Abstract
We have developed ‘b’ and ‘c’ isoform-specific chicken FGF receptor 1–3 probes for in situ hybridization. We rigorously demonstrate the specificity of these probes by using both dot blot hybridization and whole mount in situ hybridization during neurulation and early postneurulation stages, and we compare expression patterns of each of the three isoform-specific probes to one another and to generic probes to each of the three (non-isoform-specific) FGF receptors. We show that the expression pattern of each receptor is represented by the collective expression of each of its two isoforms, with the expression of each FGF receptor being most similar to that of its ‘c’ isoform at two of the three stages studied, and that tissue and stage differences exist in the patterns of expression of the six isoforms. We demonstrate the usefulness of these probes for defining the differential tissue expression of FGF receptor 1–3 isoforms.
Keywords: FGFs, Isoforms, FGF Receptors, In Situ Hybridization, Dot Blots
INTRODUCTION
Whole mount in situ hybridization has been widely used to investigate patterns of gene expression during embryogenesis (e.g., see the online chick expression database GEISHA: http://geisha.arizona.edu/geisha; also see links to expression databases of other species at: http://geisha.arizona.edu/geisha/links.jsp?category=5; Bell et al., 2004; Darnell et al., 2007). Particularly useful for understanding signaling during development are comprehensive expression studies comparing transcripts of multiple members of a family of ligands across several stages or different species, as well as the comparison of patterns of expression of transcripts of both ligands and their receptors or other downstream or related components. Owing to the typically high conservation of nucleotide sequence between family members, synthesizing specific probes for in situ hybridization can be challenging. This is especially true for isoforms of a gene.
The fibroblast growth factor (FGF) family plays critical roles in development by regulating cell proliferation, differentiation, and cell migration, and FGF signaling directs the development of a number of organ rudiments (Goldfarb, 1996; Ornitz and Itoh, 2001; Böttcher and Niehrs, 2005; Itoh and Ornitz, 2010). The FGF family consists of 22 ligands in mammals and 4 receptors (R1-4), with receptors 1–3 each having two isoforms, termed ‘b’ and ‘c’ isoforms (Ornitz and Itoh, 2001). Although studies of the expression of transcripts for many of the FGF ligands have been conducted during early chick development (e.g., Karabagli et al., 2002a, b; Paxton et al., 2010), as well as those for FGF receptors 1–3 (Walshe and Mason, 2000; Lunn et al., 2007), expression of transcripts for isoform-specific FGF receptors have not been described. Thus, progress in understanding potential roles for these isoforms in FGF signaling during early chick development has been hindered.
We have developed isoform-specific chick FGF receptor probes to detect FGF receptors 1–3 by whole mount in situ hybridization. We describe their patterns of expression in chick embryos during neurulation and early postneurulation stages, and we demonstrate that at these stages ‘b’ and ‘c’ isoforms have different patterns of expression that change over time, suggesting potentially unique roles for individual isoforms during early development.
RESULTS AND DISCUSSION
FGF receptor isoforms are structurally similar
FGF receptors (FGFRs) contain several common structural features including a hydrophobic leader sequence, three immunoglobulin–like (Ig) domains, an acidic box, a cell adhesion molecule homology domain, a transmembrane domain, and a divided tyrosine kinase domain (Fig. 1A). FGFRs normally exist as inactive monomers, and upon ligand binding, they become dimerized and activated (Spivak-Kroizman et al., 1994). Activated FGFRs serve as binding sites for proteins with SH2 domains (Mohammadi et al., 1991). The recruitment of SH2 domain proteins results in phosphorylation and activation of downstream signaling intermediates that together trigger a network of complex signal transduction cascades (Eswarakumar et al., 2005).
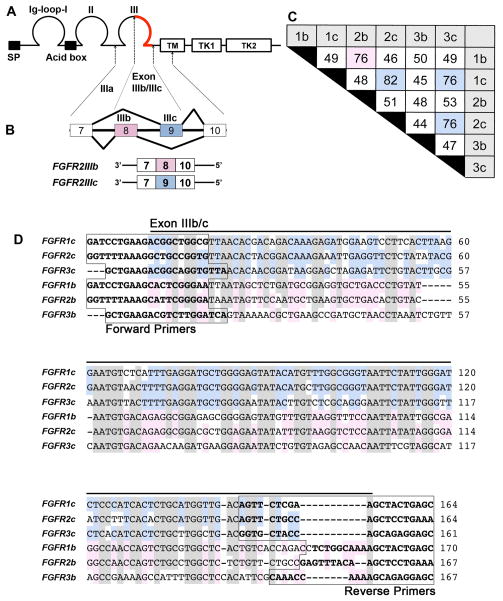
Figure 1. The FGF receptor isoforms generated by alternative splicing.
