Abstract
The structure of the potent selective mGlu5 ligand, SP203 (1, 3-fluoro-5-[[2-(fluoromethyl)thiazol-4-yl]ethynyl]benzonitrile), was modified by replacing the 2-fluoromethyl substituent with an amino or halo substituent and by variation of substituents in the distal aromatic ring to provide a series of new high-affinity mGlu5 ligands. In this series, among the most potent ligands obtained, the 2-chloro-thiazoles 7a and 7b and the 2-fluorothiazole 10b showed sub-nanomolar mGlu5 affinity. 10b also displayed >10,000-fold selectivity over all other metabotropic receptor subtypes plus a wide range of other receptors and binding sites. The 2-fluorothiazoles 10a and 10b were labeled using [18F]fluoride ion (t1/2 = 109.7 min) in moderately high radiochemical yield to provide potential radioligands that may resist troublesome radiodefluorination during the imaging of brain mGlu5 with position emission tomography. The iodo compound 9b has nanomolar affinity for mGlu5 and may also serve as a lead to a potential 123I-labeled ligand for imaging brain mGlu5 with single photon emission computed tomography.
Introduction
Glutamate, the predominant excitatory neurotransmitter in the central nervous system, promotes fast-synaptic transmission and synaptic plasticity by activating any one of three ion channel ionotropic glutamate receptors, namely NMDA, AMPA or kainate receptors.1 Glutamate also has a modulatory influence on neuronal excitability by activating metabotropic glutamate receptors (mGluRs), which are members of the G protein-coupled seven-membrane-domain class of receptors.2 Eight mGluR subtypes have been identified and classified according to their sequence homology, signal transduction, and pharmacology.3 Among these subtypes, the metabotropic glutamate receptor subtype 5 (mGlu5) plays key roles in a variety of normal brain functions, and is also putatively involved in several neuropsychiatric disorders. Activation of mGlu5 stimulates phospholipase C, and this results in phosphoinositide hydrolysis and an increase in intracellular Ca2+ levels.3,4
Modulation of mGlu5 receptors has potential for the treatment of schizophrenia5, Fragile X syndrome6 and Alzheimer’s disease7. Further evidence supports the use of antagonists or modulators of mGlu5 in the treatment of anxiety8, depression9 and pain10. mGlu5 may also play an important role in drug-related behaviors, particularly drug abuse,11 drug addiction12 and alcohol withdrawal.13 Hence, mGlu5 antagonists may turn out to be useful therapeutics for a variety of central nervous system disorders.
We previously reported the discovery of a very potent and highly selective ligand for mGlu5 which we dubbed SP203 (1, 3-fluoro-5-[[2-(fluoromethyl)thiazol-4-yl]ethynyl] benzonitrile; Chart 1).14 [18F]1, which has a fluorine-18 (t1/2 = 109.7 min) label in the 2-fluoromethyl position, is an effective radioligand for imaging brain mGlu5 receptors in human subjects in vivo without major complications arising from radiometabolites.15 Only a low amount of radioactivity appears in skeleton and that radioactivity is associated with marrow rather than bone, thereby showing this radioactivity is not present as [18F]fluoride ion and that metabolic radiodefluorination is virtually absent.16 [18F]1 is significantly easier to prepare than previous 18F-labeled mGlu5 radioligands, such as the structurally related [18F]F 3-methyl-5-[(2-methyl-1,3-thiazol-4-yl)ethynyl]benzonitrile ([18F]F-MTEB17, [18F]2; Chart 1), and has broad potential utility, ranging from receptor occupancy studies preceding drug clinical trials to studies of mGlu5 receptor expression in neuropsychiatric patients.
Chart 1.
Structures of some mGlu5 ligands and derived PET radioligands.
In rat and in monkey, [18F]1 also shows acceptable brain entry and a sizeable receptor-specific signal.14 However, unlike in human subjects, radiodefluorination occurs,18 leading to steady accumulation of fluorine-18 in bones and skull. This radioactivity confounds measurement of rat or monkey brain mGlu5 receptors with this radioligand and positron emission tomography (PET).
Although metabolic defluorination often occurs from an aliphatic carbon,19 this is relatively rare from an aryl carbon. The thiazole moiety is strongly aromatic. We therefore considered that a 2-fluoro-thiazole moiety might resist defluorination in vivo, unlike the 2-fluoromethylthiazole moiety of 1 when in monkey or rat. One report suggests that fluorination on a thiazole ring might increase stability towards ring metabolism.20
Here we report the synthesis of a series of compounds related to 1 in which the 2-fluoromethylthiazole moiety in 1 was replaced with a halogen, including fluorine, or with an amino group. The substitution pattern in the distal phenyl ring was also varied. We report the affinities of these new ligands towards mGlu5 receptors. Finally, we describe the labeling of some of the novel 2-fluorothiazoles to produce candidate 18F-labeled PET mGlu5 radioligands.
Results and Discussion
Our primary goal was to produce high-affinity mGlu5 ligands that would be suitable for development as 18F-labeled ligands for imaging brain mGlu5 in animals without being susceptible to troublesome radiodefluorination. We considered that this aim might be achieved by modifying [18F]1 so that the fluorine-18 atom was directly bound to the thiazole ring at the 2-position. Computation indicated that this modification, the replacement of a fluoromethyl group with a single fluorine atom, would not greatly alter lipophilicity. For example, the cLogD value of the 2-fluoro compound 10b is 3.58 and similar to that of the 2-fluoromethyl analogue 1 (cLogD = 3.31). These values are within the range usually considered desirable for a prospective brain imaging agent.21 In accord with this guideline, [18F]1, in rodents and primates, shows good penetration of the blood-brain barrier, has high peak uptake in brain and shows relatively low non-specific binding.14
Chemistry
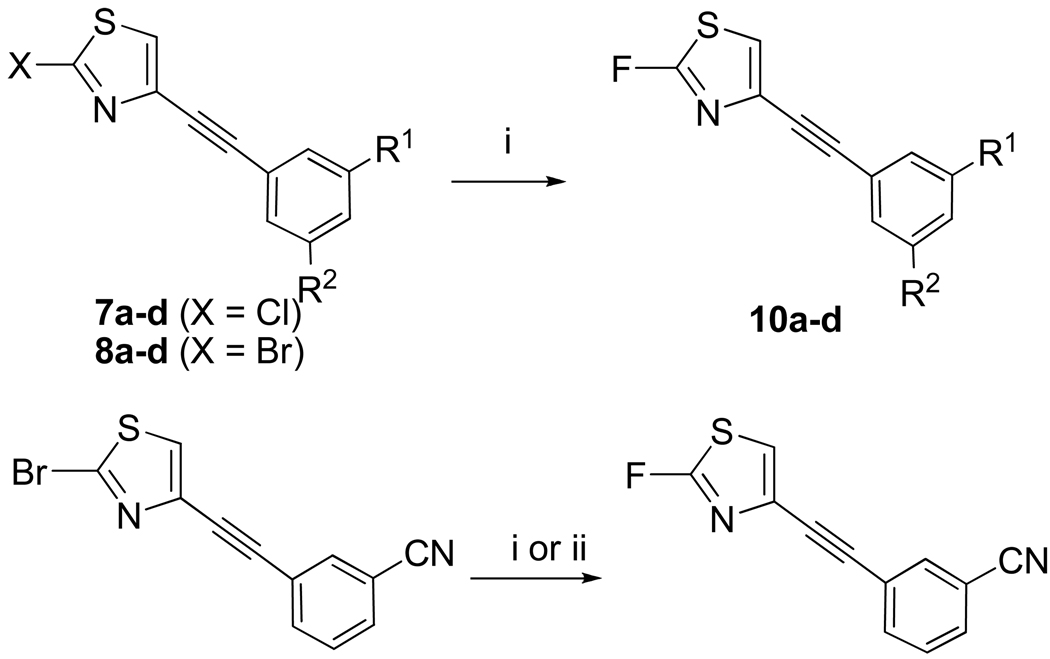
The 2-amino compounds (6a–d, Scheme 1) were prepared by Sonogashira cross-coupling of the aminothiazole 4 with the appropriate haloarenes. Yields were low from bromoarenes (< 30%), but moderate to high from iodoarenes (60 to ~ 90%). Therefore, iodoarenes were used in all coupling reactions. The amines 6a–d were then halogenated regioselectively22 with CuX (X = Cl, Br, I) in the presence of n-butyl nitrite to give the 2-chloro (7a–d), 2-bromo (8a–d) and 2-iodo derivatives (9a–d) (Table 1). Yields increased with halogen size. Thus, yields for chlorination were low and typically in the range 15–25%. Yields for bromination and iodination were usually over 50% (Table 1).
