Abstract
AMPA receptors (AMPARs) are heterotetromeric complexes composed of GluA1–4 subunits. They are glutamate-gated channels traditionally considered solely as ion carriers for postsynaptic depolarization. However, the existence and dynamic regulation of GluA2-lacking, calcium-permeable AMPARs (Cp-AMPARs) enable these special receptors to serve also as signaling molecules presumably via calcium influx. Recent studies have implicated Cp-AMPARs in several types of synaptic plasticity, including homeostatic synaptic regulation and Hebbian synaptic plasticity. Cp-AMPARs are usually expressed transiently at an early stage of synaptic plasticity, but are then replaced by normal GluA2-containing receptors, indicating a role for Cp-AMPARs in induction, rather than the maintenance, of synaptic plasticity.
Introduction
Upon glutamate release at an excitatory synapse, two primary ionotropic glutamate receptors, AMPA receptors (AMPARs) and NMDA receptors (NMDARs), will be activated to open their channel pores, allowing a rapid flux of ions into the postsynaptic spine. Since AMPARs permit only sodium influx, in contrast to NMDARs which permit both sodium and calcium, AMPARs have been attributed solely as a carrier of synaptic current, while NMDARs, via calcium, are responsible for intracellular signaling and synaptic regulation.
The lack of AMPAR calcium permeability comes from special genetic engineering by nature. In all AMPAR GluA1–4 subunit genes, there exists a conserved glutamine site at the second intramembrane domain that constitutes the inner face of the channel. At the mRNA level, this glutamine codon is edited to arginine, which confers channel resistance to calcium. This crucial mRNA editing selectively targets GluA2 subunits. Although GluA2 is assembled into AMPAR complexes by default at basal conditions in mature neurons, GluA2-lacking, and thus calcium permeable AMPARs (Cp-AMPARs), have been detected at many brain regions and synapses [1,2]. While Cp-AMPARs are more prominent in early developmental neurons [3], they have been found to exist in mature neurons as well [4]. It has become clear that the biogenesis and surface expression of Cp-AMPARs is dynamically regulated. More importantly, an increasing number of studies observe the dynamic occurrence of Cp-AMPARs and their involvement during the induction of varied forms of synaptic plasticity including long-term potentiation (LTP) and depression (LTD), as well as homeostatic synaptic plasticity [5], strongly indicating that this `abnormal' minority of AMPARs can in fact play important normal and novel roles in synaptic regulation.
Cp-AMPARs in homeostatic plasticity
Neurons have been shown to be able to restore their activity to a set-point level when challenged by external or internal perturbations. This type of regulation, or homeostatic plasticity, is important in the maintenance of neuronal or network stability during development and over time in maturation. One of the major cellular measures underlying neuronal homeostatic response is to regulate synaptic strength, largely by an alteration in AMPAR synaptic accumulation [6–10] (Figure 1). In central neurons the most studied model of functional homeostasis is activity deprivation by tetrodotoxin (TTX). When cultured cortical neurons are incubated with TTX to chronically block sodium channels and silence network activity, the synapse responds in a compensatory manner, resulting in an increase in synaptic AMPAR accumulation and the strength of synaptic transmission [11–13]. A key question in synaptic homeostatic plasticity, or synaptic scaling, is to identify the initial cue(s) generated during activity alteration that triggers recruitment of AMPAR at synapses. As a free extracellular ion whose cellular influx is intimately related to neuronal activity, calcium has been considered to be one of the signaling factors [7,9]. However, NMDAR-mediated calcium entry, which is critical for Hebbian synaptic plasticity, is not required for homeostatic regulation [11,14], and calcium from voltage-gated calcium channels has been positively linked to this regulation only in a few circumstances [15].