A, Diagram of the primary structure of FGF receptors showing a signal peptide (SP), three immunoglobulin-like (Ig) domains (labeled as Ig-loop-I, II, III), a stretch of seven conserved acidic amino acids between IgI and II (Acid box), a single transmembrane domain (TM), and two intracellular tyrosine kinase domains (TK1, 2). B, Alternative splicing of FGF receptors occurs in the IgIII domain, resulting in the corresponding ‘b’ or ‘c’ FGF receptor isoforms. As an example, FGFR2 is diagrammed to show that exons 8 and 9 are alternatively spliced to form the ‘b’ and ‘c’ isoforms. C, The nucleotide identity of the C-terminal half of the IgIII domain of each isoform is tabulated as percent identity, with an identity over 75% being highlighted in pink (isoform ‘b’) or blue (isoform ‘c’). The highest identity occurs between members of isoform ‘c’, with the highest identity of 82% occurring between FGFR1c and FGFR2c. D, The nucleotide sequence alignment of the C-terminal half of the IgIII domain of each isoform. High base pair identity occurs among all six isoforms (shown shaded in gray), with further identity within ‘c’ (blue) and ‘b’ (pink) subgroups. The boxed sequences indicate the location of primers for each isoform.
An intriguing feature of FGFRs is that a variety of isoforms are generated by alternative splicing of FGFR transcripts. Three exons encode Ig domains IIIa, b, and c of FGFRs (Givol and Yayon, 1992; Yayon et al., 1992) with two undergoing alternative splicing, such that the Ig domain III of FGFRs consists of either IgIIIa/IgIIIb or IgIIIa/IgIIIc, which constitutes the ‘b’ and ‘c’ isoforms of FGFRs, respectively (Fig. 1B). Alternative splicing in Ig domain III affects ligand-binding specificity (Miki et al., 1992; Yayon et al., 1992). For example, FGFR1b shows an affinity for binding with FGF3 and FGF10, but not with FGF4 and FGF6, whereas FGFR1c shows an affinity for binding with FGF4 and FGF6, but not with FGF3 and FGF10. Furthermore, although FGFR2b binds with FGF3 and FGF10, but not with FGF4 and FGF6, the FGFR2c isoform binds with FGF4 and FGF6, but not with FGF3 and FGF10 (Eswarakumar et al., 2005). Comparison of the nucleotide sequence of the exons encoding IIIb/c (Fig. 1C, D) showed that FGFR1b and FGFR2b have a higher sequence identity (Fig. 1C: 76%) than each has with its ‘c’ isoform (Fig. 1C: 49% and 51%, respectively), and FGFR1c and FGFR2c have a higher sequence identity (Fig. 1C: 82%) than each has with its ‘b’ isoform, consistent with the preferred combinations of receptor and ligand binding described above. Among the ‘b’ isoform of the FGF receptors, FGFR1b and FGFR2b showed the highest sequence identity (Fig. 1C: 76%; pink box), and among the ‘c’ isoform of the receptor, all three receptors share more than 75% sequence identity (Fig. 1C, blue boxes, 82%, 76%, 76%).
Nucleotide alignment within the alternative spliced region for several vertebrates is included in Supplemental Figure 1. The figure demonstrates a high level of nucleotide identity in this region among species.
Isoform-specific probes hybridize with high specificity in dot blots
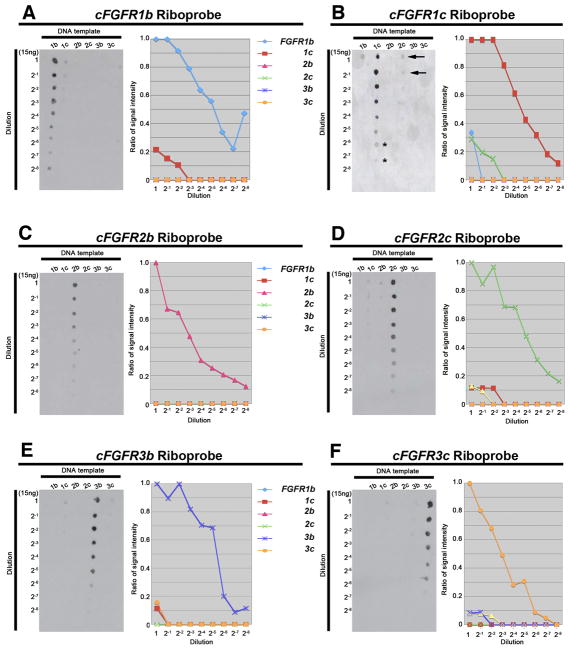
As just discussed, analysis of nucleotide sequence revealed a high degree of identity among some of the FGF receptor isoforms. It has been reported that if a non-target sequence has >70–80% identity to the target sequence, the probe generated against the target sequence might hybridize indiscriminately to the non-target sequence (Evertsz et al., 2001; Xu et al., 2001). To test the specificity of our riboprobes, we performed dot blot hybridization.