Scheme 1a.
aReagents and conditions: i) CuCl, n-BuNo2; 30–67% ii) PdPPh4, Arl, Et3N, TBA, DME, 62–89%; iii) CuX, n-BuNO2 (X = Br or I), 32–83%, See Table 1 for identify of R1 and R2.
Table 1.
Ligand yields, lipophilicities and mGlu5 affinities.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | X | R1 | R2 | Yield (%) | cLogDa | Ki (nM)b |
| 1 | FCH2 | F | CN | NA | 3.66 (2.18)14 | 0.03614 |
| 2 | Me | F | CN | NA | 3.42 | 0.08 ± 0.0217 |
| 6a | NH2 | H | CN | 71 | 2.52 | 0.90 ± 0.19 |
| 6b | NH2 | F | CN | 62 | 3.06 | 0.25 ± 0.04 |
| 6c | NH2 | H | CH2CN | 86 | 2.49 | 5.90 ± 0.95 |
| 6d | NH2 | H | OMe | 89 | 2.72 | 18.6 ± 0.38 |
| 7a | Cl | H | CN | 56c | 2.88 | 0.27 ± 0.04 |
| 7b | Cl | F | CN | 64c | 3.41 | 0.40 ± 0.09 |
| 7c | Cl | H | CH2CN | 67c | 2.99 | 3.10 ± 0.46 |
| 7d | Cl | H | OMe | 48c | 3.44 | 8.10 ±1.36 |
| 8a | Br | H | CN | 32d | 3.38 | 3.70± 0.25 |
| 8b | Br | F | CN | 48 | 3.95 | 0.95 ± 0.10 |
| 8c | Br | H | CH2CN | 61 | 3.48 | 3.30 ± 0.53 |
| 8d | Br | H | OMe | 59 | 3.86 | 22.3 ± 0.50 |
| 9a | I | H | CN | 83 | 3.56 | 4.20 ± 0.50 |
| 9b | I | F | CN | 71 | 4.21 | 1.30 ± 0.23 |
| 9c | I | H | CH2CN | 62 | 3.63 | 23.3 ± 3.53 |
| 9d | I | H | OMe | 50 | 3.96 | 132.9 ± 2.54 |
| 10a | F | H | CN | 24, 75e | 3.12 | 1.60 ± 0.11* |
| 10b | F | F | CN | 35, 72e | 3.58 | 0.28 ± 0.05* |
| 10c | F | H | CH2CN | 43, 78 e | 3.18 | 3.90 ± 0.56 |
| 10d | F | H | OMe | 33, 65e | 3.56 | 22.7 ± 0.28 |
| 10e | F | H | H | 46, 78f | 3.38 | 122 ± 3.3 |
The cLogD was calculated with Pallas 3.4.0 software (Compudrug), except value in parenthesis which is a measured value.
Values were determined in triplicate from rat brain homogenates, except those marked
(n = 6).
Obtained by coupling from compound 5.
Value reported in reference 22.
From the 2-bromo and 2-chloro-thiazoles, respectively (each treated with KF in DMSO at 130 °C).
From the 2-bromo- and 2-chloro-thiazoles, respectively.24
NA = Not applicable.
Because the 2-chloro derivatives 7a–d could only be obtained in small amounts and their purifications were tedious, we decided to explore an alternative synthesis to improve their yields. We considered that the use of the 2-chlorothiazole synthon 5 (Scheme 1) in a Sonogashira cross-coupling reaction might lead to higher yields. Synthon 5 was prepared by reaction of the easily accessible compound 4 with CuCl and n-butyl nitrite and submitted to Sonogoshira cross-coupling. Yields of the 2-chloro compounds 7a–d from cross-coupling reactions with 5 were greatly improved (48–67%). The purifications of the 2-chloro products were also easier than for the amino compounds 6a–d. Noticeably, during the cross-coupling reactions, no coupling was observed at position 2 of the 2-chloro-thiazole acetylene 5.
With the 2-chloro, 2-bromo, and 2-iodo compounds available, we aimed to prepare the corresponding 2-fluorothiazoles. The use of electrophilic reagents, either Accufluor23 or Selectfluor24, gave almost negligible yields (1–5%) of the desired fluorinated products. Therefore, we next investigated direct nucleophilic substitution in the halo precursors with potassium fluoride. We recently reported a procedure for the rapid fluorination of 2-chloro and 2-bromothiazole derivatives under microwave irradiation25 and we looked to apply this method to obtain the target 2-fluoro ligands. By treating bromides 8a–d with a potassium fluoride-Kryptofix 2.2.2 (K 2.2.2) complex in DMSO under microwave irradiation (30 W, 130 °C, 8–15 min), the respective 2-fluoro compounds 10a–d were obtained in useful 24–43% yields (Table 1).
When the 2-chloro-precursors 7a–d were treated similarly (KF-K 2.2.2, DMSO, MW, 130 °C, 10–15 min), the fluorination yields were dramatically improved, reaching 78% in the case of 10c. By contrast, only negligible product was detected when the iodo precursor 9a was submitted to nucleophilic fluorination (yield, < 5%). These results accord with our previous observations on leaving group ability in the nucelophilic fluorination of simpler 2-halo-thiazoles.25
For the synthesis of 10a, we also experimented with the use of a KF-AgF with the bromo precursor 8a under microwave irradiation (Scheme 2). After eight minutes irradiation, the yield of 10a reached 62%, as measured with HPLC. However, this fluorination method was less reproducible and we also observed the reduction of DMSO into the irritant and volatile dimethyl sulfide.
Scheme 2.
aReactions and conditions. i) KF, K2.2.2 DMSO or MeCN, MW, 130 °C, 10 min, 24–78%; ii) KF-AgF, DMSO, MW, 130 °C, 8 min, 62%. See Table 1 for identities of R1 and R2.
