Abstract
Failure of physiologic transformation of the spiral arteries has been studied using placental bed biopsies in several obstetrical syndromes. Contrary to what was originally believed, this lesion is not restricted to preeclampsia and/or intrauterine growth restriction. A review of published evidence indicates that failure of physiologic transformation can be observed in women with spontaneous second trimester abortions, preterm labor with intact membranes, prelabor rupture of membranes and abruptio placentae. Therefore, disorders of deep placentation are present in a wide range of complications of pregnancy, emphasizing the importance of understanding the physiology and pathology of transformation of the spiral arteries. We propose that changes in the population and function of immunocytes at the maternal-fetal interface can be part of the mechanism of disease of obstetrical disorders, and this requires further investigation.
Keywords: failure of physiologic transformation, spiral arteries, maternal-fetal interface
Disorders of deep placentation are now recognized as a major mechanism of disease for several complications of pregnancy. The definition, classification, diagnostic criteria and mechanisms of disease responsible for disorders of deep placentation have been covered by other authors in this issue of the journal1;2 and elsewhere.3 Moreover, a recent review has examined the key concepts and proposed a classification of defective deep placentation.4
The article “Defective deep placentation” by Khong and Brosens in this issue reviews the information about disorders of deep placentation in preeclampsia and intrauterine growth restriction (IUGR).1 The frequency of physiologic remodeling of the spiral arteries in normal pregnancy and in those with preeclampsia from 8 articles is summarized in Table 1.4 The mechanisms proposed to be responsible for disorders of deep placentation are reviewed in detail by Pijnenborg et al.2
Table 1.
Remodeling of myometrial segment in normal pregnancy versus preeclampsia in placental bed biopsy studies
| Study | Normal pregnancy n/N (%) |
Preeclampsia n/N (%) |
|---|---|---|
| Gerretsen1 | 22/23 (96%) | 1/30 (3%) |
| Khong2 | 18/18 (100%) | 3/14 (21%) |
| Frusca3 | 13/14 (93%) | 6/24 (25%) |
| Meekins4 | 16/21 (76%) | 3/24 (12%) |
| Sagol5 | 16/20 (80%) | 7/17 (41%) |
| Kim6 | 55/59 (93%) | 9/31 (29%) |
| Kim7 | 89/103 (86%) | 18/43 (19%) |
| Guzin8 | 16/20 (80%) | 11/32 (33%) |
| Mean (range) | 88% (76–100%) | 27% (3–41%) |
Reference List
Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol 1981;88:876–81.
Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986;93:1049–59.
Frusca T, Morassi L, Pecorelli S, Grigolato P, Gastaldi A. Histological features of uteroplacental vessels in normal and hypertensive patients in relation to birthweight. Br.J Obstet Gynaecol. 1989;96:835–39.
Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994;101:669–74.
Sagol S, Ozkinay E, Oztekin K, Ozdemir N. The comparison of uterine artery Doppler velocimetry with the histopathology of the placental bed. Aust.N.Z.J Obstet Gynaecol 1999;39:324–29.
Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 2002;187:1137–42.
Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2003;189:1063–69.
Guzin K, Tomruk S, Tuncay YA, Naki M, Sezginsoy S, Zemheri E et al. The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Arch.Gynecol Obstet 2005;272:283–88.
It is now clear that disorders of deep placentation are present not only in preeclampsia and IUGR, but in other obstetrical syndromes. This article will review the evidence that failure of physiologic transformation is present in spontaneous abortion, preterm labor with intact membranes, preterm prelabor rupture of membranes (PROM) and abruptio placentae. We will also propose how such defects may play a role in the genesis of the different phenotypes of complications of pregnancy.
Spontaneous abortions in the first trimester
Khong et al.5 were the first to examine the morphology of the placental bed in idiopathic, sporadic and recurrent spontaneous abortion. In this pioneering study, twelve patients had spontaneous abortions, four composed the control group, three had elective terminations of pregnancy and one had a late spontaneous abortion with a live fetus (due to cervical insufficiency). All patients in the control group had physiologic transformation of the spiral arteries. In contrast, failure of physiologic transformation of the decidual segments was seen in 58% (7/12) cases of spontaneous abortion. Only 33% (4/12) of cases had myometrial segments of the spiral arteries in the biopsy material. None had evidence of physiologic transformation. Karyotype of the products of conception was performed in six of the spontaneous abortions, and there was no relationship between the karyotype results and the histology of the placental bed.
Similarly, Gun et al.6 reported a study in which the histology of the decidua was compared in women (n=25) with spontaneous abortion (5 to 12 weeks) and the decidua of 40 women undergoing elective terminations of pregnancy (between 5 and 11 weeks). The authors found that transformation of the spiral arteries occurred in 90% of elective abortions, whereas lack of physiologic transformation in the decidual segments was present in 48% of patients with spontaneous abortions (p<0.001).
Michel et al.7 examined the placental bed of patients who had an elective termination of pregnancy (n=12), women who had a history of recurrent spontaneous abortion (defined as 3 or more unexplained miscarriages with the same partner, and not more than one live birth; n=10), and women who had a “mis-abortion”, diagnosed with ultrasound (n=6). Physiologic transformation in the decidual segments of the spiral arteries was observed in 91.6% (11/12) of patients who had an elective termination of pregnancy. In contrast, 83% (5/6) of women who had a spontaneous abortion had inadequate development of most spiral arteries in the decidua. In the group of patients with recurrent spontaneous abortions, physiologic transformation in the decidual segments of the spiral arteries was observed in 4 women, incomplete physiologic transformation was present in the other two, and poorly developed vasculature was observed in the remaining four. Moreover, the number of cells with large granules, probably corresponding to uterine natural killer cells, was reported in the study.7 The proportion of these cells was significantly higher in women undergoing elective terminations of pregnancy than in those who had spontaneous abortions (either sporadic or recurrent).
Hustin et al.8 evaluated 184 specimens of complete spontaneous abortions. A complete spontaneous abortion was defined as one in which the gestational sac was expelled “en bloc”. The control group consisted of 219 patients who underwent voluntary termination of pregnancy in the first trimester by uterine aspiration. In addition, there were three hysterectomy specimens with pregnancies in situ (before 10 weeks of gestation).
Limited trophoblastic infiltration and inadequate physiologic changes of the spiral arteries was observed in 64% of those with embryonic demise and 77% of abnormal conceptuses, while such findings were not observed in patients with normal pregnancies. The authors concluded that most cases of spontaneous abortion are associated with defective trophoblast invasion into the decidua and spiral arteries. They proposed if the trophoblast growth is reduced, intra-arterial plugs will be less well-formed. Therefore untimely initiation of blood circulation in the intervillous space may be associated with pregnancy failure and complete abortion. Indeed, maternal blood was found with higher frequency in the intervillous space in pathologic cases than in normal pregnancy. This study is unique because it was based on the examination of the complete aborted material, rather than a biopsy of the placental bed.8 For further detail for pathophysiology we referred the readers to the article by Burton et al. in this issue of the journal.9
In contrast to the conclusion of the previous studies, Ball et al.10 reported a large study in which placental bed biopsies were obtained under ultrasound visualization from 50 women who had a spontaneous abortion, and from 78 women who underwent elective terminations of pregnancy. Frozen sections were immunostained for cytokeratin, desmin and von Willebrand factor (to detect trophoblast, vascular and myometrial smooth muscle, and endothelium, respectively). Inclusion in the study required the presence of at least one spiral artery in the biopsy specimen. Trophoblast invasion of the decidua and physiologic transformation of spiral arteries did not differ between spontaneous abortion and elective terminations of pregnancy. Similarly, there were no differences in the histologic features between patients with euploidy and aneuploidy conceptuses.