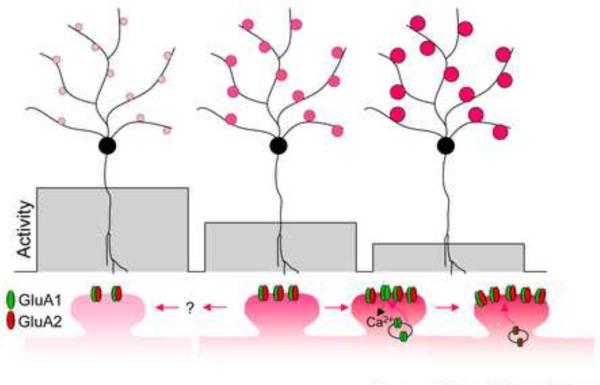
Figure 1.
Expression of GluA2-lacking AMPARs in homeostatic synaptic regulation. At basal conditions (middle neuron), synaptic strength (indicated by red circles) is maintained by a certain level of AMPARs at the postsynaptic spines. During activity suppression (right), Cp-AMPARs are inserted into the synaptic membrane, and are then replaced by GluA2-containing AMPARs, resulting in enhanced synaptic strength. On the other hand, elevated neuronal activity induces a reduction in receptor number via an unknown signaling cascade, leading to weaker synaptic strength (right).
Treatment with TTX alone or together with the NMDAR antagonist APV can reliably induce an increase in AMPAR synaptic accumulation, which is often concluded by a change in GluA1 abundance. Surprisingly, when alterations between GluA1 and GluA2 subunits are compared, a discrepancy is often detected [16]. Following the induction of a homeostatic response, the increase in AMPAR expression is preferable on GluA1, not GluA2 [14,16,17], which can potentially lead to the generation of GluA2-lacking AMPARs. In support of this idea, AMPAR-mediated currents show inward rectification and become sensitive to antagonists specific against Cp-AMPARs such as philanthotoxin-433 (PhTx) or Naspm [14,16–19]. Indeed, homeostatic synaptic regulation is blocked in neurons chronically incubated with PhTx [19]. Interestingly, among the recently identified mediators of homeostatic synaptic plasticity including Tumor necrosis factor-alpha (TNFα), retinoic acid, Arc/Arg3.1 and integrin β3, most are capable of causing imbalanced GluA1 and GluA2 regulation and Cp-AMPAR expression. The glia-derived TNFα is the first signaling molecule identified to mediate TTX-induced synaptic scaling. TNFα can mimic the TTX effect, and knockout of TNFα abolishes inactivity-dependent homeostatic regulation [20]. TNFα is known to cause rapid membrane insertion of GluA2-lacking AMPARs [21,22], although their involvement in TNFα-mediated synaptic scaling has not been directly determined. In retinoic acid-mediated synaptic scaling, the increase in AMPAR surface expression is GluA1 specific, and the homeostatic response in EPSCs can also be abolished by PhTx, indicating an incorporation of GluA1 homomeric AMPARs [17]. An unbalanced regulation in AMPAR subunits is also observed in Arc/arg3.1-mediated homeostatic regulation. Knockout of Arc/arg3.1 results in a typical synaptic scaling of AMPAR-mediated mEPSCs. Interestingly, Arc/arg3.1 knockout neurons reveal a significant increase in GluA1 surface expression, whereas surface GluA2 shows no change [23], suggesting membrane addition of GluR2-lacking, probably GluA1 homomeric AMPARs. In support of shared underlying mechanisms, the Arc/arg3.1-dependent synaptic regulation occludes TTX-induced homeostatic regulation [23]. β3 integrin has also been shown to be a necessary component in TTX dependent synaptic scaling. Disruption of β3 adhesion induces internalization of GluA2, but not GluA1 subunits, leaving GluA1-dominant, GluA2-lacking AMPARs at the cell surface. In agreement with a role of β3 integrin in synaptic scaling, TTX increases β3 integrin surface expression, and β3 integrin knockout abolishes TTX-dependent homeostatic synaptic plasticity [24], supporting integrin as a mediator in inactivity-dependent homeostatic regulation. In this specific paradigm, the role of Cp-AMPAR is less clear though it seems to block synaptic scaling. As a postulation, at the beginning of TTX silencing, there might be a transient drop of β3 activity to allow a minimal amount of Cp-AMPARs, followed by increased β3 integrin expression and AMPAR accumulation.