The 2-chloro and 2-bromo-(4-phenylacetylene)thiazoles 7e and 8e were also subjected to fluorination with KF in DMSO, as reported previously, to give the fluoro derivative 10e in 78 and 46% yields, respectively.25
Pharmacology
The affinity of each new ligand for rat brain tissue mGlu5 was determined in a binding assay with [3H]MPEP ([3H]6-methyl-2-phenylethenylpyridine) as reference radioligand. The majority of the prepared 2-halo-thiazoles exhibited high affinity with Ki values in the nanomolar range. In general, the 2-iodo compounds had lower affinity than their bromo, chloro or fluoro analogs. Unexpectedly, the amino compound intermediates 6a–d also exhibited high to very high affinity for mGlu5. The substituent pattern in the distal aryl group strongly influenced affinity for mGlu5. Thus, the combination of fluoro and nitrile substituents in meta position to the alkynyl group, as also exists in 1, generally gave the highest affinity among each subset of 2-substituted thiazoles. Removal of the meta-fluoro substituent generally had little effect. A single meta cyanomethyl or methoxy group reduced affinity appreciably, especially a methoxy group.
The high-affinity 2-fluoro ligand 10b was screened for affinity at other mGluR subtypes (mGlu1, mGlu2, mGlu4, mGlu6, mGlu7, and mGlu8) and a panel of other brain receptors, binding sites and ion channels as listed under Experimental. In all cases, the affinity of 10b was found to >10,000 nM. Therefore, replacement of the 2-fluoromethyl group in 1 with a fluoro substituent retained excellent mGlu5 selectivity. Such excellent selectivity is highly desirable in a prospective PET radioligand.
Currently, no effective radioligand exists for imaging brain mGlu5 with SPECT (single photon emission computed tomography). Interestingly, the iodo compounds 9a and 9b show mGlu5 affinity in the nanomolar range and are possibly suitable for labeling with iodine-123 to provide candidate radioligands for imaging brain mGlu5 with single photon emission computed tomography (SPECT). The highest affinity iodinated ligand 9b has affinity that almost matches that of PET radioligands, such as [11C]M-MTEB ([11C]3-methyl-5-[(2-methyl-1,3-thiazol-4-yl)ethynyl] benzonitrile) and [18F]F-PEB ([18F]3-fluoro-5-[2-methyl-1,3-thiazol-4-yl)ethynyl] benzonitrile), that have been shown to give strong receptor-specific signals in monkeys.17 The computed lipophilicity of 9b appears quite high for a prospective SPECT radioligand. However, this computed value, and also those for the other ligands may be considerably over-estimated, since the true value for ligand 1 is much lower than for its computed value (Table 1).
Radiochemistry
We previously reported reaction conditions for the preparation of [18F]2-fluorothiazole derivatives with cyclotron-produced no-carrier-added (NCA) [18F]fluoride ion under thermal and microwave heating conditions.25 We established that simple 2-chlorothiazole structures label efficiently with [18F]fluoride ion in the presence of K+-K 2.2.2 in DMSO or MeCN. The radiochemical yield was slightly higher for 2-chlorothiazoles than for 2-bromothiazoles. These conditions were applied successfully for the radiosynthesis of [18F]10a from the chloro compound 7a. Thus, at 130 °C in DMSO under inert atmosphere and with brief microwave heating (90 W, 150 °C, 10 min), [18F]10a was obtained in 36% decay-corrected radiochemical yields (RCY) and with a measured specific activity of 2.1 Ci/µmol at the end of synthesis.
Because of its high affinity, we then selected 10b as the major target for labeling with fluorine-18. The chloro compound 7b was selected as precursor for radiofluorination. The conversion of 7b into [18F]10b was initially attempted using the reaction conditions mentioned above. Reaction for 10 min at 130 °C gave decay-corrected RCYs of 26% (Scheme 3). Unexpectedly, under these conditions some exchange of the aryl fluoro group with [18F]fluoride ion was also observed and [18F]7b was detected as a by-product of radiofluorination. Typically, with the use of anhydrous DMSO at 130 °C, the ratio of [18F]10b to [18F]7b was 45 to 55. The use of anhydrous acetonitrile under similar conditions (K 2.2.2 - K2CO3; MW, 130 °C), switched the major labeled product to [18F]10b ([18F]10b: [18F]7b; 82: 18). In acetonitrile, when the temperature of reaction was lowered to 110 °C, the incorporation of [18F]fluoride ion was up to 48%, with the ratio of [18F]10b to [18F]7b being 92: 8.
Scheme 3a.
aReactions and conditions. i) [18F]FK, K 2.2.2., DMSO or MeCN, MW, 130 °C, 10 min, 26 % RCY; ii) [18F]FK, K 2.2.2., MeCN, MW, 110 °C, 10 min, 26–48 % RCY.
The radioligands were easily purified by HPLC in high chemical purity (> 95%) and high radiochemical purity (> 99%). The specific activity of the target compound [18F]10b, 0.5 Ci/µmol at the end of synthesis (about 150 min from the end of radionuclide production) was somewhat lower than for [18F]10a, probably because of the 18F for F exchange occurring in precursor 7b, Nonetheless this new radioligand now merits further evaluation with PET in animals to assess its resistance to radiodefluorination and efficacy for imaging brain mGlu5.
Conclusions
Several new 2-amino and 2-halo-thiazole-4(alkynylbenzenes) were synthesized and they showed moderate to high affinity as ligands at mGlu5 in rat brain tissue. The labeling of some of the 2-fluorothiazole derivatives with [18F]fluoride ion was shown to be feasible to provide new candidate radioligands, [18F]10a and [18F]10b, for mGlu5 imaging with PET. A candidate, 9b for development as a SPECT radioligand for imaging brain mGlu5, was also identified.
Experimental
Materials and general methods
5-Amino-3-fluorobenzonitrile was obtained from Oakwood Products, Inc. All other reagents and solvents were obtained from Sigma-Aldrich in the highest purity available (≥ 98%) and were used as purchased. All reactions were performed under argon atmosphere unless otherwise indicated. 4-((Trimethylsilyl)ethynyl)thiazol-2-amine (4)27, 3-((2-fluorothiazol-4-yl)ethynyl) benzonitrile (8a)22, and 2-fluoro-4-(phenylethynyl)thiazole (10e)25 were prepared as described previously. Thin layer chromatography (TLC) was performed with silica gel layers (type 60 F254; 400–630 mesh) with compounds visualized under UV light (λ = 254 nm). Column chromatography was performed on silica gel with hexane-EtOAc mixtures as eluents. Constituent proportions in chromatographic mobile phases are expressed by volume. Separation of compounds with HPLC was performed on a Luna column (10 µm, 250 × 10 mm, Phenomenex, Torrance, CA) eluted with H2O-MeCN. The purities of all new compounds were determined by HPLC on a Luna C18 column (5 µm, 250 × 4.6 mm, Phenomenex) eluted isocratically with H2O-MeCN and were greater than 95%. All HPLC eluates were monitored for absorbance at 254 nm. Yields were recorded for chromatographically pure materials (≥ 98%).