Dot blot membranes containing DNA templates of all six of the ‘b’ and ‘c’ isoforms of FGF receptors 1–3 were hybridized with each of our candidate isoform-specific riboprobes. All of our candidate isoform-specific riboprobes hybridized strongly with the appropriate template (Fig. 2), demonstrating their specificity. However, at the highest probe concentrations some non-specific hybridization occurred. For example, although our FGFR1b isoform riboprobe strongly recognized the FGFR1b target sequence, it also hybridized weakly to the FGFR1c template at the highest probe concentrations (Fig. 2A, left panel), despite having less than 50% sequence identity (Fig. 1C). The hybridization affinity of our FGFR1b probe to FGFR1b template was about 5-fold greater than that of our FGFR1b isoform probe to FGFR1c template (Fig. 2A, right panel). Similarly, our other isoform probes, particularly our FGFR1c (Fig. 2B, left panel), FGFR2c (Fig. 2D, left panel), and FGFR3b (Fig. 2E, left panel) probes, weakly hybridized with blots other than those that contained target sequence; again, hybridization was significantly greater with the target sequence (3–10 fold; Figs. 2B, D, and E, right panels). Interestingly, in only one of these three cases did cross-hybridization occur between probe and template having greater than 70% sequence identity (82%: FGFR1c probe to FGFR2c template; Fig. 1C). Thus, the strong hybridization of our riboprobes to their target DNA and their minimal and significantly weaker (i.e., only at the highest probe concentrations) hybridization to non-target DNA suggested that our probes were isoform-specific and likely to provide reliable results for whole mount in situ hybridization.
Figure 2. Dot blot hybridization demonstrates the high specificity of FGF receptor isoform riboprobes.
Isoform target DNAs were diluted serially and spotted onto nitrocellulose membranes. Hybridization was performed with a single FGF receptor isoform riboprobe using the same conditions for all riboprobes. A-F, All probes identified their corresponding targets on dot blots (left panel in each figure) with the highest specificity. Faint cross-hybridization can be seen, for example, in the FGFR1c and FGFR2c probe/target interactions (B, arrows), however, the intensity of this cross-hybridization corresponds to a 1:128–256 dilution (2−7, 2−8; asterisks), providing evidence that riboprobes have high specificity for their targets, even in the instance of FGFR1c and FGFR2c, where base pair identity is the highest (82%). The dot blot signal was quantified with ImageJ to produce the graphs (right panel in each figure).
Overview of the tissue specificity of ‘b’ and ‘c’ isoforms of FGF receptors
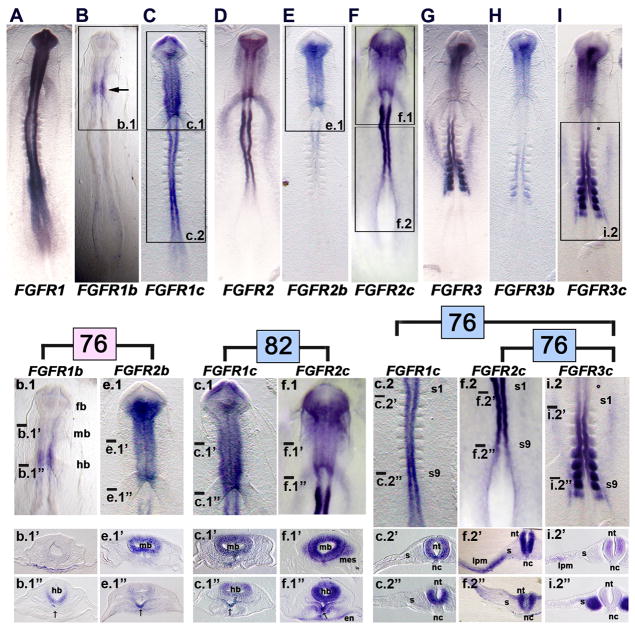
It is generally believed that in developing organs, ‘b’ isoforms of FGF receptors are expressed within epithelial tissues, whereas ‘c’ isoforms are expressed within mesenchymal tissues (e.g., reviewed by Ornitz et al., 1996; Ornitz and Itoh, 2001). This tissue relationship seems to apply specifically for FGFR2 isoforms, but not necessarily for other FGFRs (e.g., FGFR3 isoforms; Scotet and Houssaint, 1995). As detailed below for early stages of chick development, this tissue restriction in isoform expression did not exist. For example, in HH stage 9 embryos, both the ‘b’ and ‘c’ isoforms of FGFR1, 2, and 3 were expressed in the neural tube—an epithelial structure (Figs. 3B, C; 3E, F; 3H, I). However, expression of the ‘b’ isoform was consistently weaker and more spatially restricted than that of the ‘c’ isoform. Similarly, at HH stage 13, both the ‘b’ and ‘c’ isoforms of FGFR1, 2, and 3 were expressed in the neural tube, with the expression of the ‘b’ isoform being consistently weaker and more spatially restricted than that of the ‘c’ isoform (Figs. 4B, C; 4E, F; 4H, I). At both HH stage 9 and 13, isoform expression (as well as generic FGFR expression), was largely restricted to epithelial tissue, rather than mesenchyme, including areas of epithelialized mesoderm such as the developing presomitic mesoderm (somites) or lateral plate mesoderm (e.g., HH stage 9: Figs. 3f.2′, i.2″; HH stage 13: Figs. 4C.2‴, 4i.2″, 4i2″). Interestingly, only the ‘c’ isoform was expressed in epithelialized mesoderm, revealing at least some tissue specificity at these early stages.
