Abstract
Neutrophil recruitment via CXCR2 is required for innate and adaptive protective immunity to the larvae of Strongyloides stercoralis in mice. The goal of the present study was to determine the mechanism of CXCR2-mediated neutrophil recruitment to S. stercoralis. Mice deficient in the receptor for IL-17A and IL-17F, upstream mediators of CXCR2 ligand production, were infected with S. stercoralis larvae; there was no difference in larval survival, neutrophil recruitment, or production of CXCR2 ligands compared with wild type mice. In vivo and in vitro stimulation of neutrophils with S. stercoralis soluble extract resulted in significant neutrophil recruitment. In vitro assays demonstrated that the recruitment functioned through both chemokinesis and chemotaxis, was specific for CXCR2, and was a G protein coupled response involving tyrosine kinase and PI3K. Finally, neutrophil stimulation with S. stercoralis soluble extract induced release of the CXCR2 ligands MIP-2 and KC from neutrophils, thereby potentially enhancing neutrophil recruitment.
Keywords: IL-17, CXCR2, Strongyloides stercoralis, neutrophil, chemotaxis
1. Introduction
Protective immunity to Strongyloides stercoralis in mice depends on neutrophils during both the primary and secondary immune responses [1–2]. Infective third-stage larvae of S. stercoralis are killed in naïve mice within 5–7 days post-infection through an innate immune response dependent on complement activation [3] and neutrophils [1]. Adaptive immunity, induced in mice by immunization with live larvae, kills greater than 90% of larvae within 24 hours and requires CD4+ Th2 cells for IL-4 and IL-5 [4], B-1a B cells for IgM antibody [5], complement component C3 [3], and neutrophils [1]. Neutrophils from mice deficient in TLR4 kill the larvae of S. stercoralis in naive mice, but do not kill the worms in immunized mice. Neutrophils from mice deficient in TLR4, however, migrate to the larval microenvironment in both naïve and immunized animals, at rates equivalent to that seen in the wild type mice [6]. These findings show that TLR4 signaling is required for neutrophils to kill larvae in immunized mice, but not in naïve mice, and that TLR4 is not required for neutrophil recruitment in either innate or adaptive immunity. If neutrophil recruitment in mice was blocked, either because of a defect in Gαi2 signaling [2] or in the expression of CXCR2 [1], the capacity of naïve and immunized mice to kill S. stercoralis larvae was significantly decreased. Adding neutrophils isolated from CXCR2−/− mice directly into the larval microenvironment in recipient CXCR2−/− mice restores larval killing [1]. Therefore, neutrophil recruitment to the parasite requires CXCR2, while larvicidal function is independent of this receptor.
CXCR2 is a receptor for the neutrophil chemokines MIP-2 and KC (orthologs of the human chemokine IL-8) [7]. The cytokines IL-17A and IL-17F are potent inducers of the CXCR2 ligands MIP-2 and KC through signaling via IL-17R [8]. Bacteria, fungi, protozoa and viruses all can induce IL-17 responses, and these are associated with increased numbers of neutrophils associated with the pathogen and decreased pathogen burden [9–10]. IL-17R−/− mice have increased susceptibility to the pathogens Candida albicans [9, 11], Klebsiella pneumoniae [12], Salmonella typhimurium [13], HSV-1 [14], Staphylococcus aureus [15], Toxoplasma gondii [16] and to polymicrobial sepsis [17]. In each case the increased susceptibility to the pathogen was associated with decreased neutrophil recruitment to the site of infection. Both MIP-2 and KC have been associated with a variety of helminth infections, including Litomosoides sigmodontis [18], Mesocestoides corti [19] and Schistosoma mansoni [20], suggesting that IL-17 might be important for the recruitment of neutrophils to the site of helminth infections.
Neutrophils can also undergo chemotaxis in response to a variety of helminth-derived factors, thereby obviating the need for host ligand-dependent pathways [21]. Products from the nematodes Dirofilaria immitis [22], Necator americanus [23] and Onchocerca volvulus [24] have been shown to recruit neutrophils. Furthermore, Ascaris suum-derived products recruit neutrophils through a IL-8 receptor pathway [25] and Brugia malayi asparaginyl-transfer RNA synthetase induces chemotaxis of human neutrophils apparently through the receptor CXCR2 [26].
Eosinophils can also participate in protective innate immunity to S. stercoralis [1], and it has been shown that they undergo both chemotaxis and chemokinesis to soluble parasite extract in vitro. Treating the parasite extract with proteinase K or chitinase significantly inhibited its ability to induce chemotaxis, thereby demonstrating that the chemoattractants were both protein and chitin. Pretreatment of eosinophils with pertussis toxin, a G protein-coupled receptor inhibitor, blocked migration of the eosinophils to the parasite extract. Blocking PI3K, tyrosine kinase, p38 and p44/42 also inhibited eosinophil chemotaxis to parasite extract as did CCR3, CXCR4 or CXCR2 antagonists. Therefore, chemoattractants derived from S. stercoralis larvae and host derived chemokines stimulate similar receptors and second messenger signals, to induce eosinophil chemotaxis [27].
The goal of the present study was to ascertain whether the CXCR2-dependent neutrophil recruitment to S. stercoralis larvae requires host and/or parasite-derived chemotactic factors. The requirement for IL-17 to stimulate production of the neutrophil chemokines MIP-2 and KC, and thereby neutrophil chemotaxis to the parasite was evaluated in naïve and immunized mice. Also, the ability of the parasite to directly recruit neutrophils through CXCR2, and the mechanisms through which this occurred was assessed.
2. Materials and Methods
2.1 Mice and Parasites
IL-17R−/− mice on a C57BL/6 background were a gift from Amgen Corporation (Thousand Oaks, CA). C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were housed in filter-top microisolator boxes under pathogen-free, light- and temperature-controlled conditions in the Thomas Jefferson University animal facility. All injections and surgical procedures were performed while mice were anesthetized with isoflurane (Webster Veterinary, Sterling, MA).
S. stercoralis larvae were harvested from seven day old fecal-charcoal cultures from a laboratory dog infected with the parasite according to methods previously described [28]. Larvae were washed 5 times by centrifugation using media consisting of 1 part NCTC:1 part IMDM (Sigma-Aldrich, St. Louis, MO), with 100 U Penicillin, 100 μg Streptomycin (Cellgro, Manassas, VA), 100 μg Gentamicin (Invitrogen, Carlsbad, CA) and 25 μg Levaquin (Ortho-McNeil, Raritan, NJ).
