Abstract
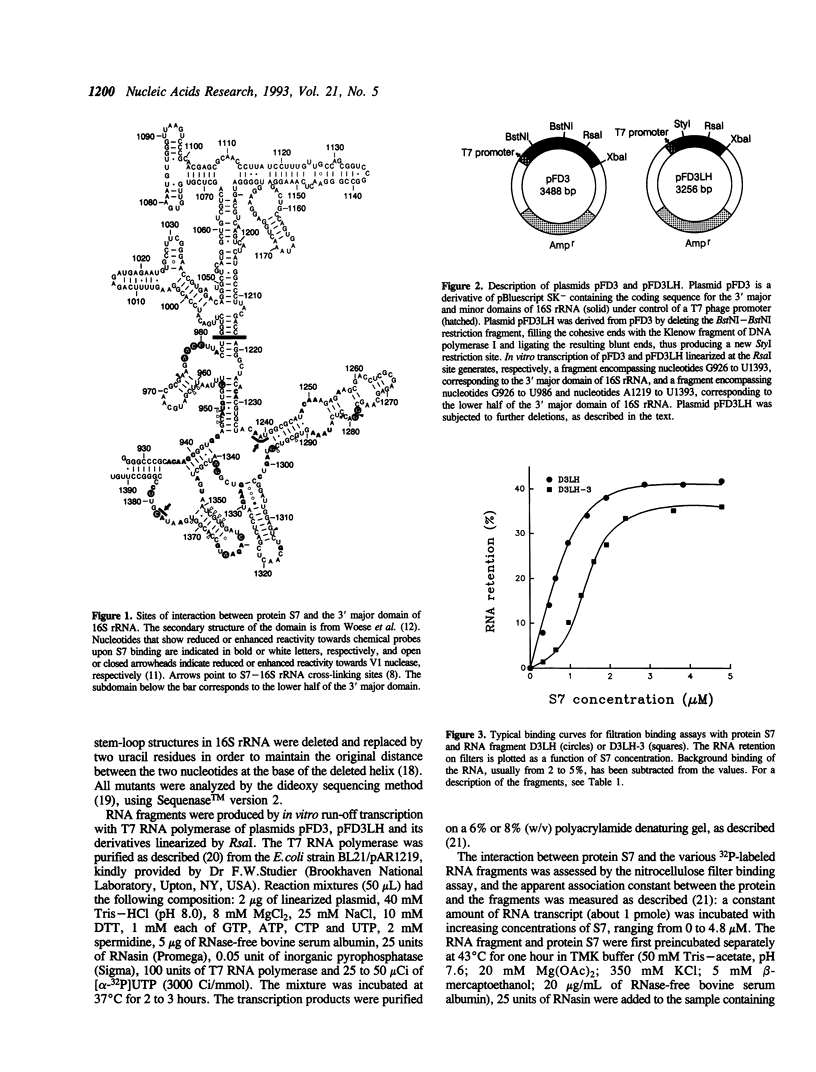
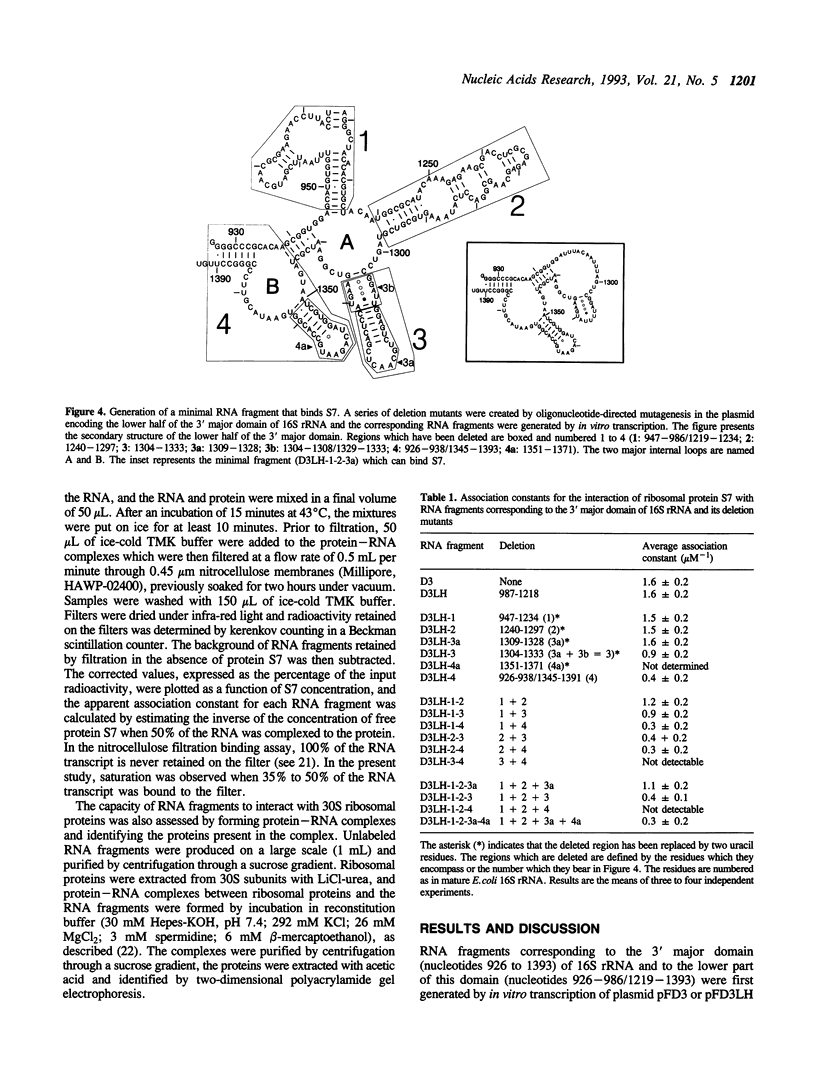
The interaction between Escherichia coli ribosomal protein S7 and 16S rRNA was investigated using in vitro synthesized RNA transcripts. It was shown by nitrocellulose membrane filtration that RNA transcripts corresponding to the 3' major domain (nucleotides 926-1393) and to the lower half of this domain (nucleotides 926-986/1219-1393) bound S7 with the same affinity as 16S rRNA. A series of deletion mutants of the DNA coding for the lower half of the 3' major domain were constructed and the corresponding RNA fragments were assayed for their capacity to bind S7. A minimal domain of 108 nucleotides which can still efficiently bind S7 was thus obtained. In this domain, the 1304-1308/1329-1333 irregular helix and the 1351-1371 irregular hairpin were found to contain important determinants for S7 binding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Brimacombe R. RNA-protein interactions in the Escherichia coli ribosome. Biochimie. 1991 Jul-Aug;73(7-8):927–936. doi: 10.1016/0300-9084(91)90134-m. [DOI] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Capel M. S., Engelman D. M., Freeborn B. R., Kjeldgaard M., Langer J. A., Ramakrishnan V., Schindler D. G., Schneider D. K., Schoenborn B. P., Sillers I. Y. A complete mapping of the proteins in the small ribosomal subunit of Escherichia coli. Science. 1987 Dec 4;238(4832):1403–1406. doi: 10.1126/science.3317832. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Christiansen J., Garrett R. A. Attachment sites of primary binding proteins L1, L2 and L23 on 23 S ribosomal RNA of Escherichia coli. J Mol Biol. 1991 Nov 20;222(2):251–264. doi: 10.1016/0022-2836(91)90210-w. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Cahill P. B., Thurlow D. L., Zimmermann R. A. Interaction of Escherichia coli ribosomal protein S8 with its binding sites in ribosomal RNA and messenger RNA. J Mol Biol. 1988 Nov 20;204(2):295–307. doi: 10.1016/0022-2836(88)90577-3. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Weiser B., Woese C. R., Noller H. F. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. doi: 10.1016/s0079-6603(08)60348-7. [DOI] [PubMed] [Google Scholar]
