% India ink (Pelikan Fount; PLK 51822A143, www.mrart.com, Houston, TX) in Howard Ringer’s solution was injected below the area opaca to visualize each embryo. Embryos were staged according to the criteria of Hamburger and Hamilton (HH) (Hamburger and Hamilton, 1951).
Constructs
KikGR (kind gift from Prof. A. Miyawaki Laboratory, Japan) driven by a CMV promoter is now commercially available as Kikume Green-Red, AM-V0082, MBL Int’l Corp, Woburn, MA). A monomeric red fluorescent protein mRFP1 (shortened here as mRFP) was obtained originally from R. Tsien, San Diego, CA, USA and made nuclear-specific and driven by a CMV promoter (H2B-mRFP1) (kind gift from S. Megason and R. Lansford, Caltech) and used to label the nuclei of individual neural crest (NC) cells. All constructs were used at a concentration of 5ug/ul.
Cell Labeling
Embryos were injected with expression constructs at HH stages 8–9 (5–7 somites) to label premigratory rhombomere3(r3)-r5 NC cells. After windowing the eggshell, a sharpened tungsten needle was used to open a small hole in the vitelline membrane above the cranial neural tube and a cocktail of KikGR and H2B-mRFP or GFP-only were microinjected directly into the lumen of the cranial neural tube, using a pulled borosilicate glass needle (Sutter; BF100-50-10, Novato, CA) attached to a Picospritzer III (Parker Hannifin Corporation, Fairfield, NJ). The constructs were delivered into NC cells in the right side of the dorsal neural tube with electroporation (using platinum electrodes and an Electro Square Porator ECM 830 (BTX, a division of Genetronics, San Diego, CA). Five, 45 millisecond pulses at one second intervals of 20 volts current, delivered the constructs into premigratory NC cells in the dorsal hindbrain. A few drops of sterile Ringer’s solution was applied to each embryo prior to the eggs being resealed with adhesive tape and re-incubated at 38°C in a humidified incubator (Model 1550, G.Q.F. Manufacturing Co., Savannah, GA) until removal at 10–14 hrs after electroporation for photoconversion or photobleaching experiments.
Imaging and Culture Preparation
For static imaging, KikGR injected embryos were collected at 24 hours post-photoconversion. Embryos showing expression after surveying using a fluorescence dissecting microscope (Leica MZFLIII) were mounted dorsal side up on 22 × 75 mm microslides (48312-024, VWR, West Chester, PA) within PBS contained by a silicone grease ring (79810-99, Dow Corning, Midland, MI) then covered with a 22 × 22 mm glass coverslip (48366 067, VWR). All imaging was performed on an inverted LSM 510 DUO microscope (Carl Zeiss Microimaging, Thornwood, NY, USA), using either a Plan-Apochromat 10X/0.45, 20X/0.8, or C-Apochromat 40X/1.2W objective (Carl Zeiss). KikGR images were collected in multi-track mode using the filter combination of 505–550nm (green) and 575–615nm (red), where the 561nm laser (2–4% power) was used to excite the photoconverted proteins and mRFP. Collected z-stacks were projected onto single fields using the AIM Software (Carl Zeiss). Multiple fields were merged using Adobe Photoshop CS2 (Adobe Systems, Inc., San Jose, CA).
For confocal time-lapse imaging, the microscope (LSM 510 Duo) was surrounded by an incubator box (Carl Zeiss) with the temperature set at 38°C for the duration of the time-lapse acquisition. Whole embryo explant cultures were prepared according to the method described in Krull and Kulesa (1998). Collected confocal images were analyzed by concatenating the consecutive time points using the AIM software (Carl Zeiss).
For neural tube cultures, neural tubes were extracted from embryos 2 hours post-microinjection. The surface ectoderm was digested with dispase and neural tubes placed on fibronectin coated glass bottom culture dishes (MatTek Corp). After the neural tubes adhered to the bottom of the cover glass, a thin layer of Matrigel (356234, BD Biosciences) was placed over the cover glass to prevent the sample from suspending. Once the Matrigel set, neural tubes were covered with neural basal media with 1/50 B-27 supplement, Pen/Strep and L-glutamine. The cultures were incubated overnight at 37°C to allow neural crest migration.