Efficacy of the antibiotic treatment was assessed by placing antibiotic treated and untreated larvae on blood agar plates at 37°C for 4 8 hours. The number of bacterial colonies found on the blood agar plates was then estimated. In addition, the final wash fluid was placed in culture for 48 hours and then assessed for bacterial growth.
2.2 Diffusion chambers
Diffusion chambers were constructed from 2.0 μm pore-size membranes (Millipore, Bedford, MA) as previously described [1]. Briefly, 14-mm Lucite rings (Millipore) were covered with 2.0 μm pore-size membranes (Millipore, Bedford) using cyanoacrylate adhesive (Superglue Corp., Hollis, NY), fused together with an adhesive consisting of a 1:1 mixture of 1,2-dichloroethane (Fisher Scientific, Pittsburgh, PA) and acryloid resin (Rohm and Haas, Philadelphia, PA), and then sterilized using 100% ethylene oxide.
2.3 Extract Preparation
Parasite extract used in chemotactic assays was prepared using previously described methods [29]. Briefly, frozen larvae were ground using a homogenizer with protease inhibitor cocktail (Sigma) followed by sonication. The homogenate was incubated in PBS overnight at 4°C, after which the soluble proteins were removed. The concentration of extract soluble in PBS was quantified using a Micro BCA Protein Assay kit (Pierce Biotechnology, Rockford, IL). Determination of endotoxin levels within the extract was done using a Limulus amebocyte lysate (LAL) test (Cambrex, Charles City, IA).
2.4 Experimental infections
Diffusion chambers were filled with Hanks’ Balanced Salt Solution (HBSS), 50 larvae, 300 μg of the S. stercoralis soluble extract or 300 μl of the protease inhibitor cocktail (Sigma) used to make the extract and implanted in naïve (uninfected and unimmunized) mice. After 24 hr the diffusion chambers were removed and cells within the diffusion chambers analyzed as described below. Mice were immunized by subcutaneous injection of 5,000 larvae on day 0 and day 14. On day 21 post immunization diffusion chambers containing 50 larvae in medium were implanted subcutaneously in the dorsal flank of naïve or immunized mice. Diffusion chambers were removed after 1–5 days and larval viability was determined based on motility and morphology. The supernatant remaining in the diffusion chamber was recovered for use in chemokine measurements and the cells were counted using a hemocytometer. Differential cell analyses were performed after centrifugation onto slides using a Cytospin 3 (Shandon, Pittsburgh, PA) and staining of cells using DiffQuik (Baxter Healthcare, Miami, FL).
2.5 Isolation of murine neutrophils
Neutrophils used in chemotaxis experiments were isolated from the bone marrow of C57BL/6J mice as previously described [30]. Mouse bone marrow was strained with a 70 μm cell strainer (BD, Bedford, MA) and the cells were suspended within 45% Percoll solution at the top of a Percoll gradient column consisting of 81, 62, 55, and 50% Percoll/HBSS. The columns were centrifuged at 610 × g for 30 minutes. The layer containing the neutrophils (between 81 and 62%) was then removed and red blood cells eliminated by hypotonic lysis. If the purity of the neutrophils was less than 90% following the Percoll column step, 1μl each of anti-B220 and anti-Thy1.2 MACS (Miltenyi Biotec, Auburn, CA) beads were added per 1×106 total cells to bind contaminating lymphocytes. The cells were incubated with the beads for 15 minutes at 4°C, purified using MACS LS columns (Miltenyi) and then reanalyzed for total number and percentage of neutrophils. Purity of neutrophil populations utilized for experiments consistently exceeded 92%.
2.6 Chemotaxis assays
Transwell plates with 3.0 μm pore size membrane inserts (Corning, Acton, MA) were used to determine the chemotactic response of neutrophils to various chemoattractants. The top wells were preincubated with 50 μm of 2% fetal bovine serum (Cellgro) in HBSS (Cellgro) for 45 minutes. After preincubation, 50 μl of a suspension containing 1×106 cells was added to the top well. The chemoattractants used in the bottom of the wells included S. stercoralis PBS extract with or without Polymyxin B (Sigma) pre-incubation, the positive controls LTB4 (Sigma), MIP-2 (Peprotech Inc., Rocky Hill, NJ) and SDF-1α (Sigma) and HBSS as a negative control. Culture wells were incubated for 90 minutes and the contents of the bottom wells were collected and washed with PIPES buffer (Sigma). Cells that migrated into the bottom chamber were counted using a hemocytometer.
2.7 Chemokine concentrations and inhibition
MIP-2 and KC were measured using the mouse DuoSet ELISA kits (R&D systems, Minneapolis, MN) according to the manufacturer’s instructions. Neutrophils (1×105/well) were preincubated in granulocyte-macrophage colony-stimulating factor (GMCSF) (Peprotech, Rocky, Hill, NJ) for 90 minutes at 37°C in the presence of either highly purified TLR4-specific lipopolysaccharide (LPS) from Escherichia coli K12 (InVivoGen, San Diego, CA) at 1,10 or 100 ng/ml or S. stercoralis extract at 1,10, 100 or 1,000 μg/ml [31]. Supernatants were collected and measured for MIP-2 and KC.
Chemotaxis assays were performed, as described above, with MIP-2 and KC added to the bottom wells at the concentrations measured after neutrophils were directly incubated in the S. stercoralis and at four times that concentration as a positive control. Antibodies against MIP-2 and KC (R&D Systems, Minneapolis, MN) were added to the wells to block the activity of the chemokines. The anti-KC antibody was used at 0.5 μg/ml for the controls and the low concentration of the KC tested and 1.0 μg/ml of the antibody was used with the high concentration of KC, the S. stercoralis soluble extract and when both MIP-2 and KC antibodies were used. The anti-MIP-2 antibody was used at 8.0 μg/ml for the controls and the low concentration of the KC tested and 16 μg/ml of the antibody was used with the high concentration of KC, the S. stercoralis soluble extract and when both MIP-2 and KC antibodies were used.