Figure 3. Whole mount in situ hybridization demonstrates the specificity of hybridization of FGF receptor isoform riboprobes in HH stage 9 embryos.
A, FGFR1, B, FGFR1b (the boxed area is shown at a higher magnification in b.1; arrow, restricted expression of FGFR1b to the caudal midbrain region), and C, FGFR1c (the boxed areas are shown at a higher magnification in c.1 and c.2) show patterns of expression of FGF receptor 1. b.1, Head region of the FGFR1b labeled embryo shown in B (transverse sections at the craniocaudal levels indicated by the lines are shown in b.1′ and b.1″). c.1, Head region of the FGFR1c labeled embryo shown in C (transverse sections at the craniocaudal levels indicated by the lines are shown in c.1′ and c.1″). c.2, Trunk region of the FGFR1c labeled embryo shown in C (transverse sections at the craniocaudal levels indicated by the lines are shown in c.2′ and c.2″). fb, forebrain; mb, midbrain; hb, hindbrain; s1, somite 1; s9, somite 9; s, somite; nt, neural tube (spinal cord level); nc, notochord; arrows in b.1′ and c.1″ indicate the expression in the ventral midline foregut.
D, FGFR2, E, FGFR2b (the boxed area is shown at a higher magnification in e.1), and F, FGFR2c (the boxed areas are shown at a higher magnification in f.1 and f.2) show patterns of expression of FGF receptor 2. e.1, Head region of the FGFR2b labeled embryo shown in E (transverse sections at the craniocaudal levels indicated by the lines are shown in e.1′ and e.1″). f.1, Head region of the FGFR2c labeled embryo shown in F (transverse sections at the craniocaudal levels indicated by the lines are shown in f.1′ and f.1″). f.2, Trunk region of the FGFR2c labeled embryo shown in F (transverse sections at the craniocaudal levels indicated by the lines are shown in f.2′ and f.2″). mb, midbrain; hb, hindbrain; en, endoderm; mes, head mesenchyme; en, endoderm of the anterior intestinal portal; lpm, lateral plate mesoderm; s, somite; nt, neural tube; nc, notochord; arrows in e.1″ and f.1″ indicate expression in the ventral midline foregut.
G, FGFR3, H, FGFR3b, and I, FGFR3c (the boxed area is shown at a higher magnification in i.2) show patterns of expression of FGF receptor 3. i.2, Trunk region of the FGFR3c labeled embryo shown in I (transverse sections at the craniocaudal levels indicated by the lines are shown in i.2′ and i.2″). s1, somite 1; s9, somite 9; s, somite; lpm, lateral plate mesoderm; nt, neural tube; nc, notochord.
Enlargements and sections are arranged to facilitate comparisons of expression patterns across the receptor family membranes that share the highest identity, indicated by the pink and blue numbers (74, 81, 77, and 72), as explained in Fig. 1C. In all combinations, differences occur in the patterns of expression as detailed in the text.
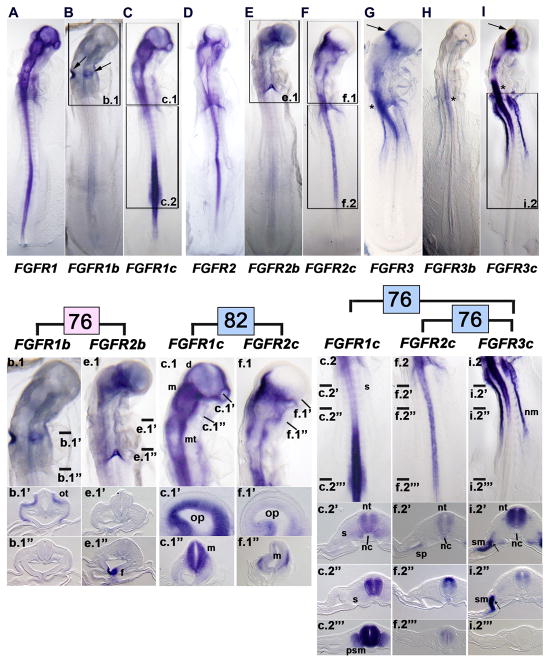
Figure 4. Whole mount in situ hybridization demonstrates the specificity of hybridization of FGF receptor isoform riboprobes in HH stage 13 embryos.
A, FGFR1, B, FGFR1b (the boxed area is shown at a higher magnification in b.1; arrows, expression of FGFR1b in the otic cups), and C, FGFR1c (the boxed areas are shown at a higher magnification in c.1 and c.2). b.1, Head region of the FGFR1b labeled embryo shown in B (transverse sections at the craniocaudal levels indicated by the lines are shown in b.1′ and b.1″). c.1, Head region of the FGFR1c labeled embryo shown in C (transverse sections at the craniocaudal levels indicated by the lines are shown in c.1′ and c.1″). c.2, Trunk region of the FGFR1c labeled embryo (transverse sections at the craniocaudal levels indicated by the lines are shown in c.2′, c.2″, and c.2‴). ot, otic cup; d, diencephalon; m, mesencephalon; mt, metaencephalon; op, optic vesicle; s, somite; nt, neural tube (spinal cord level); nc, notochord; psm, presomitic mesoderm (segmental plate mesoderm).