Photoconversion of KikGR and Fluorescence Loss in Photobleaching (FLIP)
The KikGR photoconvertible protein is sensitive to light that may cause inadvertent photobleaching and/or photoconversion. Thus, it was important to consider the amount of laser and Hg light exposed to each embryo. Cells expressing KikGR were located by using 488nm laser excitation, low laser power (1–2%) with 10X/0.45 or 20 X/0.8 objectives at 1024×1024 pixel resolution. We performed photoconversion of KikGR-labeled cells, including cell target acquisition and selection for photoconversion by using the Edit Bleach option in the confocal software (AIM; Carl Zeiss) under the parameter of 50 iterations of a 405nm laser (2–4% power) over a region of interest. Briefly, all photoconversions were performed in whole embryo explants and neural tube cultures with increased detector gain, pinhole diameter, optical zoom (2X-3X) and m=2 frame averaging to maintain brightness while preventing photobleaching.
We performed the fluorescence loss in photobleaching (FLIP) experiments in vivo on GFP and H2B-mRFP labeled NC cells using a C-Aprochromat 40X/1.2 W corr objective (Carl Zeiss), zoom=2.5, Briefly, a subregion of interest within a NC cell was selected an photobleached between every imaging cycle with 488nm laser light (10% power), with images collected every 6–11 seconds for approximately 15–20 minutes.
Analysis of KikGR-Labeled NC Cell Divisions
Sequences of dividing photoconverted KikGR-labeled NC cells were selected from short (<4hrs) time-lapse imaging sessions of r4 NC cell migratory stream dynamics. The time-lapse files were concatenated together using AIM (Carl Zeiss) and each pair of NC cells were cropped from the original data set to reduce file size for analysis. Cropped time-lapse images were imported into Image-Pro Plus (Media Cybernetics) or Imaris (Bitplane) for measurements. Each cell was selected individually by a color cube based approach or by manual drawing of the region of interest. The following measurements were recorded: area, position of the center of the cell, average fluorescence intensity in green and red over the cell area, cell width, cell length, and orientation angle (with respect to horizontal). Measurements were exported into Microsoft Excel for comparative analysis between the in vitro and in vivo data.
Using the centroid positions for each NC cell, the distance between each NC cell was measured beginning from cytokinesis to the end time point of the time lapse. The average distance between divided NC cell pairs was compared for in vitro, in vivo, and random NC cells that bumped into each other (control).
Model Simulations of NC Cell Cytoplasmic Transfer Dynamics
To simulate the diffusion of the photoconverted and non-converted KikGR molecules, a Brownian motion simulation was created in Matlab (Mathworks Inc). In the program, a volume was created with two equal 3D boxes representing the NC cells connected by a thin cellular bridge similar to the shape of a dumbbell. The geometry of the boxes and cellular bridge was estimated from NC cells and bridges from our experimental data. At the start of the simulation, 5000 red particles were randomly distributed in the left box and 5000 green in the right box. At each time step, the particles moved in each of the three dimensions with a step size equal to a Gaussian distributed random number with standard deviation of square root of 2*D*t, where D is the diffusion constant and t is the value of the time step (usually 0.5 seconds). The volume had reflective boundary conditions on all edges, except where a box met a cellular bridge the particles were allowed to travel through in either direction. If the cellular bridge changed shape significantly over time in a given experiment, the simulation was altered such that the width or length of the cellular bridge grew or shrunk over time. This reflected the true experimental data to give a more accurate representation. The fluorescence intensity of each box was calculated from the number of green or red particles inside each box divided by 5000. Simulations were performed with various values of D to find the best fit to the experimental data.
Supplementary Material
Time-lapse confocal microscopy imaging sequence of neural crest cells labeled with nuclear localized H2B-mRFP and the photoconvertible fluorescent protein, KikGR. Photoconversion (excitation with 405nm laser light) was performed, followed sequentially by a single excitation scan at 488nm and this process was repeated every 30sec for approximately 10 minutes. Notice that the photoconverted fluorescent molecules (red) travel throughout the cell and an extending filopodium tip that is in contact with a neighboring NC cell.
A time-lapse confocal imaging sequence of neural crest cells labeled with H2B-mRFP and cytoplasmic KikGR. Notice that there is cytoplasmic transfer of photoconverted fluorescent molecules (red) from one NC cell into its neighbors below and to the left. Photoconversion was performed (excitation with 405nm laser light) and images were collected every 7.5 seconds for approximately 6 minutes.