The 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra of all compounds were acquired in CDCl3 using the chemical shift of residual deuterated solvent as internal standard; chemical shifts (δ) for the proton and carbon resonance are reported in parts per million (ppm) downfield from TMS (δ = 0). The following abbreviations were used to describe peak splitting patterns when appropriate: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = double of doublet. 19F-NMR (376.5 MHz) spectra were acquired in CDCl3 in the presence of CFCl3 as internal standard (δ = 0). Mass spectra were acquired with either a GC-MS instrument equipped with a capillary RTX-5ms column (30 m × 0.25 mm; flow rate, 1 mL/min; carrier gas, He) or by LC-MS. LC-MS analyses were performed on a LCQ Deca MS instrument interfaced with a Surveyor HPLC pump and autosampler (Thermo Fisher Scientific, Waltham, MA). High-resolution mass spectra were acquired with ESI.
3-Fluoro-5-iodobenzonitrile (3)
3-Amino-5-fluorobenzonitrile (2.72 g, 20 mmol) was treated with n-butyl nitrite (3.09 g, 35.6 mmol) and CuI (5.71 g, 30 mmol) in MeCN (30 mL) at 80 °C. The mixture was refluxed for 20 min, and allowed to cool to r.t. Then the solvent was evaporated off. The residue was suspended in water (20 mL) and extracted twice with DCM (2 × 25 mL). The organic layers were combined, washed with brine (15 mL), dried over MgSO4 and evaporated to dryness under vacuum. Chromatography of the residue on silica gel (hexane-EtOAc; 90: 10, v/v) gave 3 as yellow crystals (2.32 g; yield, 47%). m.p.: 29–30 °C; 1H-NMR: δ 7.80 (s, 1H) 7.71 (m, 1H), 7.36 (m, 1H); 13C-NMR: δ 161.6 (d, J = 256.03 Hz), 136.7 (d, J = 3.8 Hz), 129.1 (d, J = 23.20 Hz), 118.7 (d, J = 24.34 Hz), 115.9 (d, J = 3.23 Hz), 115.3 (d, J = 9.59 Hz), 93.9 (d, J = 8.23 Hz); 19F-NMR: δ −107.54 (s, 1F); GC-MS m/z: 247.94 [M + H] +.
2-Chloro-4-((trimethylsilyl)ethynyl)thiazole (5)
4-(Trimethylsilyl)ethynyl)thiazol-2-amine (4; 2.25 g, 11.48 mmol) and CuCl (2.85 g, 28.78 mmol) were dissolved in MeCN (25 mL) at r.t. n-Butyl nitrite (1.6 mL, 13.7 mmol) was added with stirring, and the solution heated to 75 °C. TLC showed the reaction to be complete after 45 min. The reaction mixture was then evaporated to dryness under vacuum. The residue was dissolved in ethyl acetate (20 mL) and washed with ammonia solution (0.1M; 2 × 50 mL). The organic layer was dried over MgSO4 and evaporated to dryness under vacuum. Chromatography of the residue on silica gel (hexane-EtOAc; 95: 5, v/v) gave 5 as a white powder (750 mg; yield, 30%). m.p.: 67–68 °C; 1H-NMR: δ 7.34 (s, 1H), 0.25 (s, 9H); 13C-NMR: δ 151.1, 136.7, 125.2, 97.4, 96.3, 0.0; HRMS (ESI+): calc’d for C8H11NClSSi 207.0070, found 207.0067. HPLC purity: 98.45%.
3-[(2-Aminothiazol)-4-ethynyl]benzonitrile (6a)
Compound 4 (375 mg, 1.91 mmol), 3-iodobenzonitrile (650 mg, 2.85 mmol), CuI (25 mg, 0.12 mmol), Pd(PPh3)4 (74 mg, 0.7 mmol), and Et3N(1.2 mL) were added to dimethylethylene glycol (10 mL) under argon. Argon was bubbled into the resulting dark solution while it was heated to 80 °C. A solution of TBAF (1.0 M) in THF (2.5 mL) was then added via syringe over 25 min. The reaction mixture was heated at 80°C until TLC showed no starting material present (15 min), and then cooled to r.t. and evaporated to dryness. The residue was dissolved in EtOAc (25 mL), and then washed with water (2 × 40 mL) and brine (1 × 40 mL). The combined organic fractions were dried over MgSO4, and evaporated to dryness in vacuo. Chromatography of the residue on silica gel (hexane-EtOAc, 70: 30, v/v) gave 6a (230 mg; yield, 54%) as a brown solid; m.p.: 154–155 °C; 1H-NMR: δ7.52 (m, 2H), 7.33 (m, 2H), 6.78 (s, 1H); 13C-NMR: δ 166.6, 133.1, 131.7, 128.5, 128.3, 122.6, 113.15, 88.1, 83.9; HRMS (ESI+): calc’d for C11H9N2S 201.0486, found 201.0485. HPLC purity: 99.75%.
3-[(2-Aminothiazol)-4-ethynyl]-5-fluorobenzonitrile (6b)
This compound was prepared from 4 and 3-fluoro-5-iodobenzonitrile with the method and chromatography described for 6a and gave 6b as a brown powder (288 mg; yield, 62%); m.p.: 209–210°C; 1H-NMR: δ 7.64 (m, 1H), 7.59 (s, 1H), 7.55 (m, 1H), 7.44 (m, 1H); 13C-NMR: δ 166.5 (d, J = 256.10 Hz), 160.7, 152.3, 135.1, 131.2 (d, J = 3.56 Hz), 125.9, 123.1 (d, J = 22.86 Hz), 118.9 (d, J = 24.76 Hz), 116.7, 115.3 (d, J = 10.10 Hz), 85.9, 84.6; 19F-NMR: δ −109.18 (s, 1F); HRMS (ESI+): calc’d for C11H9N2S 244.0345, found 244.0334. HPLC purity: 97.32%.
2-(3-((2-Aminothiazol-4-yl)ethynyl)acetonitrile (6c)
This compound was prepared from 4 and 2-(3-iodophenyl)acetonitrile with the method and chromatography described for 6a and gave 6c as a brown powder (395 mg; yield, 86%); m.p.: 154–156 °C; 1H-NMR: δ 7.47 (m, 2H), 7.30 (t, J = 7.80 Hz, 1H), 7.31 (t, J = 7.8 Hz, 1H), 6.82 (s, 1H), 3.75 (s, 2H); 13C-NMR: δ 166.7, 159.5, 133.3 (2 C), 129.6, 124.4, 123.8, 116.5, 115.5, 113.5, 88.3, 83.9, 22.4; HRMS (ESI+): calc’d for C12H7FN3S 240.0591, found 240.0595. HPLC purity: 96.40%.
4-(3-Methoxyphenyl)ethynyl(thiazol)-2-amine (6d)
This compound was prepared from 4 and 1-iodo-3-methoxybenzene with the method and chromatography described for 6a and gave 6d as a yellow powder (392 mg; yield, 89%); m.p.: 94–95 °C; 1H NMR: δ 7.24 (t, J = 7.64 Hz, 1H), 7.12 (d, J = 7.60 Hz, 1H), 7.05 (s, 1H), 6.89 (m, 1H), 6.78 (s, 1H), 3.80 (s, 3H); 13C-NMR: δ 166.8, 159.5, 133.3, 129.6, 124.4, 123.8, 116.5, 115.5, 113.5, 88.3, 83.9, 55.5; HRMS (ESI+): calc’d for C12H11N2OS 231.0585, found 231.0592. HPLC purity: 97.22%.