D, FGFR2, E, FGFR2b (the boxed area is shown at a higher magnification in e.1), and F, FGFR2c (the boxed areas are shown at a higher magnification in f.1 and f.2). e.1, Head region of the FGFR2b embryo shown in E (transverse sections at the craniocaudal levels indicated by the lines are shown in e.1′ and e.1″). f.1, Head region of the FGFR2c embryo shown in F (transverse sections along the indicated line are shown in f.1′ and f.1″). f.2, Trunk region of the FGFR2c labeled embryo shown in F (transverse sections at the craniocaudal levels indicated by the lines are shown in f.2′, f.2″, and f.2‴). f, expressing ventral midline of foregut (thyroid rudiment); op, optic vesicle; m, mesencephalon; nt, neural tube (spinal cord level); nc, notochord; sp, medial splanchnic mesoderm.
G, FGFR3 (arrow, strong expression at the border between the diencephalon and mesencephalon; asterisk, expression within the hindbrain/cranial spinal cord), H, FGFR3b, and I, FGFR3c (the boxed area is shown at a higher magnification in i.2′; arrow, strong expression at the border between the diencephalon and mesencephalon; asterisk, expression within the hindbrain/cranial spinal cord). i.2, Trunk region of the FGFR3c labeled embryo (transverse sections at the craniocaudal levels indicated by the lines are shown in i.2′, i.2″, and i.2‴). nm, nephrogenic mesoderm (nephrotome or intermediate mesoderm); nt, neural tube (spinal cord level); nc, notochord; sm, somatic mesoderm; arrows in i.2′ and i.2″ indicate nephrogenic mesoderm
Enlargements and sections are arranged to facilitate comparisons of expression patterns across the receptor family membranes that share the highest identity, indicated by the pink and blue numbers (74, 81, 77, and 72), as explained in Fig. 1C. In all combinations, differences occur in the patterns of expression as detailed in the text.
In later stages of chick development, a higher level of tissue specificity exists. For example, in HH stage 24 chick wing buds, as assessed with RT-PCR, FGFR2b is expressed only in the ectoderm, whereas FGFR2c and FGFR3c are expressed only in the mesenchyme. In contrast, FGFR1b, FGFR1c, and FGFR3c are expressed in both ectoderm and mesenchyme (Sheeba et al., 2010). Moreover, in the developing chick mandible (HH stages 22–30), as assessed with section in situ hybridization, FGFR2b is expressed mainly in the epithelium, whereas FGFR2c is expressed in the mesenchyme, including Meckel’s cartilage (Havens et al., 2006). Furthermore, in chick proventriculus and gizzard (6.5 days of incubation), as assessed with RT-PCR, FGFR1b and FGFR2b are expressed in the epithelium, whereas FGFR2c and FGFR3c are mainly expressed in the mesenchyme; FGFR1c is expressed at high levels in both the epithelium and mesenchyme (Shin et al., 2005).
Table 3 summarizes the detailed patterns of expression of generic and ‘b’ and ‘c’ isoform of FGF receptors at HH stages 7, 9, and 13. A more detailed description of these patterns at each HH stage studied is given below.
Table 3.
Summary of the tissue expression of FGFRs at three stages of chick development. ND, not detected.