The discrepancy among studies could be attributed to the nature of the specimens collected, the requirement for the presence of an embryo and gestational age. Further investigation is required to elucidate the role of trophoblast plugging of the spiral arteries and physiologic transformation of the decidual and myometrial segments in early spontaneous abortions. Since myometrial invasion of the spiral arteries does not occur until after 14 weeks of gestation, one may not expect that failure of transformation of this particular segment may be associated with spontaneous abortion. Systematic studies of the cell populations in the decidua are warranted. The difficulties in interpreting whether the changes in cell populations represent a causal shift or epiphenomenon resulting from pregnancy failure are acknowledged.
Spontaneous abortions in the second trimester
In addition to the study of Khong et al.5, a recent study by Ball et al.11 provides support for a relationship between failure of physiologic transformation of the spiral arteries and spontaneous abortion in the midtrimester. This study included 26 late spontaneous miscarriages between 13 and 23.9 gestational weeks and gestational age-matched normal pregnancies undergoing elective termination (n=74). Myometrial spiral arteries from patients with late miscarriage showed a lower number of endovascular trophoblasts (4% vs. 31%, p=0.001), intramural trophoblasts (76% vs. 88%, p=0.05), and less extensive fibrinoid change (4% vs. 18%, p=0.01) compared to myometrial spiral arteries from women with normal pregnancies. In contrast, the number of endovascular trophoblasts in the decidual spiral arteries was higher in patients who had a spontaneous abortion than in the control group (66% vs. 40%, p=0.04).
Preterm Birth
Preterm birth is traditionally defined as delivery occurring before 37 weeks of gestation, and is the leading cause of perinatal morbidity and mortality worldwide.12 Spontaneous preterm labor (with intact membranes or following preterm PROM) accounts for 2/3 of preterm births. The remaining 1/3 is due to preeclampsia and intrauterine growth restriction, in which preterm delivery is accomplished for maternal or fetal indications.12
Spontaneous preterm parturition is considered a syndrome13 whether it occurs with intact or ruptured membranes. The spectrum of the phenotype may range from cervical insufficiency, preterm labor with intact membranes or preterm PROM. The causes of spontaneous preterm parturition have been proposed to include infection/inflammation,14–26 ischemia due to vascular disease,27;28 cervical disease,29–31 uterine overdistension,32 abnormal allograft reaction,33;34 allergy,35–37 and endocrine disorders (deficient progesterone action).38–41 Growing evidence suggests that vascular disorders may play a role in a subset of patients who have preterm labor with intact membranes or preterm PROM.
Spontaneous preterm labor leading to preterm delivery
The most common lesions found in the placenta of patients with spontaneous preterm parturition are those of acute inflammation (acute chorioamnionitis and funisitis). Vascular lesions are the second most common pathology of the placenta in these patients.27;28 Arias et al.27 reported that 34.1% of women with spontaneous preterm labor and intact membranes had vascular lesions in decidual vessels attached to the placenta, while this only occurred in 11.8% of control women (term gestation without complications, p=0.001). Placental vascular lesions in the decidual vessels of the placenta were more frequent in patients with preterm labor with intact membranes (odds ratio 3.8, 95% CI 1.3–11.1, p=0.007). Moreover, abruptio placentae was more frequent in patients with preterm labor than in controls (9.5% vs. 0%, respectively; p=0.001). Similarly, Germain et al.42 reported a higher frequency of ischemic lesions in placentas from patients with preterm labor who delivered preterm than in those with an episode of preterm labor who delivered at term (25.4% vs. 3.7%, p<0.05).
Kim et al.43 reported the only systematic study of the placental bed of patients presenting with preterm labor who had a preterm delivery. Placental bed biopsies were obtained at the time of cesarean section under direct visualization. Specimens were immunostained for cytokeratin and counterstained with periodic acid Schiff (PAS) to detect trophoblasts and fibrinoid, respectively. Three groups of patients were studied: 1) patients who had a cesarean section at term; 2) patients who had a spontaneous preterm labor/delivery with a cesarean section for obstetrical indications; and 3) patients with preeclampsia (positive control).
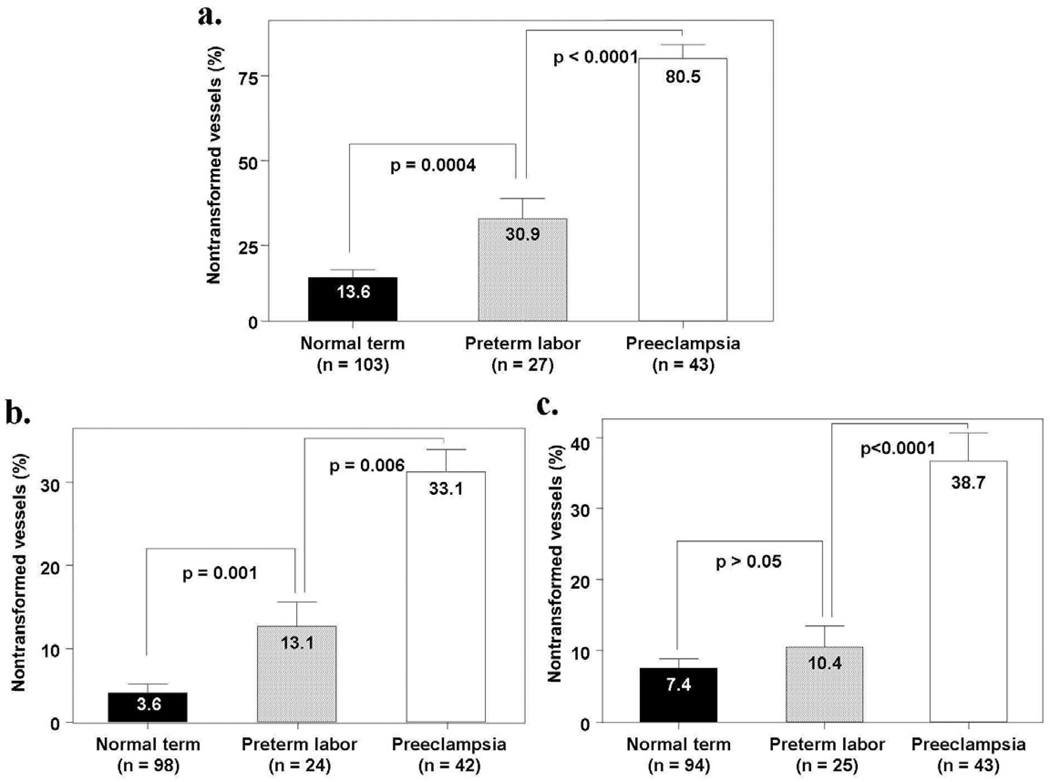
The rate of failure of physiologic transformation of the myometrial segment of the spiral arteries was significantly higher in patients with spontaneous preterm labor/delivery than in those who had a term delivery (30.9% vs. 13.6%, p=0.004). The same was the case for the decidual segment in the placental bed (13.1% vs. 3.6%, p=0.001), but not for the decidual segment in the basal plate of the placenta (10.4% vs. 7.4%, p>0.05). Figure 1 describes the mean percentage of non-transformed spiral arteries in three different locations (myometrium, decidual segment of the placental bed, and decidua in the basal plate).