2.8 Inhibition assays
Pertussis toxin was used to inhibit all Gα protein signaling, wortmannin to inhibit signaling via PI3K [32] and herbimycin A to block signaling through tyrosine kinase [33]. SB225002 was used to inhibit CXCR2 associated chemotaxis [34], while AMD 3100 was used to inhibit CXCR4 [35]. Cells were incubated with pertussis toxin (1.0 μg/ml) (Calbiochem, Inc., San Diego, CA), wortmannin (1×10−7M) (Sigma), herbimycin A (1×10−6M) (Sigma), SB225002 (1×10−5M) (Calbiochem), or AMD 3100 (4×10−8M) (Sigma). The cells were incubated in the tested inhibitor/antagonist for 45 minutes at 37°C prior to use in chemotactic assays.
2.9 Statistics
All in vivo experimental results are representative data of at least two experiments, except as noted. A minimum of 4 mice per group were included in each experiment. In vitro chemotaxis data represents the mean of a minimum of 3 experiments. Statistical differences were identified by a p value of ≤ 0.05 as determined by ANOVA with post hoc LSD testing using Systat software (Systat software, Richmond, CA).
3. Results
3.1 Role of IL-17 in neutrophil recruitment to larval S. stercoralis
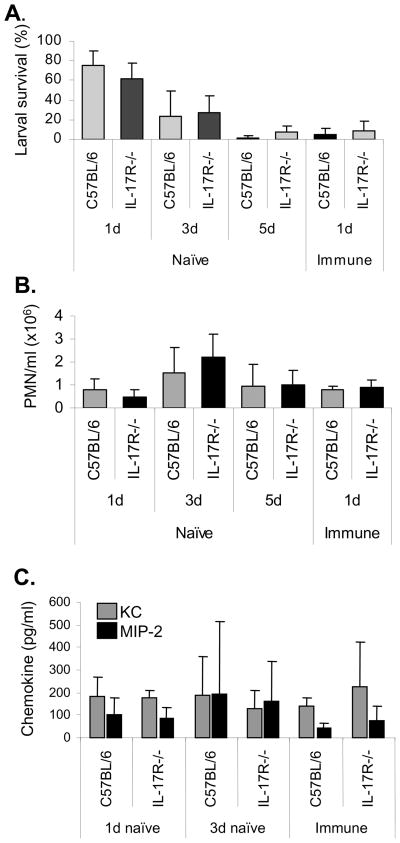
Naïve and immunized IL-17R−/− mice were infected with S. stercoralis larvae within diffusion chambers and larval survival was determined. Parasites survived in naïve C57BL/6J and IL-17R−/− mice for 1, 3 or 5 days at equal rates. Furthermore, immunized C57BL/6J and IL-17R−/− mice also killed challenge larvae at equivalent rates within one day post challenge (Figure 1A). The number of neutrophils recruited into the diffusion chambers did not differ between wild type and IL-17R−/− mice, in either naïve or immunized animals (Figure 1B). There were similar levels of MIP-2 and KC in the diffusion chamber fluids collected from naïve and immunized IL-17R−/− and C57BL/6J mice, indicating that production of CXCR2 ligands occurs independently of IL-17R during S. stercoralis infection in mice (Figure 1C). Thus, IL-17R−/− mice had intact innate and adaptive killing responses to S. stercoralis larvae and neutrophil recruitment to the parasite microenvironment in IL-17R−/− mice was not impaired.
Figure 1. IL-17R−/− and wild type mice have equivalent ability to recruit neutrophils and kill larvae.
(A) Naïve and immunized C57BL/6J and IL-17−/− mice were challenged with 50 larvae in diffusion chambers and the surviving larvae were determined at 1, 3, or 5 days post challenge. (B) Diffusion chamber fluid contents were collected and the number of neutrophils (PMN) recruited to the diffusion chambers was assessed. (C) Levels of the neutrophil chemotactic factors KC and MIP-2 were measured in the recovered diffusion chamber fluid using ELISA. Data is presented as mean ± standard deviation and data is representative of two experiments with ≥5 mice per group.
3.2 Recruitment of neutrophils to larval S. stercoralis extract
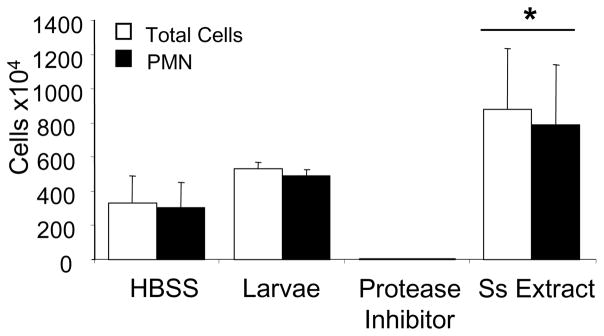
Diffusion chambers containing culture medium, live larvae, S. stercoralis soluble extract or the protease inhibitors, which were used to prepare the S. stercoralis soluble extract, were implanted in naïve mice for 24 hr. Diffusion chambers containing S. stercoralis soluble extract recruited statistically significant increased numbers of neutrophils to the interior of the diffusion chambers as compared to diffusion chambers containing only culture medium or live larvae. Interestingly, if the diffusion chambers contained only the protease inhibitors neutrophil recruitment was blocked (Figure 2). These observations indicate that S. stercoralis soluble extract is capable of recruiting neutrophils in vivo.
Figure 2. Neutrophils are recruited to S. stercoralis soluble extract in vivo.
Diffusion chambers were filled with Hanks’ Balanced Salt Solution (HBSS), 50 larvae, 300 μg of the S. stercoralis soluble extract (Ss Extract) or 300 μl of the protease inhibitor cocktail used to make the extract and implanted in naïve mice. After 1 day the diffusion chambers were removed and total cells and neutrophils (PMN) within the diffusion chambers analyzed. Data is presented as mean ± standard deviation and data is from one experiment with 5 mice per group.
Chemotaxis studies were performed in vitro to study the ability of neutrophils to migrate to the S. stercoralis soluble extract. Neutrophils migrated to S. stercoralis soluble extract in a dose-dependent manner (Table 1). Zigmond-Hirsch checkerboard assays were performed to distinguish between neutrophil chemotaxis and chemokinesis. Extract was added to the top and bottom wells of transwell plates in increasing serial dilutions. Increased cell migration to the lower wells, associated with increased extract in the lower well (going down the columns of the checkerboard assay), indicated chemotactic migration. Increased migration to the lower wells, associated with equivalent extract in the top and bottom wells (going diagonally across the checkerboard assay), indicated chemokinetic migration. The results indicate that neutrophils react to S. stercoralis soluble extract through both chemokinesis and chemotaxis (Table 1).
Table 1.
Neutrophil chemotaxis and chemokinesis to S. stercoralis soluble extract.