Cp-AMPARs in Hebbian plasticity
At the excitatory input to interneurons in the basolateral amygdala, AMPARs show a rectified current-voltage relationship, indicating the expression of Cp-AMPARs. Tetanus stimulation-induced LTP at these inputs requires postsynaptic calcium rises, but is independent of NMDARs and voltage-gated calcium channels [25]. Importantly, Cp-AMPARs have also been implicated in hippocampal LTP [26–29]. At CA1 pyramidal neurons, LTP protocol induces a rapid, but often transient incorporation of GluA2-lacking AMPARs, which are necessary for the initiation and/or stabilization of LTP. Since the stable expression of LTP is based on insertion of conventional GluA2-containing AMPARs, the early expression of Cp-AMPARs may function as signaling components via AMPAR-gated calcium. The requirement of Cp-AMPARs in LTP expression is age dependent. LTP in both young (within 2 wk old) and mature (more than 8 wks old) neurons requires Cp-AMPARs, but not in neurons of ages in between, presumably due to coupled PKA activity and GluA1 phosphorylation [27]. However, despite increasing evidence of Cp-AMPAR participation in LTP, some carefully executed studies do show failure in detecting Cp-AMPAR expression at LTP synapses [30,31]. The exact reasons remain unclear, but it is suggested that the disparity may arise from recording conditions (whether NMDARs are blocked during recording), or alternatively, due to the age of animals given that the LTP dependency on Cp-AMPAR varies during development [27]. Since elevated AMPAR synaptic expression underlies both LTP and silencing-induced homeostatic plasticity, it is intriguing to postulate that Cp-AMPAR-dependent calcium signaling maybe coupled to machineries for AMPAR surface targeting. In support of this, LTP expression in hippocampal CA1 synapses from GluA2 knockout animals requires Cp-AMPAR activity and postsynaptic calcium, and is blocked by postsynaptic infusion of tetanus toxin to suppress vesicle fusion [29], suggesting the existence of Cp-AMPAR-mediated AMPAR membrane insertion. Little is known regarding the removal of Cp-AMPARs from synapses, but they might be selectively targeted for internalization [32], or simply diffuse laterally out of the synapse presumably by an unanchoring mechanism to detach the receptors from their postsynaptic associated proteins (Figure 2).
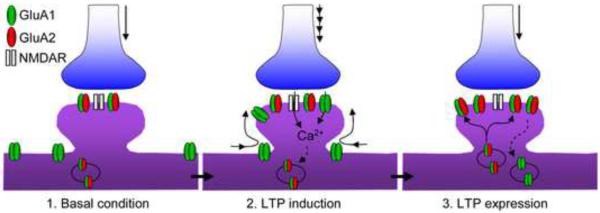
Figure 2.
Transient expression of Cp-AMPARs for the induction of Hebbian LTP. At the beginning of LTP stimulation, GluA2-lacking AMPARs are recruited to synaptic regions from nearby dendritic shafts. Calcium from both Cp-AMPARs and NMDARs triggers a signaling cascade that leads to membrane insertion of GluA2-containing AMPARs, while the Cp-AMPARs are removed from the cell surface via receptor internalization.
In addition to neuronal synapses, Cp-AMPARs have also been shown to mediate synaptic plasticity in certain neuron-glia transmission. Stimulation of the Schaffer collateral inputs to NG2 glial cells in the hippocampal CA1 region elicits typical LTP [33]. Because the glial synapses do not express NMDARs, this glial LTP is NMDAR-independent, but requires Cp-AMPARs and calcium. Distinct from CA1 synapses, at basal conditions NG2 synapses contain both GluA2-contaiing and GluA2-lacking AMPARs, but lack NMDAR components. An LTP protocol by theta burst stimulation leads to an increased rectification of synaptic currents and thus a higher level of synaptic Cp-AMPAR accumulation [33]. Thus in this case, signaling of Cp-AMPARs leads to a subsequent insertion of the same GluA2-lacking, rather than normal GluA2-containing receptors.
