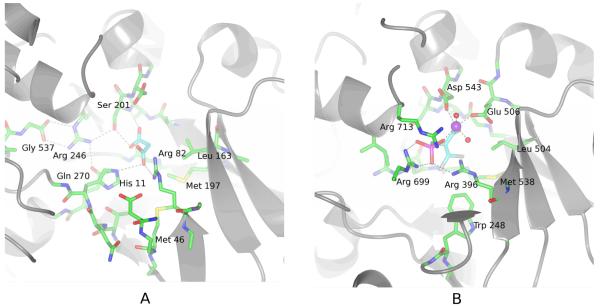
FIGURE 2.
A Malonate binds to the active-site of PepcA via interactions with His11, Arg82, Ser201 and Gln270. Several strands of the β-barrel are visible in the lower right of the figure. The C-terminal Gly537 positions Arg246, which in turn positions Ser201 and Gln270 (visible under His11) in the active-site.
B An inhibitory PEP analog binds to the active-site of the E. coli Pepc via interactions with Arg396, Arg699, Arg713 and a Mn++ ion (shown in purple). In this view into the active-site, the carboxylate of the inhibitor is directly below the Mn++ ion. His138 and Arg587 (corresponding to C. perfringens His11 and Arg246) are missing from the active-site in this T-state structure.