Zigmond-Hirsch checkerboard chemotaxis assays were performed to determine whether neutrophil movement in response to S. stercoralis soluble extract was due to chemokinesis or chemotaxis. S. stercoralis soluble extract was added to the top and bottom wells of transwell plates in increasing serial dilutions. Increases in migration of cells to the lower wells with associated increases in extract concentration in the lower well (going down the columns of the checkerboard assay) indicated chemotactic migration. Increases in cell migration to the lower wells with equivalent extract concentration in the top and bottom wells (going diagonally across the checkerboard assay) indicated chemokinetic migration (bold). Numbers presented are mean fold increase in neutrophil migration compared to control in which 3250±750 cells migrated. The data displayed is the mean ± standard error of the mean for 5 independent experiments.
| Extract in top wells (mg/ml) | |||||
|---|---|---|---|---|---|
| 0 | 0.25 | 0.5 | 1 | ||
| Extract in bottom wells (mg/ml) | 0 | 1 ± 0 | 1 ± 0.1 | 1 ± 0.2 | 3 ± 0.6 |
| 0.25 | 2 ± 0.2 | 1 ± 0.2 | 2 ± 0.3 | 8 ± 1 | |
| 0.5 | 12 ± 2 | 5 ± 1 | 8 ± 2 | 13 ± 1 | |
| 1 | 25 ± 4 | 16 ± 1 | 14 ± 2 | 19 ± 5 | |
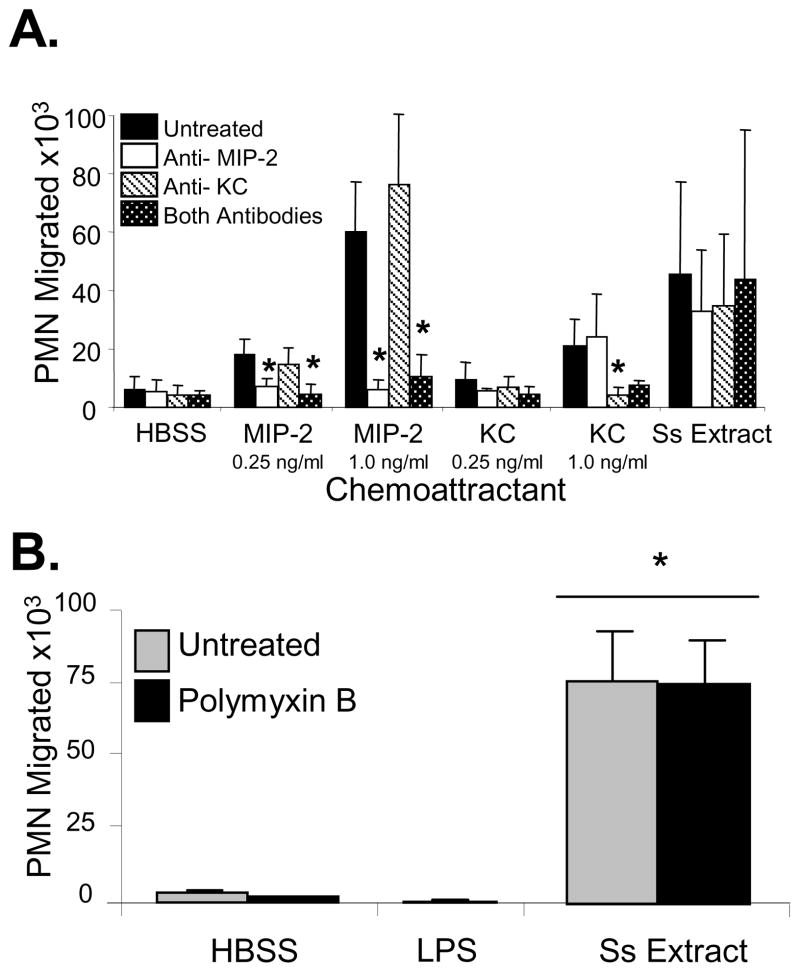
Neutrophil chemotaxis to S. stercoralis soluble extract may be directed to the molecules found within the extract or it may directed towards host-derived chemoattractants induced by the extract. To answer this question, neutrophils were incubated with S. stercoralis soluble extract for the same duration as the chemotaxis assay and it was determined that the neutrophils were induced to produce both MIP-2 (0.25ng/ml) and KC (0.25ng/ml). Stimulation of neutrophils with LPS at concentrations equal to or up to 1,000 times greater than those measured in the S. stercoralis soluble extract preparation failed to induce significant MIP-2 or KC release from neutrophils. Antibodies that block MIP-2 and KC were used to test whether neutrophil chemotaxis was directed towards the S. stercoralis soluble extract or the MIP-2 and KC induced by the extract. Anti-MIP-2 antibody blocked migration of the neutrophils to MIP-2, at the concentration of MIP-2 produced by neutrophils incubated with the S. stercoralis soluble extract and four times that concentration. KC, at the concentration produced by neutrophils incubated with S. stercoralis soluble extract, did not recruit neutrophils. KC at four times that concentration recruited neutrophils and antibody to KC blocked this neutrophil recruitment. Treatment of the S. stercoralis soluble extract with either anti-MIP-2, anti-KC antibodies or with both antibodies did not block recruitment of neutrophils to the parasite extract (Figure 3A). This indicates that neutrophil recruitment was directly to the S. stercoralis soluble extract and not to MIP-2 and KC induced by the parasite extract.
Figure 3. Neutrophils are recruited to S. stercoralis soluble extract in vitro and not to the chemokines MIP-2 and KC released by the neutrophils after culture with the S. stercoralis soluble extract and not to contaminating LPS.
(A) Chemotaxis assays were performed with MIP-2 and KC added to the bottom wells at the concentrations of 0.25 and 1.0 ng/ml. Antibodies against MIP-2 and KC were added to the wells to block the activity of the chemokines and to test if the antibodies would block the migration of the neutrophils (PMN) to the S. stercoralis soluble extract (Ss Extract). An asterisk indicates a statistically significant decrease (p<0.05) in the number of neutrophils recruited after treatment with antibody. (B) Neutrophil chemotaxis analyses were performed using transwell migration assays to 1, 10 or 100 ng/ml of LPS or S. stercoralis soluble extract (SsExtract) in HBSS with or without polymyxin B pretreatment to block LPS in the bottom well. All doses of LPS gave equivalent results and the response to100 ng/ml is reported. An asterisk indicates a statistically significant difference (p<0.05) compared to migration of cells to the control HBSS. The data presented is the mean ± standard error of five independent experiments.