| Stage and domain of expression | FGFR1 | FGFR1b | FGFR1c | FGFR2 | FGFR2b | FGFR2c | FGFR3 | FGFR3b | FGFR3c | |
|---|---|---|---|---|---|---|---|---|---|---|
| HH stage 7, 24 hours | ||||||||||
| neural plate | forebrain level | +++ | +++ | +++ | +++ | + | +++ | ++ | ++ | ++ |
| midbrain level | +++ | +++ | +++ | +++ | + | +++ | ++ | ++ | ++ | |
| hindbrain level | ++ | ++ | ++ | ++ | + | ++ | ++ | + | ++ | |
| spinal cord level | ++ | ++ | ++ | ++ | ND | ++ | ND | ND | ND | |
| endoderm | ND | ND | ND | ++ | ND | ++ | ND | ND | ND | |
| extraembryonic tissues | ND | ND | ND | ND | ND | ND | ++ | ND | ++ | |
| somite | ++ | ND | ++ | ND | ND | ND | +++ | + | +++ | |
| preotic placode | ND | ++ | ND | ND | ND | ND | ND | ND | ND | |
| HH stage 9, 29 hours | ||||||||||
| neural tube | forebrain | +++ | ND | ++ | +++ | ++ | +++ | +++ | ++ | +++ |
| midbrain | +++ | ND | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| hindbrain | +++ | ++ | +++ | ++ | ++ | ++ | ++ | + | ++ | |
| spinal cord | +++ | ND | +++ | +++ | ND | +++ | ++ | + | ++ | |
| head mesenchyme | ND | ND | ND | ++ | ND | ++ | ND | ND | ND | |
| anterior intestinal portal lateral | + | ND | + | ++ | ND | + | ND | ND | ND | |
| lateral plate mesoderm | ND | ND | ND | + | ND | + | ++ | ND | + | |
| intermediate mesoderm | ND | ND | ND | ND | ND | ND | ++ | ND | ++ | |
| somite | ND | ND | ND | ND | ND | ND | +++ | + | +++ | |
| primitive streak | ++ | ND | ++ | ND | ND | ND | ND | ND | ND | |
| HH stage 13, 50 hours | ||||||||||
| neural tube | diencephalon | +++ | ND | ++ | ++ | ++ | ++ | ND | ND | ND |
| mesencephalon | +++ | + | ++ | ++ | ++ | ++ | ND | ND | ND | |
| met/myelencephalon | +++ | ND | +++ | +++ | ND | +++ | +++ | ND | +++ | |
| spinal cord | +++ | ND | +++ | ++ | ND | ++ | +++ | + | +++ | |
| head mesenchyme | ND | ND | ND | ++ | ND | ++ | ND | ND | ND | |
| lateral plate mesoderm | somatic mesoderm | ND | ND | ND | ND | ND | ND | +++ | ND | +++ |
| splanchnic mesoderm | ND | ND | ND | + | ND | + | ND | ND | ND | |
| intermediate mesoderm | ND | ND | ND | ND | ND | ND | + | ND | + | |
| foregut | ND | ND | ND | ++ | ++ | ++ | ND | ND | ND | |
| somite | ND | ND | ND | ND | ND | ND | + | ND | + | |
| presomitic mesoderm | + | ND | ++ | ND | ND | ND | ND | ND | ND | |
| otic cup | ND | +++ | ND | ND | ND | ND | ND | ND | ND | |
| optic vesicle | +++ | ND | +++ | ND | ND | ND | ND | ND | ND | |
In situ hybridization of FGFR isoform riboprobes in HH stage 9 chick embryos reveals unique patterns of expression of various probes
To begin to ask whether our isoform-specific FGFR riboprobes had unique patterns of expression in developing embryos, we performed whole mount in situ hybridization on HH stage 9 chick embryos. The generic FGFR1 riboprobe (like the generic FGFR2 and FGFR3 riboprobes) was generated against sequence outside of the alternatively spliced region to recognize both isoforms. Generic FGFR1 was expressed throughout the newly formed neural tube along its entire craniocaudal extent, and extended into the primitive streak (Fig. 3A). The expression of the FGFR1b isoform was markedly different than that of generic FGFR1, being restricted to a short craniocaudal span of the hindbrain (Fig. 3B). In contrast, expression of the FGFR1c isoform essentially mirrored that of generic FGFR1 (Fig. 3C), clearly showing that most of the expression pattern observed with the generic FGFR1 probe was due to the expression of the FGFR1c isoform, and that the FGFR1b and FGFR1c expression patterns were additive, collectively accounting for the generic FGFR1 expression pattern.
Generic FGFR2, like generic FGFR1, was also expressed throughout most of the craniocaudal extent of the neural tube, beginning cranially in the caudal half of the forebrain and stopping caudally before extending into the primitive streak. In addition, generic FGFR2 was expressed in the head mesenchyme flanking the developing brain, and in the caudal part of the straight heart tube (sinoatrial region at the anterior intestinal portal). Expression of the FGFR2b isoform was restricted to the brain (beginning cranially in the caudal half of the forebrain) and cranial spinal cord (Fig. 3E), whereas expression of the FGFR2c isoform mirrored that of generic FGFR2, occurring throughout the craniocaudal extent of the neural tube (with cranial and caudal borders essentially the same as those of generic FGFR2) and in the head mesenchyme and caudal heart tube (Fig. 3F). This again shows (as for FGFR1) that most of the expression pattern observed with the generic FGFR2 probe was due to the expression of the FGFR2c isoform, and that the FGFR2b and FGFR2c expressions patterns were additive.
Generic FGFR3 was expressed throughout most of the craniocaudal extent of the neural tube, from the caudal forebrain to the spinal cord up to the level of the last formed somite (Fig. 3G). In addition, generic FGFR3 was expressed in the last 6 or so formed somites, in the flanking nephrotome (intermediate mesoderm), and in two wings of lateral plate mesoderm that extended cranially from the most cranially expressing somite pair. Expression of the FGFR3b isoform was restricted to the brain and spinal cord, with a gap in expression at the hindbrain level, and in the caudal 2–3 somite pairs (Fig. 3H). Expression of the FGFR3c isoform (Fig. 3I) closely paralleled that of generic FGFR3, again showing (as for FGFR1 and FGFR2) that most of the expression pattern observed with the generic FGFR3 probe was due to the expression of the FGFR3c isoform, and that the FGFR3b and FGFR3c expression patterns were additive.