3-((2-Chlorothiazol)-4-yl)ethynyl)benzonitrile (7a)
Compound 5 (431 mg, 2.0 mmol), 3-iodobenzonitrile (650 mg, 2.85 mmol), CuI (25 mg, 0.12 mmol), Pd(PPh3)4 (74 mg, 0.7 mmol), and Et3N (1.2 mL) were added to dimethylethylene glycol (10 mL) under argon. Argon was bubbled into the resulting dark solution while it was heated to 80 °C. A solution of TBAF (1.0 M) in THF (2.5 mL) was added via syringe over 25 min. The reaction mixture was stirred at 80°C. After 15 min TLC showed no starting material present. The reaction mixture was then cooled to r.t. and evaporated to dryness. The residue was dissolved in EtOAc (25 mL), and then washed with water (2 × 40 mL) and brine (1 × 40 mL). The combined organic fractions were dried over MgSO4, and evaporated to dryness in vacuo. Chromatography of the residue on silica gel (hexane-EtOAc, 85: 15, v/v) gave 7a as a brown solid (274 mg; 56%); m.p.: 118–120 °C; 1H-NMR: δ 7.81 (t, J = 1.4 Hz, 1H), 7.75 (dt, J1 = 7.88 Hz, J2 = 1.34 Hz, 1H), 7.65 (dt, J1 = 7.84 Hz, J2 = 1.33 Hz, 1H), 7.58 (s, 1H), 7.49 (t, J = 7.86 Hz, 1H); 13C-NMR: δ 138.1, 136.6, 135.2, 133.2, 132.7, 129.9, 126.1, 123.7, 117.1, 114.4, 87.5, 84.8; HRMS (ESI+): calc’d for C12H6 79BrN2S 244.9936, found 244.9940. HPLC purity: 99.11%.
3-((2-Chlorothiazol)-4-yl)ethynyl)-5-fluorobenzonitrile (7b)
This compound was prepared from 5 and 3-fluoro-5-iodobenzonitrile with the method and chromatography described for 7a and gave 7b as a yellow powder (335 mg; yield, 64%); m.p.: 120–121 °C; 1H-NMR: δ 7.62 (s, 1H), 7.49 (s, 1H), 7.46 (m, 1H), 7.36 (m, 1H); 13C–NMR: δ161.9 (d, J = 251.85 Hz), 152.3, 135.1, 131.2 (d, J = 3.56 Hz), 125.9, 123.2 (d, J = 23.07 Hz), 119.5 (d, J = 24.80 Hz), 116.7, 114.4 (d, J = 10.22 Hz), 85.9, 84.6; 19F-NMR: δ −109.12 (s, 1F); HRMS (ESI+): calc’d for C12H5ClFN2S 262.9841, found 262.9844. HPLC purity: 98.75%.
2-(3-((2-Chlorothiazol)-4-yl)ethynyl)-phenyl)acetonitrile (7c)
This compound was prepared from 5 and 2-(3-iodophenyl)acetonitrile with the method and chromatography described for 7a and gave 7c as a beige powder (346 mg; yield, 67%); m.p.: 108–110 °C; 1H-NMR: δ 7.52 (m, 1H), 7.50 (m, 1H), 7.43 (s, 1H), 7.36 (m, 2H), 3.75 (s, 2H); 13C-NMR: δ 150.7, 135.0, 130.4, 130.1, 129.3, 128.3, 127.4, 123.5, 122.0, 116.3, 87.6, 82.2, 22.4; HRMS (ESI+): calc’d for C13H8ClN2S 259.0097, found 259.0098. HPLC purity: 98.62%.
2-Chloro-4-((3-methoxyphenyl)ethynyl)thiazole (7d)
This compound was prepared from 5 and 1-iodo-3-methoxybenzene with the method and chromatography described for 7a and gave 7d as a light yellow powder (238 mg; yield, 48%); m.p.: 88 °C; 1H-NMR: δ 7.39 (s, 1H), 7.26 (t, J = 7.96 Hz, 1H), 7.15 (m, 1H), 7.07 (m, 1H), 6.93 (m, 1H), 3.81 (s, 3H); 13C-NMR: δ 159.3, 151.6, 136.4, 129.5, 124.4, 124.1, 122.9, 116.5, 115.9, 89.7, 82.1, 55.3; HRMS (ESI+): calc’d for C12H9ClNOS 250.0093, found 250.0097. HPLC purity: 99.08%.
3-((2-Bromothiazol)-4-ethynyl)-5-fluorobenzonitrile (8b)
The 2-aminothiazole derivative 6b (0.245 g, 1 mmol) and CuBr (0.2 g, 1.39 mmol) were dissolved in MeCN (8 mL) at r.t. n-Butyl nitrite (162 µL, 1.39 mmol) was added with stirring, and the solution heated to 60 °C. The reaction was complete after 15 min, as monitored by TLC. The reaction mixture was then evaporated to dryness in vacuo. The residue was dissolved in EtOAc (20 mL) and washed with ammonia solution (0.1 M; 2 × 50 mL). The organic layer was dried over MgSO4 and evaporated to dryness in vacuo. Chromatography of the residue on silica gel (hexane-EtOAc; 97: 3, v/v) gave 8b (150 mg; yield, 48%); m.p.: 139–140 °C; 1H-NMR: δ7.62 (t, J1 = 1.28 Hz, 1H), 7.54 (s, 1H), 7.47 (ddd, J1 = 8.72 Hz, J2 = 1.36 Hz, J2 = 1.12 Hz, 1H), 7.36 (ddd, J1 = 7.82 Hz, J2 = 1.40 Hz, J2 = 1.12 Hz, 1H); 13C-NMR: δ 161.9 (d, J = 251.71 Hz),136.6, 136.3 (d, J = 32.20 Hz), 131.2 (d, J = 3.56 Hz), 127.6, 125.7 (d, J = 10.08 Hz), 123.2 (d , J = 23.50 Hz), 119.5 (d, J = 24.57 Hz), 116.7, 114.4 (d, J = 10.20 Hz), 86.2 (J = 3.45 Hz), 85.3; 19F-NMR: δ −108.7 (s, 1F); HRMS (ESI+) calc’d for C12H5BrFN2S 306.9335, found 306.9338. HPLC purity: 98.45%.
2-(3-((2-Bromothiazol-4-yl)ethynyl)phenyl)acetonitrile (8c)
This compound was prepared from the 2-aminothiazole derivative 6c with the method described for 8b. Chromatography (hexane-EtOAc, 95: 5, v/v) gave 8c as a white powder (185 mg; yield, 61%); m.p.: 110–111 °C; 1H-NMR δ 7.51 (m, 2H), 7.47 (s, 1H), 7.37 (m, 2H), 3.76 (s, 2H); 13C-NMR δ 137.9, 136.1, 131.7, 131.4, 130.5, 129.5, 128.7, 126.4, 123.3, 117.5, 89.2, 83.2, 23.6; HRMS (ESI+): calc’d for C13H8BrN2S 302.9592, found 302.9587. HPLC purity: 98.08%.