The presence of bacteria or their products in the S. stercoralis soluble extract was evaluated through the following experiments. S. stercoralis larvae were treated with antibiotics to eliminate contaminating bacteria before the extract was prepared. To verify that the antibiotics were effective, the medium used to wash the larvae after they were treated with the antibiotic mixture was incubated for 24 hours. There was no evidence of any bacterial growth in the liquid medium. Antibiotic treated and untreated larvae were placed on blood agar plates for 24 hours. Few bacteria colonies were observed on plates in which antibiotic-treated larvae were placed as compared to innumerable bacterial colonies on plates with untreated larvae. Furthermore, when the S. stercoralis soluble extract was placed in diffusion chambers and implanted in mice for 24 hr, there was no sign of bacterial growth in the diffusion chambers. Finally, treatment of the S. stercoralis soluble extract with polymyxin B to block LPS did not alter the neutrophil chemotactic response. Likewise, neutrophil chemotaxis was not seen in response to LPS at the concentration of LPS found in the S. stercoralis soluble extract as determined by LAL test (0.1ng/ml), nor at doses 10 or 100 times those levels (Figure 3B). These findings suggest that neutrophils are attracted to products from the parasite and not to the few remaining naturally occurring bacteria.
3.3 Mechanism of neutrophil recruitment to S. stercoralis soluble extract
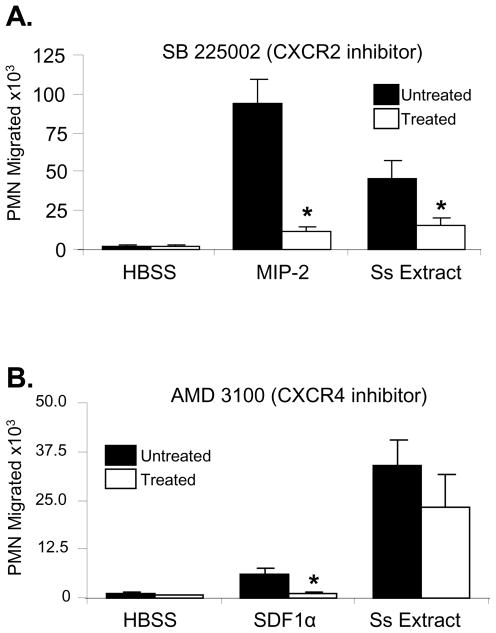
The role of the receptors CXCR2 and CXCR4 in neutrophil chemotaxis to S. stercoralis soluble extract was investigated. Treatment of neutrophils with the CXCR2-specific antagonist, SB 225002, significantly inhibited migration to S. stercoralis soluble extract and the positive control MIP-2 (Figure 4A). Treatment with AMD 3100, a CXCR4 inhibitor, significantly inhibited chemotaxis to the positive control SDF-1α [36], but not to S. stercoralis soluble extracts (Figure 4B).
Figure 4. Neutrophil chemotaxis to S. stercoralis soluble extract is mediated by CXCR2 and not CXCR4.
Neutrophils (PMN) were treated with the CXCR2 inhibitor SB 225002 (1×10−5M) (A) or the CXCR4 inhibitor AMD 3100 (4×10−8M) (B) and examined for ability to migrate towards SDF-1α (1 nM), MIP-2 (100ng/ml), and S. stercoralis soluble extract (Ss Extract - 1mg/ml). The data presented is the mean ± standard error of five independent experiments. An asterisk indicates a statistically significant difference (p<0.05) compared to migration of cells to the control HBSS.
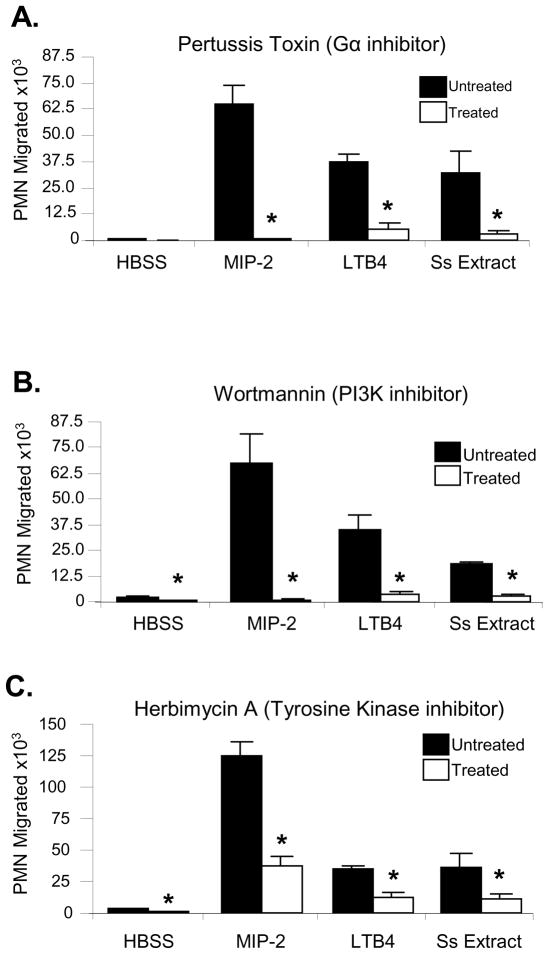
Host-mediated signaling through CXCR2 requires G protein-coupled signaling, especially via Gαi2 [37]. Treatment of neutrophils with pertussis toxin, a potent inhibitor of the Gα subunit and subsequent G protein receptor-based chemotaxis, significantly inhibited migration of cells to the S. stercoralis soluble extract and to the positive controls LTB4 [38] and MIP-2 (Figure 5A). Association of PI3K with the tyrosine kinase-containing protein Cbl is also important for neutrophil chemotaxis in response to host ligands, and inhibition of either tyrosine kinase or PI3K blocks chemotaxis [39]. Treatment of neutrophils with the PI3K inhibitor wortmannin (Figure 5B) or the tyrosine kinase inhibitor herbimycin A (Figure 5C) also caused a significant decrease in the number of neutrophils migrating to the S. stercoralis soluble extract. These data support the hypothesis that larval extract recruits neutrophils through pathways similar to those used by host-derived chemokines.