- Ikeda R. A., Richardson C. C. Enzymatic properties of a proteolytically nicked RNA polymerase of bacteriophage T7. J Biol Chem. 1987 Mar 15;262(8):3790–3799. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mandiyan V., Tumminia S., Wall J. S., Hainfeld J. F., Boublik M. Protein-induced conformational changes in 16 S ribosomal RNA during the initial assembly steps of the Escherichia coli 30 S ribosomal subunit. J Mol Biol. 1989 Nov 20;210(2):323–336. doi: 10.1016/0022-2836(89)90334-3. [DOI] [PubMed] [Google Scholar]
- Melançon P., Gravel M., Boileau G., Brakier-Gingras L. Reassembly of active 30S ribosomal subunits with an unmethylated in vitro transcribed 16S rRNA. Biochem Cell Biol. 1987 Dec;65(12):1022–1030. doi: 10.1139/o87-134. [DOI] [PubMed] [Google Scholar]
- Nowotny V., Nierhaus K. H. Assembly of the 30S subunit from Escherichia coli ribosomes occurs via two assembly domains which are initiated by S4 and S7. Biochemistry. 1988 Sep 6;27(18):7051–7055. doi: 10.1021/bi00418a057. [DOI] [PubMed] [Google Scholar]
- Powers T., Changchien L. M., Craven G. R., Noller H. F. Probing the assembly of the 3' major domain of 16 S ribosomal RNA. Quaternary interactions involving ribosomal proteins S7, S9 and S19. J Mol Biol. 1988 Mar 20;200(2):309–319. doi: 10.1016/0022-2836(88)90243-4. [DOI] [PubMed] [Google Scholar]
- Raué H. A., Klootwijk J., Musters W. Evolutionary conservation of structure and function of high molecular weight ribosomal RNA. Prog Biophys Mol Biol. 1988;51(2):77–129. doi: 10.1016/0079-6107(88)90011-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J., Craven G. R. Apparent association constants for E. coli ribosomal proteins S4, S7, S8, S15, S17 and S20 binding to 16S RNA. Nucleic Acids Res. 1981 May 11;9(9):2223–2237. doi: 10.1093/nar/9.9.