Figure 1.
Mean percentage on non-transformed spiral arteries weighted by the number of vessels. Figure 1a: The mean percentage of the spiral arteries that had failure of physiologic transformation in the myometrial segment was significantly higher in patients with preterm labor and those with preeclampsia than in normal pregnant women at term (preterm labor; mean ± SEM = 30.9% ± 5.8% vs. normal pregnant women; mean ± SEM = 13.6% ± 2.1%; p=0.0004 and preeclampsia; mean ± SEM = 80.5% ± 3.8% vs. normal pregnant women; mean ± SEM = 13.6% ± 2.1%; p<0.0001). Figure 1b: Patients with preterm labor and intact membranes, as well as patients with preeclampsia, had a significantly higher mean percentage of the spiral arteries that had failure of physiologic transformation in the decidual segment of the placental bed than normal pregnant women (preterm labor; mean ± SEM = 13.1% ± 4.4% vs. normal pregnant women; mean ± SEM = 3.6% ± 1.2%; p=0.001 and preeclampsia; mean ± SEM = 33.1% ± 4.5% vs. normal pregnant women; mean ± SEM = 3.6% ± 1.2%; p<0.0001). Figure 1c: The mean percentage of the spiral arteries that had failure of physiologic transformation in the decidual segments of the basal plate was not significantly different between patients with preterm labor and normal pregnant women at term (preterm labor; mean ± SEM = 10.4% ± 3.0% vs. normal pregnant women; mean ± SEM = 7.4% ± 1.3%; p>0.05). Patients with preeclampsia had a significantly higher mean percentage of the spiral arteries that had failure of physiologic transformation in the decidual segments of the basal plate than patients with preterm labor and normal pregnant women (preeclampsia; mean ± SEM = 38.7% ± 4.0% vs. preterm labor; mean ± SEM = 10.4% ± 3.0%; p<0.0001 and preeclampsia; mean ± SEM = 38.7% ± 4.0% vs. normal pregnant women; mean ± SEM = 7.4% ± 1.3%; p<0.0001). Comparisons were performed with ANOVA, followed by student’s t tests; p value >0.017 became non-significant after adjusting for multiple comparisons.
These observations indicate that patients with preterm labor and intact membranes who deliver a preterm neonate have a greater degree of failure of transformation of the spiral arteries in the myometrial and decidual segments than that of women who deliver at term. However, the extent of this defect was much greater in patients with preeclampsia than those in preterm labor with intact membranes. Of interest is that there was no difference in the frequency of lack of physiologic transformation between patients with and without histologic chorioamnionitis. The reasons why some women with failure of physiologic transformation develop preterm labor, or other obstetrical syndromes, are presently unknown. However, it is possible that the more extensive degree of vascular pathology in preeclampsia than in preterm labor may, in part, be responsible for the phenotype.
Preterm prelabor rupture of membranes
Preterm PROM accounts for one-third of all preterm births, and is often a leading cause of spontaneous preterm labor. Vascular lesions of the placental bed have been described in patients with preterm PROM, including failure of physiologic transformation of the decidual segment of the spiral arteries, thrombosis, and atherosis.
Only one study has examined the histology of the placental bed in patients with preterm PROM. Kim et al.44 determined the frequency of non-transformed spiral arteries in placental bed biopsies obtained under direct visualization at the time of cesarean section in three groups of patients: 1) normal pregnancies who delivered at term; 2) patients with preterm PROM that underwent cesarean section for obstetrical indications; and 3) patients with preeclampsia. Specimens were stained with cytokeratin and PAS. The study showed that the frequency of failure of physiologic transformation of the myometrial segment of the spiral arteries was higher in patients with preterm PROM than in patients who had a spontaneous delivery at term (completely transformed spiral arteries were observed in 59% of patients who delivered at term, 29% of those with preterm PROM, and 4.3% of patients with preeclampsia (Table 2). The lack of transformation of all vessels in the biopsy specimen was observed in only 6.8% of term deliveries, 52% of patients with preeclampsia, and in 19.4% of patients with preterm PROM. Notably, there was no difference in the rate of failure of physiologic transformation in the decidual or myometrial segments of the spiral arteries in patients with and without histologic chorioamnionitis (Table 3).
Table 2.
The number (percent) of patients who had failure of physiologic transformation of the spiral arteries in normal pregnant women at term and patients with preeclampsia and preterm PROM
| Normal Pregnancy at term |
Preeclampsia | P value | Preterm PROM |
Pa value |
Pb value |
|
|---|---|---|---|---|---|---|
| Myometrial segment of the spiral arteries | ||||||
| Total No. of patients | 59 | 23 | 31 | |||
| Completely transformed in all vessels | 31 (52.5%) | 1 (4.3%) | 9 (29%) | |||
| Partially transformed or mixed transformed and non-transformed vessels | 24 (40.7%) | 10 (43.5%) | 16 (51.6%) | |||
| Non-transformed vessels in all vessels | 4 (6.8%) | 12 (52.2%) | <.0001* | 6 (19.4%) | .016* | .003* |
| Decidual segment of the spiral arteries in the basal plate | ||||||
| Total No. of patients | 52 | 22 | 30 | |||
| Completely transformed in all vessels | 44 (84.6%) | 8 (36.4%) | 25 (83.3%) | |||
| Partially transformed or mixed transformed and non-transformed vessels | 8 (15.4%) | 13 (59.1%) | 5 (16.7%) | |||
| Non-transformed in all vessels | 0 | 1 (4.5%) | <.0001* | 0 | NS | 0.001* |
The P values for pairwise comparisons between diagnostic groups derived from the comparisons of shift in proportions of patients in each classification using Spearman rank correlation.
P = Comparison between preeclampsia and normal pregnancy at term.
Pa = Comparison between preterm PROM and normal pregnancy at term.
Pb = Comparison between preeclampsia and preterm PROM.
PROM: prelabor rupture of membranes; NS: Not significant.
P<0.05
Table 3.
Comparison of number (count) of failure of physiologic transformation of the spiral arteries between patients with preterm PROM with and without histologic chorioamnionitis
| Preterm PROM | |||
|---|---|---|---|
| Without histologic chorioamnionitis |
With histologic chorioamnionitis |
P value | |
| Myometrial segment of the spiral arteries | |||
| Total No. of patients | 11 | 20 | |
| Non-transformed vessels (mean ± SE) | 0.91 ± 0.37 | 0.90 ± 0.28 | NS |
| Completely or partially transformed vessels (mean ± SE) | 1.91 ± 0.79 | 2.75 ± 0.63 | NS |
| Decidual segment of the spiral arteries in the basal plate | |||
| Total No. of patients | 11 | 19 | |
| Non-transformed vessels (mean ± SE) | 0.09 ± 0.09 | 0.32 ± 0.22 | NS |
| Completely or partially transformed vessels (mean ± SE) | 3.18 ± 0.69 | 3.37 ± 0.45 | NS |
NS: Not significant.
Vascular lesions in decidual vessels attached to the placenta also have been detected in patients with preterm PROM. Arias et al.27 reported a higher rate of maternal vascular lesions in patients with preterm PROM than normal pregnancies (35.1% vs. 11.8%, p=0.001). An interesting observation was that the gestational age at delivery was greater in patients who had vascular lesions of the placenta than in those who had acute histologic chorioamnionitis (30.1 weeks vs. 32.3 weeks, p=0.005). This observation is consistent with the results of microbiologic studies as well as those examining the frequency of intra-amniotic inflammation as a function of gestational age.45 Thus, there are two major clusters of patients who delivered preterm: 1) patients with acute inflammatory lesions (acute chorioamnionitis); and 2) those who delivered preterm, but at a later gestational age, in whom the predominant lesions are vascular.