Figure 5. Neutrophil migration towards S. stercoralis soluble extract is mediated by G protein coupled receptor, PI3K, and tyrosine kinases.
Neutrophils (PMN) were pre-treated with varying concentrations of A) Pertussis toxin, a G protein coupled receptor inhibitor (1μg/ml); B) wortmannin, a PI3K inhibitor (1×10−7M); or C.) herbimycin A, a tyrosine kinase inhibitor (1×10−6M). Chemotaxis toward LTB4 (1 nM), MIP-2 (100 ng/ml), and S. stercoralis soluble extract (Ss Extract) (1mg/ml) was measured. The data presented is the mean ± standard error of a minimum of three independent experiments. An asterisk indicates a statistically significant difference (p<0.05) compared to migration of cells to the control HBSS.
4. Discussion
Protective immunity to S. stercoralis depends on neutrophils in both the innate and the adaptive immune responses. This was demonstrated in mice in which neutrophil recruitment to the parasite microenvironment was blocked because of defects in either Gαi2 signaling [2] or the expression of CXCR2 [1]. Neutrophils must be in proximity to the parasite to participate in the killing process. Therefore, the first step in neutrophil-dependent protective immunity is the recruitment of neutrophils to the parasite microenvironment. In this study we tested two hypotheses as to the mechanism of CXCR2 dependent recruitment of neutrophils to S. stercoralis larvae. Either neutrophils are recruited by an IL-17 dependent pathway or recruitment depends on CXCR2 recognition of parasite associated factors.
IL-17 is a potent upstream mediator of neutrophil chemotaxis, specifically through induction of MIP-2 and KC production following stimulation of the IL-17R [40]. IL-17 is a major contributor to neutrophil recruitment in inflammatory and infectious models [11–12, 16]. In the present study neutrophil recruitment to the parasite microenvironment in IL-17R−/− mice was equivalent to wild type mice, in either naïve or immunized mice. This suggests that, unlike many other infectious agents [11–12, 16], IL-17R is not required for neutrophil recruitment to larval S. stercoralis. Furthermore, IL-17R−/− mice had completely intact capacities to kill S. stercoralis larvae in naïve and immunized mice. IL-17A has additional functions aside from neutrophil recruitment, including cytokine production and induction of nitric oxide synthase [8, 41]. These alternative IL-17A functions also were not required for innate and adaptive immunity to murine S. stercoralis infections. Protective immunity to S. stercoralis infection is Th2 dependent [42]. The observation that IL-17R signaling was not required for immunity to S. stercoralis in mice supports the interpretation that there is a separate Th17 type of immune response that functions independently from Th1 and Th2 responses [43].
The alternative hypothesis tested in this study was that neutrophils are recruited directly by the parasite through CXCR2, without the participation of other cells or factors from the host. S. stercoralis soluble extract was shown to attract neutrophils in vivo after implantation in diffusion chambers in naïve mice. This observation was confirmed in vitro using Zigmond-Hirsch checkerboard assays which indicated that neutrophils reacted through both chemokinesis and chemotaxis to the S. stercoralis soluble extract.
One of the confounding observations from the studies utilizing IL-17R−/− mice was the presence of MIP-2 and KC in the fluid recovered from the diffusion chambers. Since production of these chemokines was not induced by IL-17, we tested the hypothesis that MIP-2 and KC were produced directly by neutrophils after exposure to the parasite extract. Stimulation of naïve neutrophils with S. stercoralis extract induced the release of both MIP-2 and KC. Mouse neutrophils have been reported to produce both MIP-2 and KC under other conditions [44]. Although the S. stercoralis soluble extract did induce production of both MIP-2 and KC, recruitment of neutrophils was not dependent on these molecules, since blocking MIP-2 and/or KC with antibodies did not block recruitment of neutrophils to the parasite extract. The ability of the neutrophils to amplify the response to the S. stercoralis antigens via release of MIP-2 and KC, suggests that the larval products alone are likely sufficient to evoke a maximal neutrophil recruitment response without support from other host factors.
Efforts were made to eliminate bacteria from the larvae before the extract was prepared. Antibiotics were carefully selected and the apparent absence of bacteria in the larval wash fluids and in the S. stercoralis soluble extract implanted in diffusion chambers in vivo suggest that these efforts were successful. Furthermore, treatment of the extract with polymyxin B to block LPS did not significantly alter the neutrophil chemotactic response and neutrophil chemotaxis was not seen in response to LPS at the concentration of LPS found in the soluble extract nor at doses 10 or 100 times those levels. Previous studies on immunity to S. stercoralis in C3H/HeJ mice have shown that TLR4 is not required for recruitment of neutrophils in the innate and adaptive immune responses [6]. The observation that TLR4 was not required for recruitment of neutrophils in naïve or immunized mice was confirmed in studies using TLR4−/− mice (Personal Observation), thereby demonstrating that LPS signalling was not required for neutrophil recruitment to S. stercoralis larvae in vivo. Similarly, studies of Nippostrongylus brasiliensis infections of mice concluded that neutrophil recruitment to lymph nodes was not impaired if bacteria were eliminated [45]. Thus, it appears that the chemoattractant in the S. stercoralis soluble extract is parasite derived. The alternative hypothesis is that the chemoattractant is derived from antibiotic resistant bacteria that are natural components of the parasite’s fauna. This would be similar to neutrophil recruitment to the intracellular symbiotic bacterium Wolbachia found in filarial worms [46]. Therefore, neutrophils are directly recruited to S. stercoralis larvae, either to the parasite itself or to bacteria naturally residing with the worms.
Blocking CXCR2 inhibited neutrophil migration to the S. stercoralis soluble extracts, whereas blocking CXCR4 had no effect. Likewise, CXCR2, but not CCR1 expression is essential for neutrophil recruitment to the cornea in mice stimulated with filarial worm extract [47] whereas B. malayi asparaginyl-transfer RNA synthetase activates both CXCR1 and CXCR2 on human neutrophils [26]. Eosinophil migration to S. stercoralis extract utilizes multiple receptors, including both CXCR2 and CXCR4 [27]. Eosinophils and neutrophils are both capable of killing S. stercoralis larvae in the innate immune response [1], while the transition from innate to adaptive immunity depends on the presence of eosinophils [42]. Eosinophils may have developed multiple receptors capable of recognizing the parasite to guarantee the crucial transition from innate to adaptive immunity. Alternatively, the response of neutrophils to parasite extract through CXCR2 may be highly efficient, whereas eosinophils may require multiple receptors to accomplish the same level of cell recruitment.