2-Bromo-4-((3-methoxyphenyl)ethynyl)thiazole (8d)
This compound was prepared from the amino-thiazole derivative 6d with the method and chromatography described for 8c and gave 8d as a white powder (175 mg; yield, 59%); m.p.: 98 °C; 1H-NMR: δ 7.44 (s, 1H), 7.25 (t, J = 8.01 Hz, 1H), 7.14 (m, 1H), 7.08 (m, 1H), 6.92 (m, 1H), 3.81 (s, 3H); 13C-NMR: δ 159.5, 138.2, 135.9, 129.7, 126.0, 124.5, 123.1, 116.7, 116.1, 90.2, 82.0, 55.5; HRMS (ESI+): calc’d for C12H9BrNOS 293.9588, found 293.9594. HPLC purity: 97.91 %.
3-[(2-Iodothiazol)-4-ethynyl]-benzonitrile (9a)
This compound was prepared from 6a with the method described for 8b but with CuI in place of CuBr. Chromatography (hexane-EtOAc, 95: 5, v/v gave 9a as a beige powder (280 mg; yield 83%); m.p.: 118–120°C; 1H-NMR: δ 7.81 (t, J = 1.4 Hz, 1H), 7.76 (dt, J1 = 7.60 Hz, J2 = 1.32 Hz, 1H), 7.65 (dt, J1 = 7.64 Hz, J2 = 1.32 Hz, 1H), 7.53 (s, 1H), 7.49 (t, J = 8.00 Hz, 1H); 13C-NMR: δ 138.1, 136.6, 135.2, 133.2, 132.7, 129.9, 126.1, 123.7, 117.1, 114.4, 87.5, 84.8; HRMS (ESI+): calc’d for C12H6IN2S 336.9296, found 336.9307. HPLC purity: 98.25%.
3-((2-Iodothiazol)-4-ethynyl)-5-fluorobenzonitrile (9b)
This compound was prepared from 6b with the method described for 9a. Chromatography (hexane-EtOAc, 90: 10, v/v) gave 9b as a light yellow powder (250 mg; yield 71%). m.p.: 158–160 °C; 1H-NMR: δ 7.62 (s, 1H), 7.56 (s, 1H), 7.47 (m, 1H), 7.36 (m, 1H); 13C-NMR: δ 161.9 (d, J = 251.76 Hz), 138.5, 131.2 (d, J = 3.51 Hz), 130.1, 125.8 (d, J = 10.01 Hz), 123.2 (d, J = 22.95 Hz), 119.4 (d, J = 24.76 Hz), 116.7 (d, J = 3.05 Hz), 114.4 (d, J = 10.18 Hz), 100.7, 86.5 (d, J = 3.31 Hz), 85.0; 19F-NMR: δ−108.7 (s, 1F); HRMS (ESI+): calc’d for C12H5FIN2S 354.9202, found 354.9192. HPLC purity: 99.05%.
2-(3-((2-Iodothiazol-4-yl)ethynyl)phenyl)acetonitrile (9c)
This compound was prepared from 6c with the method described for 9b. Chromatography (hexane-EtOAc, 90: 10, v/v) gave 9c as a beige powder (215 mg; yield 62%). m.p.: 120–122 °C; 1H-NMR: δ7.52 (m, 2H), 7.50 (s, 1H), 7.37 (m, 2H), 3.76 (s, 2H); 13C-NMR: δ139.7, 131.7, 131.4, 130.5, 129.5, 128.9, 128.6, 123.3, 117.5, 100.3, 89.5, 82.8, 23.6; HRMS (ESI+): calc’d for C13H8IN2S 350.9453, found 350.9443. HPLC purity: 97.77%.
2-Iodo-4-((3-methoxyphenyl)ethynyl)thiazole (9d)
This compound was prepared from 6d with that the method described for 9a. Chromatography (hexane-EtOAc, 90: 10, v/v) gave 9d as a pale yellow (170 mg; yield 50%). m.p. 106–108 °C; 1H-NMR: δ7.46 (s, 1H), 7.26 (t, J = 7.95 Hz, 1H), 7.15 (dt, J1 = 7.6 Hz, J2 = 1.16 Hz,1H), 7.08 (m, 1H), 6.92 (m, 1H), 3.81 (s, 3H); 13C-NMR:δ 159.3, 139.9, 129.5, 128.2, 124.4, 123.0, 116.5, 115.8, 99.9, 90.3, 81.4, 55.3; HRMS (ESI+): calc’d for C12H9INOS 341.9450, found 341.9449. HPLC purity: 98.65%.
3-((2-Fluorothiazol-4-yl)ethynyl)benzonitrile (10a)
8a (105 mg, 0.36 mmol) was placed in a microwave vial with Kryptofix®2.2.2 (135 mg, 0.36 mmol), anhydrous KF (42 mg, 0.72 mmol) and DMSO (1 mL). The solution was irradiated with microwaves (30 W, 130 °C 10 min). Reaction was monitored with 1H-NMR until disappearance of the signal of the proton in C5 in the thiazole ring. The reaction mixture was then poured into water (10 mL) and extracted with EtOAc (3 × 25 mL). The combined organic phases were washed with water (3 × 10 mL) and then brine (10 mL). The organic fraction was dried over MgSO4, evaporated to dryness under vacuum and chromatographed on silica gel (DCM-hexane, 50: 50, v/v) to give 10a as an off-white powder (30 mg; yield, 24%): m.p. 113–114 °C; 1H-NMR: δ7.81 (t, J = 1.4 Hz, 1H), 7.75 (dt, J1 = 7.88 Hz, J2 = 1.34 Hz, 1H), 7.65 (dt, J1 = 7.84 Hz, J2 = 1.33 Hz, 1H), 7.48 (t, J = 8.0 Hz, 1H) 7.18 (d, J2 = 1.6 Hz, 1H); 13C-NMR: δ 169.1 (d, J = 285.7 Hz), 135.8, 135.0, 132.2, 130.0 (d, J = 19.2 Hz), 129.4, 123.6, 119.7 (d, J = 5.03 Hz), 117.8, 113.1, 86.6, 84.9; 19F-NMR: δ −77.7 (s); HRMS (ESI+): calc’d for C12H6FN2S 229.0231, found 229.0236. HPLC purity: 99.85%.
3-((2-Fluorothiazol-4-yl)ethynyl)-5-fluorobenzonitrile (10b)
The method described for 10a was applied to 7b and gave 10b as a white powder (65 mg; yield, 72%); m.p.: 128–130 °C; 1H-NMR: δ 7.81 (t, J = 1.4 Hz, 1H), 7.75 (dt, J1 = 7.88 Hz, J2 = 1.34 Hz, 1H), 7.65 (dt, J1 = 7.84 Hz, J2 = 1.33 Hz, 1H), 7.48 (t, J = 8.0 Hz, 1H) 7.18 (d, J2 = 1.6 Hz, 1H); 13C-NMR: δ 169.2 (d, J = 286.0 Hz), 161.9 (d, J = 251.7 Hz), 131.2 (d, J = 3.5 Hz), 129.6 (d, J = 19.1 Hz), 125.7 (d, J = 9.7 Hz), 123.2 (d, J = 22.8 Hz), 120.5 (d , J = 4.9 Hz), 119.5 (d, J = 24.8 Hz), 115.4, 114.4 (d, J = 10.2 Hz), 86.6, 84.9 (d , J = 3.6 Hz); 19F-NMR: δ −109.02 (s, 1F), −77.2 (1F, s); HRMS (ESI+): calc’d for C12H5F2N2S 247.0142, found 247.0133. HPLC purity: 100.00%.