Cp-AMPARs have also been implicated in LTD. In young rats within 3 wks old, the synapses between hippocampal mossy fibers and CA3 pyramidal neurons contain both GluA2-containing and Cp-AMPARs, and the expression of the latter requires PICK1 [34]. At these synapses depolarization-induced LTD requires Cp-AMPARs because in PICK1 knockout animals that do not express Cp-AMPARs, no LTD can be induced. Interestingly, the expression of LTD is due to a selective removal of the Cp-AMPARs, presumably by receptor endocytosis [34]. The use of Cp-AMPARs as an LTD substrate has also been observed in dopaminergic neurons of the ventral tegmental area in cocaine addict animals [35,36]. It is not clear whether Cp-AMPARs serve as signaling molecules, and how widely employed Cp-AMPARs are for LTD in other synapses or brain regions.
Transient presence of synaptic Cp-AMPARs in plasticity induction
In many cases, the expression of Cp-AMPARs seems transient at the early stages of activity manipulation, and is needed only for the induction, rather than the maintenance of synaptic plasticity (Figure 1 and 2). In hippocampal CA1 neurons, LTP stimulation induces rectified AMPA currents that are present only for a short period of time. Current rectification is restored to control levels 20 min after LTP induction. PhTx application during and shortly after LTP stimulation blocks LTP expression. In contrast, 20 min after stimulation, when LTP has been induced, application of PhTx can no longer affect LTP [26]. A similar time course has been observed in another study in which Cp-AMPARs are present at synapses for only 10 min [28]. In homeostatic synaptic regulation, Cp-AMPAR expression is also limited to the beginning of activity suppression [16,19]. However, the expression of surface Cp-AMPARs appears markedly longer (24 hrs) in homeostatic plasticity [16,19] than in LTP (less than 30 min) [26,28].
Switching of GluA2-lacking to GluA2-containing AMPARS
The signaling cascades downstream of Cp-AMPAR activity are critical to initiate the plastic response, but the actual expression of synaptic plasticity is mediated largely by conventional GluA2-containing receptors (Figure 1 and 2). This switch has previously been observed. In the best studied model of Cp-AMPAR regulation, synaptic AMPARs at cerebellar stellate cells are oddly GluA2-lacking at basal conditions. Strikingly, synaptic activation induces a rapid removal of GluA2-lacking and a simultaneous addition of GluA2-containing receptors [37]. More evidence of an AMPAR subtype switch comes from LTP studies. LTP stimulation-induced Cp-AMPARs are soon replaced with PhTx-insensitive, GluA2-containing receptors [26,28]. Interestingly, a pause of activity following LTP stimulation protocol results in robust, seemingly normal LTP, except that the synaptic currents remain highly sensitive to PhTx, indicating that without sustained activation of newly positioned Cp-AMPARs at the synapses, receptors are unable to be replaced with normal AMPARs, leading to a potentiation that is expressed solely by GluA2-lacking AMPARs [26,28]. Thus, the switch is autoregulated by Cp-AMPARs themselves. The requirement of Cp-AMPAR activity strongly indicates a role for AMPAR-gated calcium. Although not directly studied in LTP paradigm, in cerebellar stellate cells, the switch to GluA2-containing receptors indeed requires calcium influx via Cp-AMPARs, but neither the activity of NMDARs nor calcium channels [37]. Interestingly, the subunit switch also occurs during normal neuronal development. In cortical neurons, at least a proportion of synaptic AMPARs are GluA2-lacking in the first two postnatal weeks, which are subsequently switched to regular receptors containing GluA2 subunits [3,38,39].