Having demonstrated that neutrophils respond directly to S. stercoralis soluble extract through CXCR2, the next question was whether exposure of CXCR2 on neutrophils to S. stercoralis soluble extract resulted in the same intracellular signaling pathway observed after CXCR2 is exposed to host ligands. Neutrophil recruitment to the parasite microenvironment in vivo depends on Gαi2 [2]. The role of Gα proteins in chemotaxis to S. stercoralis soluble extracts in vitro was confirmed by inhibiting neutrophil chemotaxis with pertussis toxin. Treatment of the parasite extract with pertussis toxin also inhibited migration of eosinophils [27]. G protein-dependent chemotaxis of neutrophils has also been reported for A. suum-derived products [25] and B. malayi asparaginyl-transfer RNA synthetase [26].
PI3K signaling is important for neutrophil chemotaxis to TNFα, G-CSF, and GM-CSF [48] and affects colocalization of Akt and F-actin during neutrophil morphological responses to stimuli [49]. During CXCR2 signaling in response to the human neutrophil chemotactic factor IL-8, PI3K associates with the tyrosine kinase containing protein Cbl, and inhibition of either tyrosine kinases or PI3K blocks chemotaxis [39]. The observation that neutrophil chemotaxis, in response to S. stercoralis soluble extract, is inhibited by the PI3K inhibitor wortmannin as well as the tyrosine kinase inhibitor herbimycin A strongly suggests that the parasite extract stimulates neutrophils through similar pathways to those used by host-derived chemokines. Similarly, blocking PI3K, tyrosine kinase, p38 and p44/42 also inhibited eosinophil chemotaxis to parasite extract [27].
Products from many parasites attract neutrophils [21] while other parasite derived products inhibit neutrophil recruitment [50]. It has been hypothesized that tissue migrating larvae secrete compounds that promote inflammation, and in particular neutrophil-related edema, to increase tissue permeability thus favoring faster and more successful migration through host tissues [23]. Although it has been demonstrated that neutrophils are effective killers of S. stercoralis larvae [1] the parasite may still recruit these cells as an immune evasion mechanism. The parasite may recruit neutrophils to permeabilize the tissue and thereby facilitate active migration of the larvae away from the site of inflammation and the lethal effects associated with neutrophils.
In conclusion, CXCR2 dependent recruitment of neutrophils to larvae of S. stercoralis occurs independently of IL-17R. S. stercoralis soluble extract is capable of directly recruiting neutrophils through CXCR2, using signaling pathways similar to those used by host chemokines. In addition, the extract also induced neutrophils to release MIP-2 and KC, which could further enhance the recruitment of neutrophils. The finding that neutrophils produce increased amounts of neutrophil-recruiting chemokines following exposure to S. stercoralis soluble extract suggests an efficiently orchestrated system whereby a primary stimulus from a parasite causes an autocrine amplification of cell recruitment through release of host-derived chemokines. The efficiency of this recruitment mechanism is further highlighted by the observation that the CXCR2 receptor has the ability to respond to both parasite- and host-derived factors resulting in highly efficient neutrophil recruitment and thus control of infection with S. stercoralis.
Acknowledgments
We thank Louis Stein, Udaikumar Padigel, Laura Kerepesi and Sandra Bonne-Année for their expert and enthusiastic technical support and guidance. This work was supported in part by NIH grants 1R56 AI076345, RO1 AI47189, RO1 AI 22662, RO1 AI 50668, RO1 AI082548 and P40 RR02512. Fellowship support was also provided in part by the Measey Foundation and by a Dubbs Scholar Fellowship Award from Thomas Jefferson University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect Immun. 2006;74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padigel UM, Stein L, Redding K, Lee JJ, Nolan TJ, Schad GA, Birnbaumer L, Abraham D. Signaling through Galphai2 protein is required for recruitment of neutrophils for antibody-mediated elimination of larval Strongyloides stercoralis in mice. J Leukoc Biol. 2007;81:1120–1126. doi: 10.1189/jlb.1106695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerepesi LA, Hess JA, Nolan TJ, Schad GA, Abraham D. Complement component C3 is required for protective innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2006;176:4315–4322. doi: 10.4049/jimmunol.176.7.4315. [DOI] [PubMed] [Google Scholar]
- 4.Rotman HL, Schnyder-Candrian S, Scott P, Nolan TJ, Schad GA, Abraham D. IL-12 eliminates the Th-2 dependent protective immune response of mice to larval Strongyloides stercoralis. Parasite Immunol. 1997;19:29–39. doi: 10.1046/j.1365-3024.1997.d01-142.x. [DOI] [PubMed] [Google Scholar]
- 5.Herbert DR, Nolan TJ, Schad GA, Abraham D. The role of B cells in immunity against larval Strongyloides stercoralis in mice. Parasite Immunol. 2002;24:95–101. doi: 10.1046/j.0141-9838.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- 6.Kerepesi LA, Hess JA, Leon O, Nolan TJ, Schad GA, Abraham D. Toll-like receptor 4 (TLR4) is required for protective immunity to larval Strongyloides stercoralis in mice. Microbes Infect. 2007;9:28–34. doi: 10.1016/j.micinf.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 8.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 12.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 13.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008;83:401–408. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
- 15.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freitas A, Alves-Filho JC, Victoni T, Secher T, Lemos HP, Sonego F, Cunha FQ, Ryffel B. IL-17 receptor signaling is required to control polymicrobial sepsis. J Immunol. 2009;182:7846–7854. doi: 10.4049/jimmunol.0803039. [DOI] [PubMed] [Google Scholar]
- 18.Al-Qaoud KM, Pearlman E, Hartung T, Klukowski J, Fleischer B, Hoerauf A. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int Immunol. 2000;12:899–908. doi: 10.1093/intimm/12.6.899. [DOI] [PubMed] [Google Scholar]