Arias et al. also evaluated the histology of the placenta in 235 consecutive patients admitted with preterm PROM28 and found the presence of maternal or fetal vascular lesions was 20.4% (48/235). Acute histologic chorioamnionitis was found in 43.4% of the cases. Maternal (rather than fetal) vascular lesions accounted for the majority (41/48) of these. Interestingly, most of the vascular lesions (77.1%) occurred in patients with preterm PROM after 30 weeks of gestation. Notably, the “vascular” and “inflammatory” clusters are not exclusive: some patients had both types of lesions.
How can vascular lesions be linked to spontaneous onset of labor with intact or ruptured membranes?
Maternal vascular lesions could lead to preterm labor by causing uteroplacental ischemia. Evidence in support of this hypothesis has been reviewed elsewhere in the literature46 and consists of the following: 1) experimental studies designed to generate a primate model for preeclampsia by causing uterine ischemia showed that a proportion of animals had spontaneous preterm labor and preterm birth;47 2) abruptio placentae, a lesion of vascular origin, is more frequent in women who deliver preterm with intact membranes27;48 or rupture of membranes than in those who deliver at term;49–51 3) women presenting with preterm labor and intact membranes who have an abnormal uterine artery Doppler velocimetry are more likely to deliver preterm than those with normal Doppler velocimetry.52;53 These results are similar to those reported by other investigators studying women before the onset of labor;54 4) the frequency of small-for-gestational-age infants is increased in women who delivered after preterm labor with intact membranes and preterm PROM.55–60 Vascular lesions leading to a compromise of the uterine supply line could account for the association of both preterm labor and both intrauterine growth restriction; and 5) the plasma concentrations of soluble endoglin (sEng), a potent anti-angiogenic factor, were found to be higher in a subset of patients with preterm labor who delivered preterm than in those who delivered at term even prior to the diagnosis of preterm labor.61 Moreover, there was a relationship between the high concentration of sEng and the presence of placental lesions consistent with maternal underperfusion.61
The precise mechanisms responsible for the onset of preterm parturition in women with uteroplacental ischemia have not been determined. A role for the renin-angiotensin system has been postulated as the fetal membranes are endowed with a functional renin-angiotensin system,62 and uterine ischemia increases the production of uterine renin.63;64 Angiotensin II can induce myometrial contractility directly65 or through the release of prostaglandins.66 When uteroplacental ischemia is severe enough to lead to decidual necrosis and hemorrhage, thrombin may activate the common pathway of parturition. Evidence in support of this includes: 1) decidua is a rich source of tissue factor, the primary initiator of coagulation;67 2) intrauterine administration of whole blood to pregnant rats stimulates myometrial contractility,68 while heparinized blood does not (heparin blocks the generation of thrombin);68 3) fresh whole blood stimulates myometrial contractility in vitro, and this effect is partially blunted by incubation with hirudin, a thrombin inhibitor;68 4) thrombin stimulates myometrial contractility in a dose-dependent manner;68 5) thrombin stimulates the production of MMP-169, urokinase type plasminogen activator (uPA), and tissue type plasminogen activator (tPA) by endometrial stromal cells in culture;70 matrix metalloproteinase-1 can digest collagen directly, while uPA and tPA catalyse the transformation of plasminogen into plasmin which, in turn, can degrade type III collagen and fibronectin,71 important components of the extracellular matrix in the chorioamniotic membranes;72 6) thrombin/anti-thrombin (TAT) complexes, markers of in vivo generation of thrombin, are increased in the plasma73 and amniotic fluid74 of women in preterm labor and with preterm PROM; 7) an elevation of plasma TAT complex concentration in the second trimester is associated with subsequent preterm PROM;75 8) the presence of retroplacental hematoma detected by ultrasound examination in the first trimester is associated with adverse pregnancy outcomes, including preterm birth and fetal growth restriction;76 and 9) the presence of vaginal bleeding in the first or second trimester is associated with preterm birth and other adverse perinatal outcomes.77–80
Placental Abruption
The initial event leading to placental abruption is thought to be an ischemic lesion of the decidua, resulting in decidual necrosis, vascular disruption and bleeding. In the event of hemorrhage, laceration and dissection along a decidual plane and placental separation occur. The latter produces more vascular rhexis, arterial hemorrhage and retroplacental accumulation of blood.
Absence of physiologic transformation of the spiral arteries was reported in 58% (7/12) of patients with abruptio placentae.81 Intimal or subintimal thickening of the spiral arteries was observed in five cases, and in every case, it occurred in a non-transformed vessel. The authors indicated that the morphologic changes were similar to those described by Brosens,82 who suggested that they may be the result of previous thrombosis. In conclusion, failure of physiologic transformation is observed in a large number of patients with placental abruption.
Primary Antiphospholipid Syndrome
Primary antiphospholipid syndrome during pregnancy is associated with a higher rate of spontaneous abortion, intrauterine growth restriction, preeclampsia, preterm birth and fetal death.83;84 Studies of the placenta have demonstrated an excess of lesions associated with underperfusion; in particular, placental infarction, thrombosis, fibrinoid necrosis and atherosis85–87 as well as defective physiologic transformation of the spiral arteries.86
A study conducted by Stone et al.88 systematically examined the characteristics of the placental bed obtained at the time of cesarean section in 12 patients with primary antiphospholipid syndrome and 16 controls. The biopsy specimens were stained with a panel of antibodies including vascular cell adhesion molecule-1 (VCAM-1) as a marker of endothelial cell activation, and also antibodies to CD68 for detection of macrophages. In addition, trophoblast was identified using cytokeratin, human alpha smooth muscle actin to identify smooth muscle, and PAS to detect fibrinoid. Orcein was used to detect the presence of elastin. The presence of extravillous trophoblasts was considered indicative that the sample was obtained from the placental bed. Decidua and myometrium were assessed for interstitial trophoblast distribution.
Placental bed biopsies of patients with antiphospholipid antibodies had a higher density of inflammatory cells (p=0.0001), and particularly macrophages (p=0.014), than those of the control group. These inflammatory cells clustered around the blood vessels; however, there was no difference in the frequency of remodeled spiral arteries between cases and controls. Atherosis was not observed in any case. In three cases, the placental bed biopsies showed necrosis and hyperplastic vessels and one case had arterial thromboses.
This study is important because decidual vasculopathy of the basal plate of the placenta was described in cases with antiphospholipid antibodies and poor pregnancy outcome.85;86 However, the decidual segments may not be representative of the events occurring in the myometrial segment of the spiral arteries. Perhaps the most striking observations in this study were the absence of impaired trophoblast invasion in women with antiphospholipid syndrome, the absence of endothelial cell activation (as determined by staining with VCAM-1) and the presence of an inflammatory reaction in the perivascular area. It should be noted that women with antiphospholipid syndrome were treated with 75mg of aspirin from the pre-conceptional period and low molecular-weight heparin after a positive pregnancy test if there was a history of thrombosis, previous late fetal loss, or recurrent miscarriages. An effect of these therapies on the placental bed findings cannot be excluded.