The replacement of Cp-AMPARs has also been observed in homeostatic regulation. Within 12 hrs of TTX/APV incubation, the potentiated AMPAR mEPSCs are markedly suppressed by Naspm. However, following 24 hr inactivity, when the homeostatic enhancement in mEPSC remains, the synaptic currents lose much of their sensitivity to Naspm [16]. Similarly, at individual synapses whose activity is chronically suppressed, homeostatic AMPAR accumulation is abolished only when PhTx is applied at the early stage of synaptic inhibition. When applied one day after synaptic inhibition, PhTx no longer produces any effect [19]. Other than activity blockade, when treated with TNFα, an established mediator of synaptic scaling, neurons show a rapid increase in surface expression of Cp-AMPARs in the early stage, followed by an increase in GluA2-containing receptors at a later time [22]. Similarly, when GluA2 expression is specifically monitored, it shows a more robust increase at a later time of TNFα incubation compared to a modest change during early treatment, consistent with the delayed expression of GluA2-contaiing receptors [24]. Thus, AMPAR subunit composition needs to be transformed from early GluA2-lacking to late GluA2-containing along the time course of homeostatic plasticity. Indeed, knockdown of GluA2 by siRNA completely blocks the expression of synaptic scaling, suggesting the ultimate need for GluA2-containing receptors [40].
Sources of Cp-AMPARs in synaptic plasticity
GluA2-lacking AMPARs may be generated at multiple sites over the life span of receptors. Presumably, AMPAR subunit composition can be altered at the ER during receptor assembly [41–43], where GluA2 is selectively removed from receptor complexes, or GluA2-lacking receptors are preferentially released from the ER for surface targeting. However, given a critical role for AMPAR translocation in synaptic plasticity, Cp-AMPARs are more likely formed by modulation of their dynamic trafficking. Association with postsynaptic proteins readily regulates AMPAR subcellular localization and subunit composition. Thus, cell surface GluA2 could be altered by its intracellular interaction with PICK1 and GRIP [44,45,46]. PICK1 interaction facilitates AMPAR endocytosis [47–49] and selectively removes GluA2-containing AMPARs from the cell surface, leading to PhTx-sensitive, rectifying synaptic currents [44,50]. It is possible that during TTX-induced homeostatic regulation, neuronal inactivity modulates GluA2-PICK1 interaction, leading to GluA2 removal. In agreement with this possibility, when the C-terminal of GluA2, but not GluA1, is over-expressed to presumably disrupt GluA2 C-terminal interaction, synaptic scaling is completely abolished [40]. However, the involvement of PICK1 seems complex and is cell type specific. At cerebellar parallel fiber-stellate synapses, PICK1 is required in activity-induced delivery of GluA2-containing AMPARs [45,46]. In addition to the specific removal of GluA2 from surface, intracellular GluA2-lacking receptors can be directly inserted to the plasma membrane such as during AMPAR inhibition [14] and TNFα stimulation [22].
Synaptic Cp-AMPARs may also be recruited from the neighboring dendritic plasma membrane via lateral migration (Figure 2). At basal conditions, less than 10% of AMPARs exist as GluA2-lacking [4] and are widely distributed at dendritic shafts [51], avoiding synaptic sites [52]. Synaptic activation has been shown to inhibit outward lateral diffusion of AMPARs from synaptic domains [53]. As a mirror process, the reserve pool of Cp-AMPARs at the extrasynaptic dendritic membrane may traffic laterally into synapses. At hippocampal CA1 neurons, PhTx-sensitive AMPARs can be detected at the perisynaptic region upon paired pulse stimulation or single stimulation combined with a glutamate transporter inhibitor to allow glutamate spillover [32]. Cp-AMPARs at the perisynaptic area have also been observed immediately after LTP stimulation protocol, which are then translocated into synapses [28,52]. These Cp-AMPARs are likely recruited by lateral diffusion of the dendritic pool into the synaptic domain via CaM-kinase I signaling [52], although direct membrane insertion cannot be excluded. A similar process has been observed in another study in which input activation during LTP stimulation recruits Cp-AMPAR from the dendritic shaft to perisynaptic and later synaptic domains [27]. In line with lateral recruitment of preexisting cp-AMPARs to the LTP synapses, a developmental switch of calcium permeable to GluA2-containing AMPARs occurs around postnatal day 14 [3], the same time when LTP property changes from Cp-dependent to independent [27]. Not much is known regarding the surface stability of Cp-AMPARs, but the status of GluA1 phosphorylation has been shown to be a determining factor. GluA1 dephosphorylation at serine 845 causes internalization and probably subsequent degradation of GluA2-lacking AMPARs [32]. In line with this finding, in fear conditioning-induced synaptic potentiation at lateral amygdala neurons, the GluA1 serine 845 phosphorylation site is required for cell surface expression of Cp-AMPARs [54].