- 19.Cardona AE, Gonzalez PA, Teale JM. CC chemokines mediate leukocyte trafficking into the central nervous system during murine neurocysticercosis: role of gamma delta T cells in amplification of the host immune response. Infect Immun. 2003;71:2634–2642. doi: 10.1128/IAI.71.5.2634-2642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park MK, Hoffmann KF, Cheever AW, Amichay D, Wynn TA, Farber JM. Patterns of chemokine expression in models of Schistosoma mansoni inflammation and infection reveal relationships between type 1 and type 2 responses and chemokines in vivo. Infect Immun. 2001;69:6755–6768. doi: 10.1128/IAI.69.11.6755-6768.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horii Y, Owhashi M, Fujita K, Nakanishi H, Ishii A. A comparative study on eosinophil and neutrophil chemotactic activities of various helminth parasites. Parasitol Res. 1988;75:76–78. doi: 10.1007/BF00931196. [DOI] [PubMed] [Google Scholar]
- 22.Owhashi M, Futaki S, Kitagawa K, Horii Y, Maruyama H, Hayashi H, Nawa Y. Molecular cloning and characterization of a novel neutrophil chemotactic factor from a filarial parasite. Mol Immunol. 1993;30:1315–1320. doi: 10.1016/0161-5890(93)90048-g. [DOI] [PubMed] [Google Scholar]
- 23.Bower MA, Constant SL, Mendez S. Necator americanus: the Na-ASP-2 protein secreted by the infective larvae induces neutrophil recruitment in vivo and in vitro. Exp Parasitol. 2008;118:569–575. doi: 10.1016/j.exppara.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio de Kromer MT, Kromer M, Luersen K, Brattig NW. Detection of a chemotactic factor for neutrophils in extracts of female Onchocerca volvulus. Acta Trop. 1998;71:45–56. doi: 10.1016/s0001-706x(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 25.Falcone FH, Rossi AG, Sharkey R, Brown AP, Pritchard DI, Maizels RM. Ascaris suum-derived products induce human neutrophil activation via a G protein-coupled receptor that interacts with the interleukin-8 receptor pathway. Infect Immun. 2001;69:4007–4018. doi: 10.1128/IAI.69.6.4007-4018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez BL, Howard OM, Dong HF, Edamatsu T, Gao P, Hartlein M, Kron M. Brugia malayi asparaginyl-transfer RNA synthetase induces chemotaxis of human leukocytes and activates G-protein-coupled receptors CXCR1 and CXCR2. J Infect Dis. 2006;193:1164–1171. doi: 10.1086/501369. [DOI] [PubMed] [Google Scholar]
- 27.Stein LH, Redding KM, Lee JJ, Nolan TJ, Schad GA, Lok JB, Abraham D. Eosinophils utilize multiple chemokine receptors for chemotaxis to the parasitic nematode Strongyloides stercoralis. J Innate Immun. 2009;1:618–630. doi: 10.1159/000233235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham D, Rotman HL, Haberstroh HF, Yutanawiboonchai W, Brigandi RA, Leon O, Nolan TJ, Schad GA. Strongyloides stercoralis: protective immunity to third-stage larvae in BALB/cByJ mice. Exp Parasitol. 1995;80:297–307. doi: 10.1006/expr.1995.1036. [DOI] [PubMed] [Google Scholar]
- 29.Herbert DR, Nolan TJ, Schad GA, Lustigman S, Abraham D. Immunoaffinity-isolated antigens induce protective immunity against larval Strongyloides stercoralis in mice. Exp Parasitol. 2002;100:112–120. doi: 10.1016/S0014-4894(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 30.Hart PH, Spencer LK, Nulsen MF, McDonald PJ, Finlay-Jones JJ. Neutrophil activity in abscess-bearing mice: comparative studies with neutrophils isolated from peripheral blood, elicited peritoneal exudates, and abscesses. Infect Immun. 1986;51:936–941. doi: 10.1128/iai.51.3.936-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol. 2007;81:786–792. doi: 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukazawa H, Li PM, Yamamoto C, Murakami Y, Mizuno S, Uehara Y. Specific inhibition of cytoplasmic protein tyrosine kinases by herbimycin A in vitro. Biochem Pharmacol. 1991;42:1661–1671. doi: 10.1016/0006-2952(91)90500-5. [DOI] [PubMed] [Google Scholar]
- 34.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 35.Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, Moore JP. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 36.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- 37.Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 38.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 39.Lane HC, Anand AR, Ganju RK. Cbl and Akt regulate CXCL8-induced and CXCR1- and CXCR2-mediated chemotaxis. Int Immunol. 2006;18:1315–1325. doi: 10.1093/intimm/dxl064. [DOI] [PubMed] [Google Scholar]
- 40.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Miljkovic D, Trajkovic V. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 2004;15:21–32. doi: 10.1016/j.cytogfr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Herbert DR, Lee JJ, Lee NA, Nolan TJ, Schad GA, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2000;165:4544–4551. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- 43.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong DA, Major JA, Chudyk A, Hamilton TA. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol. 2004;75:641–648. doi: 10.1189/jlb.0803370. [DOI] [PubMed] [Google Scholar]
- 45.Pesce JT, Liu Z, Hamed H, Alem F, Whitmire J, Lin H, Liu Q, Urban JF, Jr, Gause WC. Neutrophils clear bacteria associated with parasitic nematodes augmenting the development of an effective Th2-type response. J Immunol. 2008;180:464–474. doi: 10.4049/jimmunol.180.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner JD, Langley RS, Johnston KL, Gentil K, Ford L, Wu B, Graham M, Sharpley F, Slatko B, Pearlman E, Taylor MJ. Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J Biol Chem. 2009;284:22364–22378. doi: 10.1074/jbc.M901528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall LR, Diaconu E, Patel R, Pearlman E. CXC chemokine receptor 2 but not C-C chemokine receptor 1 expression is essential for neutrophil recruitment to the cornea in helminth-mediated keratitis (river blindness) J Immunol. 2001;166:4035–4041. doi: 10.4049/jimmunol.166.6.4035. [DOI] [PubMed] [Google Scholar]
- 48.Kamata N, Kutsuna H, Hato F, Kato T, Oshitani N, Arakawa T, Kitagawa S. Activation of human neutrophils by granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha: role of phosphatidylinositol 3-kinase. Int J Hematol. 2004;80:421–427. doi: 10.1532/ijh97.04122. [DOI] [PubMed] [Google Scholar]
- 49.Hannigan M, Zhan L, Li Z, Ai Y, Wu D, Huang C-K. Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. PNAS. 2002;99:3603–3608. doi: 10.1073/pnas.052010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith P, Fallon RE, Mangan NE, Walsh CM, Saraiva M, Sayers JR, McKenzie AN, Alcami A, Fallon PG. Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J Exp Med. 2005;202:1319–1325. doi: 10.1084/jem.20050955. [DOI] [PMC free article] [PubMed] [Google Scholar]