Recent observations in an animal model of antiphospholipid syndrome by Girardi et al.89 have demonstrated C5a receptors and neutrophils as causal factors in the generation of the pro-thrombotic phenotype in this mouse model. In a second study, Girardi et al. reported that heparin prevented antiphospholipid antibody-induced fetal loss by inhibiting complement activation.90 These two seminal studies suggest that inflammation plays a role in fetal death in this condition, and that heparin is acting not only by inhibiting thrombin, but also interfering with the local inflammatory process.
Sebire et al.91 examined the products of conception obtained at 6 to 14 weeks of gestation from patients with a history of recurrent pregnancy loss classified by the following groups: 1) fetal chromosomal abnormality (n=34); 2) primary antiphospholipid syndrome and normal karyotype (n=31); and 3) normal karyotype without primary antiphospholipid syndrome (n=50). Patients without a history of recurrent pregnancy loss and who underwent voluntary termination of pregnancy were included as controls (n=20). Among patients with a normal karyotype, normal endovascular trophoblast invasion was identified only in 23% of patients with primary antiphospholipid syndrome, compared to 61% (p=0.02) of those with a history of recurrent pregnancy loss but without primary antiphospholipid syndrome, and to 75% (p<0.01) of those with a voluntary termination of pregnancy. The authors suggest that defective early trophoblast invasion, rather than placental thrombosis, may be the main cause of pregnancy failure in this condition.91 It is important to realize that the observations derived from this study depend upon the examination of the products of conception rather than placental bed biopsies. It may be argued that this mode of sampling does not yield equivalent tissue to that obtained from a placental bed biopsy. Moreover, this study reports on the events in the first trimester of pregnancy rather than those observed at the end of pregnancy.
Vascular Pathology as a Mechanism of Disease in Obstetrics
Pregnancy requires vasculogenesis and angiogenesis in the fetal compartment and angiogenesis in the maternal compartment. A mono-allelic deletion of one of the many angiogenic factors, vascular endothelial growth factor (VEGF) causes embryo lethality due to the inability to form a vascular tree.92 Similarly, deletion of several genes is incompatible with the development of a placenta. The maternal circulation must also undergo dramatic changes for a successful pregnancy, including the development of the uteroplacental arteries from the spiral arteries to increase blood delivery to the intervillous space. Although vasodilatation of the spiral arteries can occur before physiologic transformation is completed, most investigators agree that remodeling and transformation of the spiral arteries is required to ensure adequate delivery of nutrients and oxygen to maintain pregnancy to term.
A unique feature of the biology of pregnancy (in contrast to that of other states such as angio-invasion in cancer) is that extravillous trophoblast invades the decidua and myometrium. This represents invasion of semi non-self cells into “self” tissue. A counterpart of this phenomenon seems to be the microchimerism reported after successful transplantation of solid organs. Yet, extravillous invading trophoblast seems to play an important role in remodeling the maternal circulation, and probably in orchestrating the immunological dialogue that must occur in the maternal-fetal interface. These processes are believed to be important, if not essential, to meet the requirements of the conceptus.
Inadequate angiogenesis, thrombosis, and/or inadequate physiologic transformation of the spiral arteries can lead to ischemia of the placenta and the uterus. A catalogue of lesions in the human placenta has been considered the result of maternal underperfusion of the placenta. This includes not only the uteroplacental or spiral arteries, but also the complex cellular and molecular network that operates in the decidua and myometrium to allow normal placentation.
What are the consequences of uteroplacental ischemia? Experimental evidence indicates that decreased blood supply can lead to fetal death, fetal growth restriction, maternal hypertension and preterm labor. The specific clinical phenotype in response to ischemia is probably a function of the severity, timing and duration of the ischemic insult. It is easy to understand that total occlusion of the uterine arteries will lead to sudden fetal death. This can be easily demonstrated in animal models. It could be argued that the closest human counterpart to this phenomenon can be a massive maternal floor infarction,93;94 in which extensive fibrin deposits in the intervillous space makes impossible the exchange of gases and nutrients required for fetal survival. If the degree of ischemia is less severe, a different phenotype will result. Surgical removal of the caruncle in sheep is a well-established model for fetal growth restriction.95–97 An inadequate supply of nutrients can be expected to decrease the rate of fetal growth. Deceleration of fetal growth can be considered to be adaptive if survival is the ultimate goal. In cases of a compromised supply line, fetal growth at a normal rate could lead to fetal death. The parallels between this and the necrosis observed in ischemic regions of tumors provide an understandable scenario of the consequences of mismatched growth (a mismatch between the availability of nutrients and the requirement of the growing fetus). If a reasonable compromise is reached between underperfusion and reduction of fetal growth, then the birth of a small-for-gestational-age infant would be the expected result. On the other hand, if pregnancy continues and the delivery of nutrients is insufficient, nature has another mechanism to support the fetus: the induction of maternal hypertension to maintain or increase uterine blood flow. The phenotype would be gestational hypertension, and when this homeostatic response is driven to the pathologic range, preeclampsia with maternal multi-organ damage. Thus, this adaptive mechanism is employed to support the fetus, but it occurs by placing the mother at risk. This situation is more likely to occur in preterm gestations than in term gestations. When a fetus has undergone chronic underperfusion, reduction in fetal growth often results in accelerated maturation of multiple organ systems. Therefore, when the fetus is close to maturity, the onset of preterm parturition (with intact or ruptured membranes) would solve the conundrum to both hosts.
We propose that the different phenotypes are the result of adaptive mechanisms that, in general, have survival value. In early pregnancy, a severe anti-angiogenic state is likely to lead to fetal death.98–100 Why continue to invest maternal resources when there is not enough of an angiogenic drive to maintain the placenta and fetus? In milder cases of uteroplacental ischemia, isolated small-for-gestational-age fetus (SGA), SGA plus hypertension or late preterm labor could provide a solution.
The nature of obstetrical disease: the Great Obstetrical Syndromes
We have proposed that the nature of obstetrical disease is fundamentally different from that which affects children and adults. Viviparity challenges the physiology of multiple organ systems (not only the reproductive system) to maximize survival of both mother and conceptus. Under ordinary circumstances, the interests of both hosts (mother and fetus) should converge; yet, under pathologic circumstances, they could conflict. For example, during pregnancy, the metabolic system undergoes changes to make glucose, amino acids and other fuels available for fetal growth and development.101;102 Gestational diabetes can be considered a state in which the normal adaptation is pushed to a pathologic extreme, and this change results not only in consequences for the mother (glucose intolerance)102–105 but also for the fetus (e.g. macrosomia, stillbirth, neonatal hypoglycemia, etc.).102;103;106 Women who develop gestational diabetes are also at increased risk of developing Type II diabetes later in life.102;105 This can be interpreted as indicating that the metabolic demands of pregnancy uncover a subclinical condition that would otherwise remain “silent” until much later in life. This example is illustrative of the complexity of defining obstetrical disease, because although gestational diabetes is defined by an abnormal glucose tolerance test of the mother, the fetus/placenta drives the metabolic adaptations of the mother – hence, the occurrence of gestational diabetes. Thus, the fundamental feature of obstetrical disease is that it is the result of interaction between the mother and fetus.
Another challenge of the current taxonomy of disease in obstetrics is that it is based on the clinical presentation of the mother and/or fetus, and not on the mechanism of disease responsible for clinical manifestations. For example, the term “preterm labor” does not indicate whether this condition is caused by an infection, a vascular insult, uterine overdistension, abnormal allogeneic recognition, stress or other pathological processes. The same applies to preeclampsia, small-for-gestational-age, preterm PROM, fetal death, placental abruption, nausea and vomiting during pregnancy, miscarriage, and failure to progress in labor, in which the diagnoses simply describe the clinical manifestations without consideration of the specific etiology.