2-(3-((2-Fluorothiazol-4-yl)ethynyl)phenyl)acetonitrile (10c)
The method described for 10a was applied to 7c and gave 10c as a beige powder (68 mg; yield, 78%); m.p.: 106–108 °C; 1H-NMR: δ 7.52 (m, 2H), 7.47 (s, 1H), 7.35 (m, 2H), 3.76 (s, 2H); 13CNMR: δ 170.1 (d , J = 285.1 Hz), 137.9, 136.1, 131.5 (d , J = 30.1 Hz), 131.4, 130.5, 129.5, 128.7, 126.4, 123.3, 117.5, 89.2, 83.2, 23.6; 19F-NMR: δ −78.05 (s, 1F); HRMS (ESI+): calc’d for C13H8FN2S 243.0389, found 243.0383. HPLC purity: 98.85%.
2-Fluoro-4-((3-methoxyphenyl)ethynyl)thiazole (10d)
The method described for 10a was applied to 7d and gave 10d as a white crystals (70 mg; yield 65%); m.p.: 64–66 °C. 1H-NMR: δ 7.26 (t, 1H, J = 7.94 Hz), 7.12 (dt, 1H, J = 7.96 Hz, J = 1.20 Hz), 7.10 (s, 1H), 7.06 (dd, 1H, J = 2.6 Hz, J = 1.40 Hz), 6.92 (ddd, 1H, J = 8.32 Hz, J = 2.60 Hz), 3.81 (s, 3H); 13C-NMR: δ 169.2 (d, J = 284.80 Hz), 159.5, 130.9 (d, J = 18.87 Hz), 129.7, 124.6, 123.1 (1C), 118.6 (d , J = 4.70 Hz), 116.7, 116.0, 89.4, 82.7, 55.5; 19F-NMR: δ −78.23 (s); HRMS (ESI+): calc’d for C12H9FNOS 234.0389, found 234.0383. HPLC purity: 98.95%.
mGlu5 Binding assay and pharmacological screen
The prepared ligands were assayed for binding to rat brain mGlu5 receptors by the National Institute of Mental Health (NIMH) Psychoactive Drug Screening Program (PDSP) in an assay using [3H]MPEP (1.0 nM) as radioligand.
The high-affinity ligand 10b was also screened by the PDSP for binding to a wide variety of receptor and binding sites (5-HT1A, 1B, 1D, 1E, 2A, 2B, 2C, 3, 5A, 6, 7, α1A, 1B, 1D, 2A, 2B, 2C, β1–3, BZP rat brain site, D1–5, DAT, DOR, GABAA, KOR, H1–4, M1–5, mGlu1, mGlu2, mGlu4, mGlu6, mGlu8, MOR, NET, NMDA PCP site, SERT, σ 1–2). Full details of the assays used by the PDSP may be found at their website (http://pdsp.med.unc.edu/indexR.html).
Radiochemistry
NCA [18F]fluoride ion was obtained through the 18O(p,n) 18F nuclear reaction by irradiating [18O]water (95 atom %) for 90 to120 min with a proton beam (17 MeV; 20 µA) produced with a PET trace cyclotron (GE Medical Systems, Milwaukee, MI). Radioactivity was measured with a calibrated ionization calibrator (Atomlab 300; Biodex Medical Systems, Shirley, NY), and corrected for background and physical decay. Radiochemistry was performed in a lead-shielded hot-cell for personnel safety with a Synthia MKII Lab System apparatus equipped with a microwave-heated reactor cavity.26 The reactor cavity was linked via an RF coaxial cable to a controller of both irradiation time and power placed on the outside of the hot-cell. Reactions were performed in a V-vial (1-mL) equipped with a screw-on cap and septum (Tuf-Bond Teflon/silicone). The septum was pierced with a vent needle that was connected to a glass vial (20 mL) to collect any emitted solvents, and also to a charcoal trap to retain any break-through of volatile radioactive species. Liquid handling was achieved with an Aspec auto-injector/dispenser which forms part of the Synthia apparatus. Other operations in the radiosyntheses and purification procedures were controlled by a Visual Chemistry-based recipe.
Cyclotron-produced [18F]fluoride ion (50–100 mCi) in 18O-enriched water (350–500 µL) was dried in the V-vial in the presence of Kryptofix 2.2.2 (13 µmol; 5 mg), K2CO3 (3.6 µmol, 0.5 mg) in MeCN-H2O (9: 1 v/v, 100 µL). Microwave heating (90 W in 3 pulses of 2 min) was applied under nitrogen gas flow (200 mL/min) to speed up the removal of MeCN-H2O azeotrope. The acetonitrile (500 µL) addition and evaporation were repeated twice. Precursor for labeling (~ 1.0–1.5 mg) in MeCN (100 µL) was then introduced into the sealed V-vial and heated at 130 °C (50 to 90 W in five pulses of 2 min). The reaction mixture was then diluted with water (0.75 mL) and injected onto a Luna C18 column (5 µm, 250 × 10 mm) eluted at 4 mL/min with MeCN-H2O with MeCN at 45% (v/v) for 2 min, then 75% for 20 min and finally 25% for 30 min. The radioactive product was collected and measured for the calculation of decay-corrected radiochemical yield.
Measurement of specific radioactivity
The specific radioactivity of [18F]10b was calculated by injecting a sample of known radioactivity content (0.1–0.4 mCi) into a mass-calibrated analytical HPLC system, comprising a Luna C18 column (5 µm, 4.6 × 250 mm; Phenomenex) eluted with MeCN-10 mM HCOONH4 (60: 40 v/v) at 2 mL/min with eluate monitored for absorbance at 254 nm. Specific radioactivity was calculated as the measured mass of 10b divided by the activity of [18F]10b, corrected for physical decay to the time of product isolation. The specific radioactivity of [18F]10a was calculated similarly.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH). We are grateful to NIH Clinical Center PET Department for cyclotron irradiations (Chief: Dr. Peter Herscovitch). We are also grateful to the National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP) (Contract #: NO1MH32004) for ligand Ki determinations and receptor screening. The NIMH PDSP is directed by Dr. Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer, Mr. Jamie Driscol, at NIMH, Bethesda MD, USA.