What signals Cp-AMPAR biogenesis remains unknown. In homeostatic induction, TTX and/or AMPAR and NMDAR antagonists result in activity blockade of voltage-gated calcium channels and NMDARs, causing a dramatic reduction in calcium rises. Given that calcium signaling is known to be involved in AMPAR trafficking [55] and synaptic plasticity; it is intriguing to postulate that a drop in NMDAR or/and calcium channel-gated calcium influx [14] triggers Cp-AMPAR expression. In addition, activity-dependent expression of other homeostatic molecules such as TNFα, retinoic acid, Arc/Arg3.1 and β3 integrin may also initiate signaling cascades ultimately causing Cp-AMPAR formation.
Conclusion
Emerging evidence demonstrates a transient expression of GluA2-lacking AMPARs during the early stage of synaptic plasticity, suggesting an important role for Cp-AMPAR-dependent signaling in the initiation of synaptic regulation [56]. A key role of Cp-AMPARs seems to initiate signaling for lateral recruitment or insertion of GluA2-containing AMPARs, resulting in a switch to, and an increase in synaptic accumulation of regular GluA2-containing receptors. The transient expression of Cp-AMPARs may be important for temporal precision of signaling, but is also obviously a necessary measure to avoid neuronal excitotoxicity resulting from an overload of AMPAR-gated calcium influx, which constitutes a critical step in the pathology of ischemia/stroke-induced neuronal death [57–59] and neurodegenerative diseases [60]. In addition to a role of Cp-AMPARs in Hebbian and homeostatic synaptic plasticity, an increasing amount of evidence also point to an involvement of Cp-AMPARs in pathological synaptic plasticity. GluA2-lacking AMPARs can either be switched off by fear-inducing stimulus in cerebellar stellate cells [60], or rapidly expressed during cocaine administration [36,61] and fear conditioning [54], and are required for drug craving behavior following prolonged cocaine withdrawal [61].
Perhaps the most puzzling question is why AMPAR-gated calcium is crucial in synaptic plasticity, given that NMDAR-mediated calcium has been firmly established as the key mediator. Since Cp-AMPARs possess higher channel conductance [62], their synaptic presence can cause stronger postsynaptic depolarization, leading to an enhanced calcium influx via NMDARs and calcium channels, which when combined with calcium from Cp-AMPARs, may constitute calcium rises of different complexity. Further, AMPAR-gated calcium may take a distinct intraspinal compartmentalization [63,64] that enables it to activate a new set of signaling molecules. Like NR2A- and NR2B-containing NMDARs that have different preferences in synaptic localization and are coupled to distinct signaling pathways [65–67], Cp-AMPARs may be distributed at a discrete site at the postsynaptic domain away from NMDARs and GluA2-containing AMPARs. It is therefore important to understand Cp-AMPARs' subsynaptic localization and to identify novel signaling cascades downstream of Cp-AMPAR channel activity.