The possibility that mechanisms of disease not yet discovered may be responsible for pregnancy complications must be considered given the unique biology of pregnancy requiring pacific co-existence of two hosts; therefore, the challenges presented by this intimate relationship could create conditions in which novel mechanisms of disease may emerge. The placental bed is strategically placed (anatomically and functionally) to be a site for the development of pathological processes. It is likely that with the application of modern molecular pathology techniques, and a deeper understanding of the immunobiology of the maternal-fetal interface, disorders in the stroma of the placental bed will be discovered.
We have proposed that the term “syndrome” is more appropriate to refer to the previously mentioned obstetrical disorders. “Syndrome” is defined as “a combination of symptoms and/or signs that form a distinct clinical picture indicative of a particular disorder.” Syndromes can be caused by more than one mechanism of disease and etiology. We have argued that obstetrical disorders responsible for maternal death and perinatal morbidity and mortality are syndromes; hence, the designation of the “Great Obstetrical Syndromes.” 107;108 Key features of these syndromes are: 1) multiple etiologies; 2) a long preclinical stage; 3) frequent fetal involvement; 4) predisposition to a particular syndrome influenced by gene-environment interaction and/or complex gene-gene interactions involving maternal and/or fetal genotypes; and 5) clinical manifestations, which are often adaptive in nature. The concept of the “great obstetrical syndromes” has been discussed elsewhere.107;108
We propose that pathology of the placental bed, primarily through ischemia, but perhaps through other mechanisms (e.g. immune-related), may give rise to preeclampsia, small-for-gestational age fetuses, preterm labor with intact or ruptured membranes, abruptio placentae and fetal death. Why a similar insult would result in a different clinical phenotype is dependent upon genetic and environmental factors, as well as the time of onset, duration and extent of the ischemic insult. Evolutionary pressures derived from the potential conflictual relationship between the fetus and mother is likely to play a role in determining the phenotypic expression of disorders of the placental bed.
Acknowledgment
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that they have no conflict of interest.
Reference List
- 1.Khong Y, Brosens I. Defective deep placentation. Best.Pract.Res.Clin.Obstet Gynaecol. 2010 doi: 10.1016/j.bpobgyn.2010.10.012. in press. [DOI] [PubMed] [Google Scholar]
- 2.Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best.Pract.Res.Clin.Obstet Gynaecol. 2011 doi: 10.1016/j.bpobgyn.2010.10.009. in press. [DOI] [PubMed] [Google Scholar]
- 3.Espinoza J, Romero R, Mee KY, Kusanovic JP, Hassan S, Erez O, et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J.Perinat.Med. 2006;34:447–458. doi: 10.1515/JPM.2006.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2010 doi: 10.1016/j.ajog.2010.08.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khong TY, Liddell HS, Robertson WB. Defective haemochorial placentation as a cause of miscarriage: a preliminary study. Br J Obstet Gynaecol. 1987;94:649–655. doi: 10.1111/j.1471-0528.1987.tb03169.x. [DOI] [PubMed] [Google Scholar]
- 6.Gun BD, Numanoglu G, Ozdamar SO. The comparison of vessels in elective and spontaneous abortion decidua in first trimester pregnancies: importance of vascular changes in early pregnancy losses. Acta Obstet.Gynecol.Scand. 2006;85:402–406. doi: 10.1080/00016340500501731. [DOI] [PubMed] [Google Scholar]
- 7.Michel MZ, Khong TY, Clark DA, Beard RW. A morphological and immunological study of human placental bed biopsies in miscarriage. Br.J Obstet.Gynaecol. 1990;97:984–988. doi: 10.1111/j.1471-0528.1990.tb02468.x. [DOI] [PubMed] [Google Scholar]
- 8.Hustin J, Jauniaux E, Schaaps JP. Histological study of the materno-embryonic interface in spontaneous abortion. Placenta. 1990;11:477–486. doi: 10.1016/s0143-4004(05)80193-6. [DOI] [PubMed] [Google Scholar]
- 9.Burton GJ, Jauniaux E. Oxidative stress. Best.Pract.Res.Clin.Obstet Gynaecol. 2010 doi: 10.1016/j.bpobgyn.2010.10.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball E, Robson SC, Ayis S, Lyall F, Bulmer JN. Early embryonic demise: no evidence of abnormal spiral artery transformation or trophoblast invasion. J.Pathol. 2006;208:528–534. doi: 10.1002/path.1926. [DOI] [PubMed] [Google Scholar]
- 11.Ball E, Bulmer JN, Ayis S, Lyall F, Robson SC. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J.Pathol. 2006;208:535–542. doi: 10.1002/path.1927. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113 Suppl 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin.Obstet.Gynaecol. 1982;9:593–607. [PubMed] [Google Scholar]
- 15.Minkoff H. Prematurity: infection as an etiologic factor. Obstet.Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 16.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin.Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 17.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am.J.Obstet.Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N.Engl.J.Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 21.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 22.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment.Retard.Dev.Disabil.Res.Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 23.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet.Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 24.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, et al. Clinical significance of the presence of amniotic fluid 'sludge' in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet.Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero R, Kusanovic JP, Espinoza J, Gotsch F, Nhan-Chang CL, Erez O, et al. What is amniotic fluid 'sludge'? Ultrasound Obstet.Gynecol. 2007;30:793–798. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero R, Schaudinn C, Kusanovic JP, Gorur A, Gotsch F, Webster P, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet.Gynecol. 2008;198:135. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 28.Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet.Gynecol. 1997;89:265–271. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 29.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am.J.Obstet.Gynecol. 1995;172:1097–1103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 30.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet.Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet.Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J.Perinatol. 1990;10:347–350. [PubMed] [Google Scholar]
- 33.Ogge G, Romero R, Lee DC, Gotsch F, Nandor GT, Lee J, et al. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol. 2011;223:553–565. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod.Pathol. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Kusanovic JP, Gomez R, Lamont R, Bytautiene E, Garfield RE, et al. The clinical significance of eosinophils in the amniotic fluid in preterm labor. J Matern.Fetal Neonatal Med. 2010;23:320–329. doi: 10.3109/14767050903168465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garfield RE, Bytautiene E, Vedernikov YP, Marshall JS, Romero R. Modulation of rat uterine contractility by mast cells and their mediators. Am.J.Obstet.Gynecol. 2000;183:118–125. doi: 10.1067/mob.2000.105741. [DOI] [PubMed] [Google Scholar]