Single color (GFP-labeled NC cells) FLIP experiments show loss in fluorescence in a NC cell neighboring the photobleached NC cell. Photobleaching (488nm excitation) was performed between every imaging cycle. Images were collected every 6 seconds for approximately 20 minutes.
Dual color (GFP- and H2B-mRFP-labeled NC cells) FLIP experiments show loss in fluorescence in a NC cell neighboring the photobleached NC cell. Nuclear localized H2B-mRFP confirmed there were 2 individual NC cells of which only one was photobleached. Photobleaching (488nm excitation) was performed between every imaging cycle. Images were collected every 11 seconds for approximately 17 minutes.
Time-lapse confocal microscopy images of dividing NC cells in vitro labeled with nuclear localized H2B-mRFP and cytoplasmic KikGR. Dividing NC cells were determined by visual observation of migratory NC cells that appeared to round up and divide. Photoconversion of KikGR in a subregion of the lead NC cell (box) was performed in between two imaging frames and then the cells were imaged every 30 seconds.
Time-lapse confocal microscopy images of dividing NC cells in vivo labeled with nuclear localized H2B-mRFP and cytoplasmic KikGR. Dividing NC cells were determined by visual observation of migratory NC cells that appeared to round up and divide. Photoconversion of KikGR in a subregion of the NC cell was performed in between two imaging frames and then the cells were only every 30 seconds for approximately 80 minutes.
A typical model simulation of the cytoplasmic movement of fluorescent molecules between neighboring NC cells that formed a cellular bridge.
Acknowledgments
This work was funded by NIH grant 1R01HD057922 and the Stowers Institute for Medical Research.
References
- Alfandari D, Cousin H, Marsden M. Mechanism of Xenopus cranial neural crest cell migration. Cell Adh Mig. 2010;4(4):553–60. doi: 10.4161/cam.4.4.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Kulesa PM, Fraser SE. Vital labeling of embryonic cells using fluorescent dyes and proteins. Methods Cell Biol. 2008;87:187–210. doi: 10.1016/S0091-679X(08)00210-0. [DOI] [PubMed] [Google Scholar]
- Clay MR, Halloran MC. Control of neural crest cell behavior and migration: Insights from live imaging. Cell Adh Mig. 2010;4(4):586–94. doi: 10.4161/cam.4.4.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM, Sowinski S. Membrane nanotubes: dynamic long distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9(6):431–6. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- Davis EM, Trinkaus JP. Significance of cell-to-cell contacts for the directional movement of neural crest cells within a hydrated collagen lattice. J Embryol Exp Morphol. 1981;63:29–51. [PubMed] [Google Scholar]
- Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–66. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Tosney KW, Weston JA. Analysis of migratory behavior of neural crest and fibroblastic cells in embryonic tissues. Dev Biol. 1980;77:142–56. doi: 10.1016/0012-1606(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat Cell Biol. 2002;4(9):715–9. doi: 10.1038/ncb848. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437(7058):560–3. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Kulesa PM, Lefcort F. Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia. Development. 2005;132:235–45. doi: 10.1242/dev.01553. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M, Rossi CC, Artinger KB. Mechanisms driving neural crest induction and migration in the zebrafish and Xenopus laevis. Cell Adh Mig. 2010;4(4):595–608. doi: 10.4161/cam.4.4.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull CE, Kulesa PM. Embryonic explant and slice preparations for studies of cell migration and axon guidance. Curr Top Dev Biol. 1998;36:145–59. doi: 10.1016/s0070-2153(08)60500-1. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127:1161–72. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Bailey CM, Kasemeier-Kulesa JC, McLennan R. Cranial neural crest migration: new rules for an old road. Dev Biol. 2010;344(2):543–54. doi: 10.1016/j.ydbio.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Gammill LS. Neural crest migration: patterns, phases and signals. Dev Biol. 2010;344(2):566–68. doi: 10.1016/j.ydbio.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RP, Bak-Maier M, Fraser SE. Differences in protein mobility between pioneer versus follower growth cones. PNAS. 2007;104(4):1207–12. doi: 10.1073/pnas.0610142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. 2. Cambridge Univ. Press; Cambridge: 1999. [Google Scholar]
- Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010;20(6):319–28. doi: 10.1016/j.tcb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki A, Uyeda TQ. Chemotaxis-mediated scission contributes to efficient cytokinesis in dictyostelium. Cell Motil Cytoskel. 2008;65:896–903. doi: 10.1002/cm.20311. [DOI] [PubMed] [Google Scholar]
- Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. J Immunol. 2004;173(3):1511–3. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97(5):599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- Rorth P. Communication by touch: role of cellular extensions in complex animals. Cell. 2003;112(5):595–8. doi: 10.1016/s0092-8674(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes H. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Saurin AT, Durgan J, Cameron AJ, Faisal A, Marber MS, Parker PJ. The regulated assembly of a PKCepsilon complex controls the completion of cytokinesis. Nat Cell Biol. 2008;10(8):891–901. doi: 10.1038/ncb1749. [DOI] [PubMed] [Google Scholar]
- Shimozono S, Tsutsui H, Miyawaki A. Diffusion of Large Molecules into Assembling Nuclei Revealed Using an Optical Highlighting Technique. Biophys J. 2009;97(5):1288–94. doi: 10.1016/j.bpj.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10(2):211–9. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Stark DA, Kulesa PM. Photoactivatable green fluorescent protein as a single cell marker in living embryos. Dev Dyn. 2005;233(3):983–92. doi: 10.1002/dvdy.20385. [DOI] [PubMed] [Google Scholar]
- Stark DA, Kulesa PM. An in vivo comparison of photoactivatable fluorescent proteins in an avian embryo model. Dev Dyn. 2007;236(6):1583–94. doi: 10.1002/dvdy.21174. [DOI] [PubMed] [Google Scholar]
- Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–51. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- Tosney KW. The segregation and early migration of cranial neural crest cells in the avian embryo. Dev Biol. 1982;89:13–24. doi: 10.1016/0012-1606(82)90289-5. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Shimizu H, Nukina N, Miyawaki A. Semi rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 2005;6(3):233–8. doi: 10.1038/sj.embor.7400361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–18. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time-lapse confocal microscopy imaging sequence of neural crest cells labeled with nuclear localized H2B-mRFP and the photoconvertible fluorescent protein, KikGR. Photoconversion (excitation with 405nm laser light) was performed, followed sequentially by a single excitation scan at 488nm and this process was repeated every 30sec for approximately 10 minutes. Notice that the photoconverted fluorescent molecules (red) travel throughout the cell and an extending filopodium tip that is in contact with a neighboring NC cell.
A time-lapse confocal imaging sequence of neural crest cells labeled with H2B-mRFP and cytoplasmic KikGR. Notice that there is cytoplasmic transfer of photoconverted fluorescent molecules (red) from one NC cell into its neighbors below and to the left. Photoconversion was performed (excitation with 405nm laser light) and images were collected every 7.5 seconds for approximately 6 minutes.
Single color (GFP-labeled NC cells) FLIP experiments show loss in fluorescence in a NC cell neighboring the photobleached NC cell. Photobleaching (488nm excitation) was performed between every imaging cycle. Images were collected every 6 seconds for approximately 20 minutes.
Dual color (GFP- and H2B-mRFP-labeled NC cells) FLIP experiments show loss in fluorescence in a NC cell neighboring the photobleached NC cell. Nuclear localized H2B-mRFP confirmed there were 2 individual NC cells of which only one was photobleached. Photobleaching (488nm excitation) was performed between every imaging cycle. Images were collected every 11 seconds for approximately 17 minutes.
Time-lapse confocal microscopy images of dividing NC cells in vitro labeled with nuclear localized H2B-mRFP and cytoplasmic KikGR. Dividing NC cells were determined by visual observation of migratory NC cells that appeared to round up and divide. Photoconversion of KikGR in a subregion of the lead NC cell (box) was performed in between two imaging frames and then the cells were imaged every 30 seconds.
Time-lapse confocal microscopy images of dividing NC cells in vivo labeled with nuclear localized H2B-mRFP and cytoplasmic KikGR. Dividing NC cells were determined by visual observation of migratory NC cells that appeared to round up and divide. Photoconversion of KikGR in a subregion of the NC cell was performed in between two imaging frames and then the cells were only every 30 seconds for approximately 80 minutes.
A typical model simulation of the cytoplasmic movement of fluorescent molecules between neighboring NC cells that formed a cellular bridge.