Next, we compared in detail the gene expression patterns of probes that showed high sequence identity. Although the FGFR1b and FGFR2b isoforms had 76% sequence identity, their patterns of expression were very different. FGFR1b expression was restricted to the hindbrain (Fig. 3b.1), whereas FGFR2b was expressed throughout the brain, except in the cranial forebrain (Fig. 3e.1). Comparison of sections at the midbrain and hindbrain levels showed that FGFR1b was uniquely expressed in the extraembryonic endoderm at the midbrain level, and not in the neuroectoderm (Fig. 3b.1′), whereas FGFR2b was expressed in the neuroectoderm of the midbrain, and not in the endoderm (Fig. 3e.1′). Furthermore, FGFR1b was expressed strongly in the ventral hindbrain neuroectoderm, and weakly (arrow) in the ventral midline endoderm of the foregut (Fig. 3b.1″), whereas FGFR2b was expressed weakly in the hindbrain neuroectoderm, and strongly in the ventral midline endoderm of the foregut (Fig. 3e.1″).
FGFR1c and FGFR2c, the isoforms with the highest sequence identity (82%), also had significantly different expression patterns. Although both transcripts were broadly expressed in the brain (Figs. 3c.1, 3f.1), FGFR1c expression extended to the cranial end of the forebrain neuroectoderm, but not into the lateral extremes of the optic vesicles (Fig. 3c.1), whereas FGFR2c expression extended only to the caudal half of the forebrain, but also laterally into the optic vesicles (Fig. 3f.1). In contrast to FGFR1c, FGFR2c was also expressed in the head mesenchyme (Fig. 3f.1′), caudal heart tube, and the endoderm of the underlying anterior intestinal portal (Fig. 3f.1″). Finally, both probes were expressed in the ventral midline endoderm of the foregut (thyroid rudiment; Figs. 3c.1″ and f.1″).
Differences also existed in the expression patterns of FGFR1c and FGFR3c (76% sequence identity) and FGFR2c and FGFR3c (76% sequence identity). The most notable difference between the expression patterns of FGFR1c and FGFR3c was that FGFR1c was expressed only in the neuroectoderm of the spinal cord, whereas FGFR3c was expressed both in the spinal cord and the flanking mesoderm (cf. Figs. 3c.2, 3c.2′, and 3c.2″ with Figs. 3i.2, 3i.2′, and 3i.2″). Similarly, although both FGFR2c and FGFR3c were expressed in the neuroectoderm of the spinal cord, expression of FGFR3c stopped at the level of the last formed somites, whereas expression of FGFR2c extended to the level of the primitive streak. Moreover, FGFR3c was expressed within three subdivisions of trunk mesoderm—somite, lateral plate mesoderm, and nephrotome (Figs. 3f.2, f.2′, f.2″)—whereas FGFR2c was expressed only in the lateral plate mesoderm (Figs. 3i.2, i.2′, i.2″).
Unique patterns of FGFR isoform riboprobe expression are maintained in HH stage 13 chick embryos
Both generic FGFR1 and FGFR2 continued to be uniformly expressed throughout most of the craniocaudal extent of the neural tube in HH stage 13 chick embryos (Figs. 4, A, D). Moreover, the patterns of expression of these two receptor probes were mirrored by the expression patterns of the FGFR1c and FGFR2c probes (Figs. 4C, F), but not by that of the FGFR1b and FGFR2b probes (Figs. 4B, E), which were considerably more restricted. A truly unique feature of FGFR1b at this stage is that it was also expressed in the otic cups (arrows, Fig. 4B), as no other FGFR probe was expressed in these structures.
In the mouse embryo, the expression of two isoform-specific FGF receptors, FgfR2c and FgfR3c, have been examined by in situ hybridization. In contrast to the chick embryo, in which only FGFR1b is expressed, both FgfR2c and FgfR3c are expressed in the otic cups and early otic vesicles (Wright et al., 2003).
Generic FGFR3 expression was considerably more restricted at HH stage 13 than that of the other two generic probes (cf. Figs. 4A and 4D with Fig. 4G), but again, the generic FGFR3 pattern was largely mirrored by the expression of the FGFR3c probe (Fig. 4I), with the FGFR3b probe being expressed only in the caudal hindbrain/cranial spinal cord (Fig. 4H, asterisk). Also, unlike generic FGFR1 and FGFR2 probes at this stage, the generic FGFR3 and the FGFR3c probes were robustly expressed in lateral plate mesoderm (somatic mesoderm in particular) and nephrotome (Figs. 4G, I).