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Rinke-Appel J., Brimacombe R. Site-directed cross-linking of mRNA analogues to the Escherichia coli ribosome; identification of 30S ribosomal components that can be cross-linked to the mRNA at various points 5' with respect to the decoding site. Nucleic Acids Res. 1989 Dec 11;17(23):9889–9908. doi: 10.1093/nar/17.23.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Powers T., Changchien L. M., Noller H. F. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989 May 19;244(4906):783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- Sylvers L. A., Kopylov A. M., Wower J., Hixson S. S., Zimmermann R. A. Photochemical cross-linking of the anticodon loop of yeast tRNA(Phe) to 30S-subunit protein S7 at the ribosomal A and P sites. Biochimie. 1992 Apr;74(4):381–389. doi: 10.1016/0300-9084(92)90116-v. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener L., Brimacombe R. Protein binding sites on Escherichia coli 16S RNA; RNA regions that are protected by proteins S7, S14 and S19 in the presence or absence of protein S9. Nucleic Acids Res. 1987 May 11;15(9):3653–3670. doi: 10.1093/nar/15.9.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener L., Schüler D., Brimacombe R. Protein binding sites on Escherichia coli 16S ribosomal RNA; RNA regions that are protected by proteins S7, S9 and S19, and by proteins S8, S15 and S17. Nucleic Acids Res. 1988 Feb 25;16(4):1233–1250. doi: 10.1093/nar/16.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Winker S., Gutell R. R. Architecture of ribosomal RNA: constraints on the sequence of "tetra-loops". Proc Natl Acad Sci U S A. 1990 Nov;87(21):8467–8471. doi: 10.1073/pnas.87.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Muto A., Mackie G. A. RNA-protein interactions in the ribosome. II. Binding of ribosomal proteins to isolated fragments of the 16 S RNA. J Mol Biol. 1974 Jun 25;86(2):433–450. doi: 10.1016/0022-2836(74)90029-1. [DOI] [PubMed] [Google Scholar]