Acknowledgements
I thank Steve Amato, Amy Lin and other lab members for helpful comments on the manuscript. This work is supported by National Institute of Health grant MH079407 (H.Y.M.) and NARSAD Young Investigator Award (H. Y. M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
*of special interest
- 1.Ogoshi F, Weiss JH. Heterogeneity of Ca2+-permeable AMPA/kainate channel expression in hippocampal pyramidal neurons: fluorescence imaging and immunocytochemical assessment. J Neurosci. 2003;23:10521–10530. doi: 10.1523/JNEUROSCI.23-33-10521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- 3.Brill J, Huguenard JR. Sequential changes in AMPA receptor targeting in the developing neocortical excitatory circuit. J Neurosci. 2008;28:13918–13928. doi: 10.1523/JNEUROSCI.3229-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis GW. Homeostatic Control of Neural Activity: From Phenomenology to Molecular Design. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 8.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu LM, Goda Y. Dendritic signalling and homeostatic adaptation. Curr Opin Neurobiol. 2009;19:327–335. doi: 10.1016/j.conb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 12.Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinowitch I, Segev I. Two opposing plasticity mechanisms pulling a single synapse. Trends Neurosci. 2008;31:377–383. doi: 10.1016/j.tins.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 15.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]; This study shows that in cultured hippocampal neurons, TTX plus APV incubation induces homeostatic increase in the amplitude of mEPSCs, which show sensitivity to a Cp-AMPAR inhibitor Naspm at the early stage of activity deprivation.
- 17.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- *19.Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that a homeostatic increase in postsynaptic AMPAR accumulation can be induced by selective inhibition of a single presynaptic terminal. The increase in AMPAR expression is blocked by PhTx when applied within the first day of synaptic inhibition.
- 20.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 21.Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH. Tumor necrosis-factor-alpha (TNF-alpha) induces rapid insertion of Ca2+-permeable alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp Neurol. 2005;193:384–393. doi: 10.1016/j.expneurol.2004.12.026. [DOI] [PubMed] [Google Scholar]
- *22.Leonoudakis D, Zhao P, Beattie EC. Rapid tumor necrosis factor alpha-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excitotoxicity. J Neurosci. 2008;28:2119–2130. doi: 10.1523/JNEUROSCI.5159-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; In cultured hippocampal neurons, this study shows that TNFα treatment induces a rapid surface insertion of GluA1, but not GluA2 AMPAR subunits, resulting in expression of Cp-AMPARs at the cell surface, mainly within the non-synaptic dendritic domain.
- 23.Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- *26.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]; This study shows that in hippocampal CA1 neurons, LTP protocol causes a rapid but transient incorporation of GluA2-lacking AMPARs, which are then replaced with GluA2-containing receptors.
- 27.Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW. Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. Embo J. 2007;26:4879–4890. doi: 10.1038/sj.emboj.7601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *28.Yang Y, Wang XB, Zhou Q. Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc Natl Acad Sci U S A. 107:11999–12004. doi: 10.1073/pnas.0913004107. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that in hippcampal CA1 neurons, a theta burst pairing LTP protocol recruits Cp-AMPARs to the perisynaptic region and then the synaptic site, where they are switched to GluA2-containing AMPARs in a synaptic activity-dependent manner.
- 29.Asrar S, Zhou Z, Ren W, Jia Z. Ca(2+) permeable AMPA receptor induced long-term potentiation requires PI3/MAP kinases but not Ca/CaM-dependent kinase II. PLoS One. 2009;4:e4339. doi: 10.1371/journal.pone.0004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that during hippocampal LTP, synaptic currents have no change in I–V relationship, nor sensitivity to Cp-AMPAR blocker PhTx, arguing against the involvement of GluA2-lacking AMPARs.
- 31.Gray EE, Fink AE, Sarinana J, Vissel B, O'Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol. 2007;98:2488–2492. doi: 10.1152/jn.00473.2007. [DOI] [PubMed] [Google Scholar]
- *32.He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that under basal conditions Cp-AMPARs exist at the perisynaptic region at Schaffer collateral synapses on CA1 neurons. These perisynaptic Cp-AMPARs can be removed by NMDA treatment which is accompanied by GluA1S845 dephosphorylation, or are absent in mutant mice that lack the phosphorylation site, indicating an important role for GluA1 phosphorylation in stabilizing surface Cp-AMPARs.
- *33.Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]; This study shows that LTP can be induced at an unconventional type of synapses between CA1 neurons and NG2 glia in hippocampus. The postsynaptic sites on NG2 cells contain no NMDARs, but express Cp-AMPARs, which are required for LTP expression.