- 37.Bytautiene E, Romero R, Vedernikov YP, El Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am.J.Obstet.Gynecol. 2004;191:1356–1361. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 38.Csapo AI, Pohanka O, Kaihola HL. Progesterone deficiency and premature labour. Br.Med.J. 1974;1:137–140. doi: 10.1136/bmj.1.5899.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Check JH, Lee G, Epstein R, Vetter B. Increased rate of preterm deliveries in untreated women with luteal phase deficiencies. Preliminary report. Gynecol.Obstet.Invest. 1992;33:183–184. doi: 10.1159/000294877. [DOI] [PubMed] [Google Scholar]
- 40.Mazor M, Hershkovitz R, Chaim W, Levy J, Sharony Y, Leiberman JR, et al. Human preterm birth is associated with systemic and local changes in progesterone/17 beta-estradiol ratios. Am.J.Obstet.Gynecol. 1994;171:231–236. doi: 10.1016/0002-9378(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 41.Fidel PI, Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J.Matern.Fetal Med. 1998;7:222–226. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<222::AID-MFM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet.Gynecol. 1999;94:284–289. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 43.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 44.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 45.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet.Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113 Suppl 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Combs CA, Katz MA, Kitzmiller JL, Brescia RJ. Experimental preeclampsia produced by chronic constriction of the lower aorta: validation with longitudinal blood pressure measurements in conscious rhesus monkeys. Am J Obstet Gynecol. 1993;169:215–223. doi: 10.1016/0002-9378(93)90171-e. [DOI] [PubMed] [Google Scholar]
- 48.Arias F. Placental insufficiency: an important cause of preterm labor and preterm premature ruptured membranes. Am J Obstet Gynecol. 1990 [Google Scholar]
- 49.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Preterm premature rupture of the membranes: a risk factor for the development of abruptio placentae. Am J Obstet Gynecol. 1987;156:1235–1238. doi: 10.1016/0002-9378(87)90153-0. [DOI] [PubMed] [Google Scholar]
- 50.Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol. 1988;159:390–396. doi: 10.1016/s0002-9378(88)80092-9. [DOI] [PubMed] [Google Scholar]
- 51.Major C, Nageotte M, Lewis D. Preterm premature rupture of membranes and placental abruption: is there an association between these pregnancy complications? Am J Obstet Gynecol. 1991;164:381. doi: 10.1016/0002-9378(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 52.Brar HS, Medearis AL, DeVore GR, Platt LD. Maternal and fetal blood flow velocity waveforms in patients with preterm labor: prediction of successful tocolysis. Am J Obstet Gynecol. 1988;159:947–950. doi: 10.1016/s0002-9378(88)80178-9. [DOI] [PubMed] [Google Scholar]
- 53.Brar HS, Medearis AL, De Vore GR, Platt LD. Maternal and fetal blood flow velocity waveforms in patients with preterm labor: relationship to outcome. Am J Obstet Gynecol. 1989;161:1519–1522. doi: 10.1016/0002-9378(89)90916-2. [DOI] [PubMed] [Google Scholar]
- 54.Strigini FA, Lencioni G, De Luca G, Lombardo M, Bianchi F, Genazzani AR. Uterine artery velocimetry and spontaneous preterm delivery. Obstet Gynecol. 1995;85:374–377. doi: 10.1016/0029-7844(94)00420-I. [DOI] [PubMed] [Google Scholar]
- 55.Weiner CP, Sabbagha RE, Vaisrub N, Depp R. A hypothetical model suggesting suboptimal intrauterine growth in infants delivered preterm. Obstet Gynecol. 1985;65:323–326. [PubMed] [Google Scholar]
- 56.MacGregor SN, Sabbagha RE, Tamura RK, Pielet BW, Feigenbaum SL. Differing fetal growth patterns in pregnancies complicated by preterm labor. Obstet Gynecol. 1988;72:834–837. doi: 10.1097/00006250-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Ott WJ. Intrauterine growth retardation and preterm delivery. Am J Obstet Gynecol. 1993;168:1710–1715. doi: 10.1016/0002-9378(93)90681-8. [DOI] [PubMed] [Google Scholar]
- 58.Zeitlin J, Ancel PY, Saurel-Cubizolles MJ, Papiernik E. The relationship between intrauterine growth restriction and preterm delivery: an empirical approach using data from a European case-control study. BJOG. 2000;107:750–758. doi: 10.1111/j.1471-0528.2000.tb13336.x. [DOI] [PubMed] [Google Scholar]
- 59.Bukowski R, Gahn D, Denning J, Saade G. Impairment of growth in fetuses destined to deliver preterm. Am J Obstet Gynecol. 2001;185:463–467. doi: 10.1067/mob.2001.115865. [DOI] [PubMed] [Google Scholar]
- 60.Morken NH, Kallen K, Jacobsson B. Fetal growth and onset of delivery: a nationwide population-based study of preterm infants. Am J Obstet Gynecol. 2006;195:154–161. doi: 10.1016/j.ajog.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Chaiworapongsa T, Romero R, Tarca A, Kusanovic JP, Mittal P, Kim SK, et al. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern.Fetal Neonatal Med. 2009;22:1122–1139. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poisner AM. The human placental renin-angiotensin system. Front Neuroendocrinol. 1998;19:232–252. doi: 10.1006/frne.1998.0166. [DOI] [PubMed] [Google Scholar]
- 63.Katz M, Shapiro WB, Porush JG, Chou SY, Israel V. Uterine and renal renin release after ligation of the uterine arteries in the pregnant rabbit. Am.J.Obstet.Gynecol. 1980;136:676–678. doi: 10.1016/0002-9378(80)91023-6. [DOI] [PubMed] [Google Scholar]
- 64.Woods LL, Brooks VL. Role of the renin-angiotensin system in hypertension during reduced uteroplacental perfusion pressure. Am J Physiol. 1989;257:R204–R209. doi: 10.1152/ajpregu.1989.257.1.R204. [DOI] [PubMed] [Google Scholar]
- 65.Lalanne C, Mironneau C, Mironneau J, Savineau JP. Contractions of rat uterine smooth muscle induced by acetylcholine and angiotensin II in Ca2+free medium. Br.J Pharmacol. 1984;81:317–326. doi: 10.1111/j.1476-5381.1984.tb10081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campos GA, Guerra FA, Israel EJ. Angiotensin II induced release of prostaglandins from rat uterus. Arch.Biol.Med.Exp.(Santiago.) 1983;16:43–49. [PubMed] [Google Scholar]
- 67.Lockwood CJ, Krikun G, Papp C, Toth-Pal E, Markiewicz L, Wang EY, et al. The role of progestationally regulated stromal cell tissue factor and type-1 plasminogen activator inhibitor (PAI-1) in endometrial hemostasis and menstruation. Ann.N.Y.Acad.Sci. 1994;734:57–79. doi: 10.1111/j.1749-6632.1994.tb21736.x. [DOI] [PubMed] [Google Scholar]
- 68.Elovitz MA, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am.J Obstet.Gynecol. 2000;183:799–804. doi: 10.1067/mob.2000.108897. [DOI] [PubMed] [Google Scholar]
- 69.Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern.Fetal Neonatal Med. 2002;11:11–17. doi: 10.1080/jmf.11.1.11.17. [DOI] [PubMed] [Google Scholar]
- 70.Lockwood CJ, Krikun G, Aigner S, Schatz F. Effects of thrombin on steroid-modulated cultured endometrial stromal cell fibrinolytic potential. J Clin.Endocrinol.Metab. 1996;81:107–112. doi: 10.1210/jcem.81.1.8550736. [DOI] [PubMed] [Google Scholar]
- 71.Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry (Mosc.) 2002;67:92–98. doi: 10.1023/a:1013908332232. [DOI] [PubMed] [Google Scholar]
- 72.Aplin JD, Campbell S, Allen TD. The extracellular matrix of human amniotic epithelium: ultrastructure, composition and deposition. J Cell Sci. 1985;79:119–136. doi: 10.1242/jcs.79.1.119. [DOI] [PubMed] [Google Scholar]