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- DCM
dichloromethane
- F-PEB
3-fluoro-5-[2-methyl-1,3-thiazol-4-yl)ethynyl]benzonitrile
- mGlu
metabotropic glutamate
- mGluR
metabotropic glutamate receptor
- M-MTEB
3-methyl-5-[(2-methyl-1,3-thiazol-4-yl)ethynyl]benzonitrile
- MPEP
2-methyl-6(phenylethynyl)pyridine
- NCA
no-carrier-added
- NMDA
N-methyl-d-aspartate
- PET
positron emission tomography
- RCY
decay-corrected radiochemical yields
- SPECT
single photon emission computed tomography
References
- 1.a) Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]; b) Watkins JC. L-Glutamate as a central neurotransmitter: looking back. Biochem. Soc. Trans. 2000;28:297–310. [PubMed] [Google Scholar]
- 2.Brauener-Osborne H, Egebjerg J, Nielsen EO, Madsen U, Krogsgaard-Larsen P. Ligands for glutamate receptors: design and therapeutic prospects. J. Med. Chem. 2000;43:2609–2645. doi: 10.1021/jm000007r. [DOI] [PubMed] [Google Scholar]
- 3.Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Ann. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 4.Pin J-P, Acher F. The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. Curr. Drug Targets: CNS Neurol. Disord. 2002;1:297–317. doi: 10.2174/1568007023339328. [DOI] [PubMed] [Google Scholar]
- 5.Pietraszek M, Nagel J, Gravius A, Schäfer D, Danysz W. The role of group I metabotropic glutamate receptors in schizophrenia. Amino Acids. 2006;32:173–178. doi: 10.1007/s00726-006-0319-9. [DOI] [PubMed] [Google Scholar]
- 6.Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A, Bernardi G, Bagni C, Centonze D. Abnormal mGlu5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacol. 2010;35:1500–1509. doi: 10.1038/npp.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Tsai VWW, Scott HL, Lewis RJ, Dodd PR. The role of group I metabotropic glutamate receptors in neuronal excitotoxicity in Alzheimer's disease. Neurotoxicity Res. 2005;7:125–141. doi: 10.1007/BF03033782. [DOI] [PubMed] [Google Scholar]; b) Westmark CJ, Westmark PR, Malter JS. Alzheimer’s disease and Down syndrome rodent models exhibit audiogenic seizures. J. Alzheimer’s Disease. 2010;20:1009–1013. doi: 10.3233/JAD-2010-100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodkin J, Busse C, Sukoff SJ, Varney MA. Anxiolytic-like activity of the mGlu5 antagonist MPEP: a comparison with diazepam and buspirone. Pharmacol. Biochem. Behav. 2002;73:359–366. doi: 10.1016/s0091-3057(02)00828-6. [DOI] [PubMed] [Google Scholar]
- 9.Cosford NDP, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson JJ, Bristow L, Brodkin J, Jiang XH, McDonald I, Rao S, Washburn M, Varney MA. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- 10.Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren WPJM, Stoehr N, Pagano A, Flor PI, Varanesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R. Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function I. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology. 2001;40:1–9. doi: 10.1016/s0028-3908(00)00113-1. [DOI] [PubMed] [Google Scholar]
- 11.a) Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur. J. Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]; b) Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGlu5 null mutant mice. Nature Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 12.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol. Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 13.a) Olive OM, Mcgeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGlu5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C-epsilon-dependent mechanism. Mol. Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]; b) Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGlu5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacol. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- 14.Siméon FG, Brown AK, Zoghbi SS, Patterson VM, Innis RB, Pike VW. Synthesis and simple 18F-labeling of a high affinity 2-(fluoromethyl)thiazole derivative ([18F]SP203) as a radioligand for imaging brain metabotropic glutamate subtype-5 receptors with PET. J. Med. Chem. 2007;50:3256–3266. doi: 10.1021/jm0701268. [DOI] [PubMed] [Google Scholar]
- 15.Brown AK, Kimura Y, Zoghbi SS, Siméon FG, Liow J-S, Kreisl WC, Taku A, Fujita M, Pike VW, Innis RB. Metabotropic glutamate subtype-5 (mGlu5) receptors quantified in human brain with a novel radioligand for positron emission tomography. J. Nucl. Med. 2008;49:2042–2048. doi: 10.2967/jnumed.108.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura Y, Brown AK, Siméon FG, Liow J-S, Gladding RL, Mozley PD, Pike VW, Innis RB, Fujita M. Biodistribution and radiation dosimetry of a positron emission tomography ligand, 18F-SP203, to image metabotropic glutamate subtype 5 (mGlu5) receptor in humans. Eur J. Nucl. Med. & Mol. Imaging. 2010;37:1943–1949. doi: 10.1007/s00259-010-1447-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamill TG, Krause S, Ryan C, Bonnefous C, Govek S, Seiders TJ, Cosford NDP, Roppe J, Kamenecka T, Patel S, Gibson RE, Sanabria S, Riffel K, Eng W, King C, Yang X, Green MD, O’Malley SS, Hargreaves R, Burns HD. Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGlu5) PET radiotracers. Synapse. 2005;56:205–216. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- 18.Shetty HU, Zoghbi SS, Siméon FG, Liow J-S, Brown AK, Kannan P, Innis RB, Pike VW. Radiodefluorination of 3-fluoro-5-(2-(2-[18F](fluoromethyl)-thiazol-4-yl)ethynyl) benzonitrile ([18F]SP203), a radioligand for imaging brain metabotropic glutamate subtype-5 receptors with positron emission tomography, occurs by glutathionylation in rat brain. J. Pharmacol. Exper. Ther. 2008;327:727–735. doi: 10.1124/jpet.108.143347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a) Banks RE. Fluorine the Enabler. In: Banks RE, Lowe KC, editors. Fluorine in Medicine in the 21st Century. New York: Chemserve (UMIST); 1994. [Google Scholar]; b) Campbell TF, Stephens CE. Nuclear fluorination of 2,4-diarylthiazoles with Accufluor. J. Fluorine Chem. 2006;127:1591–1594. [Google Scholar]
- 20.Briner PH, Fyfe MCT, Martin P, Murray PJ, Naud F, Procter M. Practical synthesis of 2-amino-5-fluorothiazole hydrochloride. J. Org. Process. Res. Develop. 2006;10:346–348. [Google Scholar]
- 21.Pike VW. Positron-emitting radiotracers for studies in vivo – probes for human psychopharmacology. J. Psychopharmacol. 1993;7:139–158. doi: 10.1177/026988119300700202. [DOI] [PubMed] [Google Scholar]
- 22.Siméon FG, Wendahl MT, Pike VW. Efficient and regioselective halogenations of 1,3-thiazoles with copper salts. J. Org. Chem. 2009;74:2578–2581. doi: 10.1021/jo802799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell TF, Stephens CE. Nuclear fluorination of 2,4-diarylthiazoles with Accufluor. J. Fluor. Chem. 2006;127:1591–1594. [Google Scholar]
- 24.Nyffeler PT, Gonzalez Durn S, Burkart MD, Vincent SP, Wong C-H. Selectfluor: mechanistic insight and applications. Angew. Chem. Int. Ed. 2005;44:192–212. doi: 10.1002/anie.200400648. [DOI] [PubMed] [Google Scholar]
- 25.Siméon FG, Wendahl MT, Pike VW. The [18F]2-fluoro-1,3-thiazolyl moiety - an easily-accessible structural motif for prospective molecular imaging radiotracers. Tet. Lett. 2010;51:6034–6036. doi: 10.1016/j.tetlet.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarova N, Siméon FG, Musachio JL, Lu SY, Pike VW. Integration of a microwave reactor with Synthia to provide a fully automated radiofluorination module. J. Label. Compd. Radiopharm. 2007;50:463–465. [Google Scholar]
- 27.Iso Y, Grajkowska E, Wroblewski JT, Davis J, Goeders NE, Johnson KM, Sanker S, Roth BL, Tueckmantel W, Kozikowski AP. Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. J. Med. Chem. 2006;49:1080–1100. doi: 10.1021/jm050570f. [DOI] [PubMed] [Google Scholar]