- 34.Ho MT, Pelkey KA, Topolnik L, Petralia RS, Takamiya K, Xia J, Huganir RL, Lacaille JC, McBain CJ. Developmental expression of Ca2+-permeable AMPA receptors underlies depolarization-induced long-term depression at mossy fiber CA3 pyramid synapses. J Neurosci. 2007;27:11651–11662. doi: 10.1523/JNEUROSCI.2671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- 36.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 37.Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- 38.Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin J, Shen F, Huguenard JR. Polyamines modulate AMPA receptor-dependent synaptic responses in immature layer v pyramidal neurons. J Neurophysiol. 2005;93:2634–2643. doi: 10.1152/jn.01054.2004. [DOI] [PubMed] [Google Scholar]
- *40.Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; In cultured cortical neurons, this study shows that GluA2 knockdown by RNAi blocks TTX-dependent homeostatic regulation. In addition, the intracellular C-terminus of GluA2 is shown to be required for synaptic scaling.
- 41.Greger IH, Akamine P, Khatri L, Ziff EB. Developmentally regulated, combinatorial RNA processing modulates AMPA receptor biogenesis. Neuron. 2006;51:85–97. doi: 10.1016/j.neuron.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- 43.Araki Y, Lin DT, Huganir RL. Plasma membrane insertion of the AMPA receptor GluA2 subunit is regulated by NSF binding and Q/R editing of the ion pore. Proc Natl Acad Sci U S A. 107:11080–11085. doi: 10.1073/pnas.1006584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci. 2005;8:768–775. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]
- 47.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clem RL, Anggono V, Huganir RL. PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J Neurosci. 30:6360–6366. doi: 10.1523/JNEUROSCI.6276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin HZ, Sensi SL, Carriedo SG, Weiss JH. Dendritic localization of Ca(2+)-permeable AMPA/kainate channels in hippocampal pyramidal neurons. J Comp Neurol. 1999;409:250–260. [PubMed] [Google Scholar]
- *52.Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci. 2008;28:6000–6009. doi: 10.1523/JNEUROSCI.0384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that infusion of active CaMKI into cultured hippocampal neuron induces cell-surface expression of GluA2-lacking AMPARs at non-synaptic dendritic domains. In addition, in hippocampal slices TBS-, but not HFS-induced LTP induces synaptic recruitment of Cp-AMPARs.
- 53.Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- *54.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that while fear conditioning causes expression of Cp-AMPARs in the lateral amygdala, its extinction is accompanied by a removal of the GluA2-lacking AMPARs that depends on GluA1 phosphorylation at S845 site.
- 55.Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 56.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Liu S, Lau L, Wei J, Zhu D, Zou S, Sun HS, Fu Y, Liu F, Lu Y. Expression of Ca(2+)-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Neuron. 2004;43:43–55. doi: 10.1016/j.neuron.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 58.Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MV. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc Natl Acad Sci U S A. 2005;102:12230–12235. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu B, Liao M, Mielke JG, Ning K, Chen Y, Li L, El-Hayek YH, Gomez E, Zukin RS, Fehlings MG, et al. Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J Neurosci. 2006;26:5309–5319. doi: 10.1523/JNEUROSCI.0567-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwak S, Weiss JH. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol. 2006;16:281–287. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- *61.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that cocaine withdrawal causes expression of Cp-AMPARs in the nucleus accumbens (NA). Importantly, intra-NA infusion of Naspm suppresses cue-induced cocaine-seeking behavior, indicating a role for GluA2-lacking AMPARs in cocaine craving.
- 62.Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sabatini BL, Maravall M, Svoboda K. Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol. 2001;11:349–356. doi: 10.1016/s0959-4388(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 64.Franks KM, Sejnowski TJ. Complexity of calcium signaling in synaptic spines. Bioessays. 2002;24:1130–1144. doi: 10.1002/bies.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li JH, Wang YH, Wolfe BB, Krueger KE, Corsi L, Stocca G, Vicini S. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur J Neurosci. 1998;10:1704–1715. doi: 10.1046/j.1460-9568.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- 66.Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 67.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]