- 73.Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S, et al. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern.Fetal Neonatal Med. 2002;11:368–373. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 74.Erez O, Romer R, Vaisbuch E, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, et al. Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J Matern.Fetal Neonatal Med. 2009;22:971–982. doi: 10.3109/14767050902994762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosen T, Kuczynski E, O'Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J.Matern.Fetal Med. 2001;10:297–300. doi: 10.1080/714904361. [DOI] [PubMed] [Google Scholar]
- 76.Nagy S, Bush M, Stone J, Lapinski RH, Gardo S. Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet Gynecol. 2003;102:94–100. doi: 10.1016/s0029-7844(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 77.Funderburk SJ, Guthrie D, Meldrum D. Outcome of pregnancies complicated by early vaginal bleeding. Br.J.Obstet.Gynaecol. 1980;87:100–105. doi: 10.1111/j.1471-0528.1980.tb04500.x. [DOI] [PubMed] [Google Scholar]
- 78.Williams MA, Mittendorf R, Lieberman E, Monson RR. Adverse infant outcomes associated with first-trimester vaginal bleeding. Obstet Gynecol. 1991;78:14–18. [PubMed] [Google Scholar]
- 79.Signore CC, Sood AK, Richards DS. Second-trimester vaginal bleeding: correlation of ultrasonographic findings with perinatal outcome. Am.J.Obstet.Gynecol. 1998;178:336–340. doi: 10.1016/s0002-9378(98)80022-7. [DOI] [PubMed] [Google Scholar]
- 80.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern.Fetal Neonatal Med. 2005;18:31–37. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 81.Dommisse J, Tiltman AJ. Placental Bed Biopsies in Placental Abruption. British Journal of Obstetrics and Gynaecology. 1992;99:651–654. doi: 10.1111/j.1471-0528.1992.tb13848.x. [DOI] [PubMed] [Google Scholar]
- 82.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol.Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 83.Branch DW, Dudley DJ, Scott JR, Silver RM. Antiphospholipid antibodies and fetal loss. N.Engl.J Med. 1992;326:952–954. doi: 10.1007/978-1-4471-3666-8_19. [DOI] [PubMed] [Google Scholar]
- 84.Stone S, Hunt BJ, Seed PT, Parmar K, Khamashta MA, Poston L. Longitudinal evaluation of markers of endothelial cell dysfunction and hemostasis in treated antiphospholipid syndrome and in healthy pregnancy. Am J Obstet.Gynecol. 2003;188:454–460. doi: 10.1067/mob.2003.14. [DOI] [PubMed] [Google Scholar]
- 85.Abramowsky CR, Vegas ME, Swinehart G, Gyves MT. Decidual vasculopathy of the placenta in lupus erythematosus. N.Engl.J Med. 1980;303:668–672. doi: 10.1056/NEJM198009183031204. [DOI] [PubMed] [Google Scholar]
- 86.De Wolf F, Carreras LO, Moerman P, Vermylen J, Van AA, Renaer M. Decidual vasculopathy and extensive placental infarction in a patient with repeated thromboembolic accidents, recurrent fetal loss, and a lupus anticoagulant. Am J Obstet.Gynecol. 1982;142:829–834. doi: 10.1016/s0002-9378(16)32527-3. [DOI] [PubMed] [Google Scholar]
- 87.Out HJ, Kooijman CD, Bruinse HW, Derksen RH. Histopathological findings in placentae from patients with intra-uterine fetal death and anti-phospholipid antibodies. Eur.J Obstet.Gynecol.Reprod.Biol. 1991;41:179–186. doi: 10.1016/0028-2243(91)90021-c. [DOI] [PubMed] [Google Scholar]
- 88.Stone S, Pijnenborg R, Vercruysse L, Poston R, Khamashta MA, Hunt BJ, et al. The placental bed in pregnancies complicated by primary antiphospholipid syndrome. Placenta. 2006;27:457–467. doi: 10.1016/j.placenta.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin.Invest. 2003;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat.Med. 2004;10:1222–1226. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 91.Sebire NJ, Fox H, Backos M, Rai R, Paterson C, Regan L. Defective endovascular trophoblast invasion in primary antiphospholipid antibody syndrome-associated early pregnancy failure. Hum.Reprod. 2002;17:1067–1071. doi: 10.1093/humrep/17.4.1067. [DOI] [PubMed] [Google Scholar]
- 92.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 93.Andres RL, Kuyper W, Resnik R, Piacquadio KM, Benirschke K. The association of maternal floor infarction of the placenta with adverse perinatal outcome. Am J Obstet Gynecol. 1990;163:935–938. doi: 10.1016/0002-9378(90)91100-q. [DOI] [PubMed] [Google Scholar]
- 94.Katzman PJ, Genest DR. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr.Dev.Pathol. 2002;5:159–164. doi: 10.1007/s10024001-0195-y. [DOI] [PubMed] [Google Scholar]
- 95.Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. J Dev.Physiol. 1979;1:379–398. [PubMed] [Google Scholar]
- 96.Robinson JS, Hart IC, Kingston EJ, Jones CT, Thorburn GD. Studies on the growth of the fetal sheep. The effects of reduction of placental size on hormone concentration in fetal plasma. J Dev.Physiol. 1980;2:239–248. [PubMed] [Google Scholar]
- 97.Falconer J, Owens JA, Allotta E, Robinson JS. Effect of restriction of placental growth on the concentrations of insulin, glucose and placental lactogen in the plasma of sheep. J Endocrinol. 1985;106:7–11. doi: 10.1677/joe.0.1060007. [DOI] [PubMed] [Google Scholar]
- 98.Romero R, Chaiworapongsa T, Erez O, Tarca AL, Gervasi MT, Kusanovic JP, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern.Fetal Neonatal Med. 2010;23:1384–1399. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chaiworapongsa T, Romero R, Kusanovic JP, Savasan ZA, Kim SK, Mazaki-Tovi S, et al. Unexplained fetal death is associated with increased concentrations of anti-angiogenic factors in amniotic fluid. J Matern.Fetal Neonatal Med. 2010;23:794–805. doi: 10.3109/14767050903443467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaiworapongsa T, Kusanovic JP, Savasan ZA, Mazaki-Tovi S, Kim SK, Vaisbuch E, et al. Fetal death: a condition with a dissociation in the concentrations of soluble vascular endothelial growth factor receptor-2 between the maternal and fetal compartments. J Matern.Fetal Neonatal Med. 2010;23:960–972. doi: 10.3109/14767050903410664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boden G. Fuel metabolism in pregnancy and in gestational diabetes mellitus. Obstet Gynecol Clin.North Am. 1996;23:1–10. doi: 10.1016/s0889-8545(05)70241-2. [DOI] [PubMed] [Google Scholar]
- 102.Catalano PM, Kirwan JP, Haugel-de MS, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr. 2003;133:1674S–1683S. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- 103.Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern.Fetal Neonatal Med. 2010;23:199–203. doi: 10.3109/14767050903550659. [DOI] [PubMed] [Google Scholar]
- 104.Carpenter MW. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care. 2007;30 Suppl 2:S246–S250. doi: 10.2337/dc07-s224. [DOI] [PubMed] [Google Scholar]
- 105.Lauenborg J, Hansen T, Jensen DM, Vestergaard H, Molsted-Pedersen L, Hornnes P, et al. Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care. 2004;27:1194–1199. doi: 10.2337/diacare.27.5.1194. [DOI] [PubMed] [Google Scholar]
- 106.Garcia Carrapato MR. The offspring of gestational diabetes. J Perinat.Med. 2003;31:5–11. doi: 10.1515/JPM.2003.001. [DOI] [PubMed] [Google Scholar]
- 107.Romero R. The child is the father of the man. Prenat Neonat Med. 1996;1:8–11. [Google Scholar]
- 108.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern.Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]