As at HH stage 9, detailed examination at HH stage 13 of the expression patterns of the isoforms with the highest sequence identify, showed unique patterns of expression. Whole mounts and sections through various levels of the brain showed that FGFR1b was expressed in the otic pits, whereas FGFR2b was expressed in the thyroid rudiment (cf. Figs. 4b.1, 4b.1′, and 4b.1″ with Figs. 4e.1, 4e.1′, and 4e.1″). Similarly, FGFR1c and FGFR2c had different expression patterns in the diencephalon and developing optic cups (cf. Figs. 4c.1 and 4c.1′ with Figs. 4f.1 and 4f.1′), and the expression patterns of the two other isoforms with high sequence identity differed at the spinal cord level, including the caudal extent of isoform expression in the neural tube and the particular mesodermal subdivision in which each isoform was expressed (cf. Figs. 4c.2 and sections with Figs. 4i.2 and sections; cf. Figs. 4f.2 and sections with Figs. 4i.2 and sections).
FGFR isoform riboprobe expression at later stages of chick development
The expression of isoform-specific FGF receptors has been examined in chick by in situ hybridization at later stages. This includes FGFR2b and FGFR2c in the developing chick mandible (Havens et al., 2006) and FGFR1b, FGFR1c, FGFR3b, and FGFR3c in the developing forelimb bud (Sheeba et al., 2010).
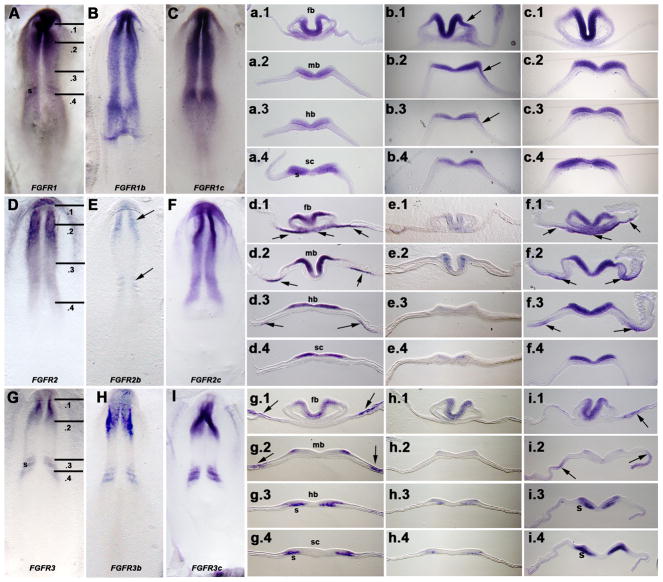
Patterns of receptor isoform expression in neurula stage embryos (HH stage 7) are less unique than at later stages
At neurula stages, all three FGF receptors were expressed almost exclusively within the neuroectoderm, with generic FGFR1 and FGFR2 being expressed throughout the craniocaudal extent of the neuraxis (Figs. 5A, D), and generic FGFR3 being restricted to the midbrain/forebrain and caudal hindbrain/cranial spinal cord levels (Fig. 5G). Our results using generic FGFR1, FGFR2, and FGFR3 probes confirmed previous published patterns of FGF receptors at these stages (Walshe and Mason, 2000; Lunn et al., 2007). In contrast to later stages (discussed above), the patterns of expression of the generic FGFR1 and FGFR3 probes were mirrored by the expression of both the FGFR1b and FGFR3b probes, respectively, and that of the FGFR1c and FGFR3c probes, rather than by just by the ‘c’ isoform probes alone, although for FGFR2, the pattern of expression of the ‘c’ isoform (and not the ‘b’ isoform) mirrored that of the generic FGFR2 pattern, as at later stages. Sections revealed more subtle differences (Figs. 5a-i, .1-.4). In particular, the FGFR1b probe was expressed in the future ectoderm of the otic placode (Fig. 5b.3), as well as more rostrally along the midbrain and forebrain, presaging its expression in the otic pits at stage 13. Moreover, some probes were expressed in extraembryonic tissues (generic FGFR2: arrows Figs. 5d.1-d.3; FGFR2c: arrows Figs. 5f.1-f.4; generic FGFR3: arrows Figs. 5g.1-g.3; FGFR3c: arrows Figs. 5i.1, i.2), somites (generic FGFR3: Figs. 5g.3, g.4; FGFR3c: Figs. 5i.3, i.4), or embryonic endoderm (and some overlying tissue) (generic FGFR2: arrows Figs. 5d.2, d.3; FGFR2c: arrows Figs. 5f.2, f.3).
Figure 5. Whole mount in situ hybridization demonstrates similarity in the patterns of expression of FGF receptor isoforms in HH stage 7 embryos.
A, FGFR1, B, FGFR1b, and C, FGFR1c. Lines in A indicate section planes shown in a.1-a.4, b.1-b.4, and c.1-c.4. fb, forebrain level; mb, midbrain level; hb, hindbrain level; sc, spinal cord level. Arrow in b.3 indicates expression in the ectoderm of the future otic placode, which extends cranially to flank both the midbrain (b.2, arrow) and forebrain (b.1, arrow) levels.
D, FGFR2, E, FGFR2b (weak expression restricted to the cranial and caudal levels of neuraxis), and F, FGFR2c. Lines in D indicate section planes shown in d.1-d.4, e.1-e.4, and f.1-f.4. fb, forebrain level; mb, midbrain level; hb, hindbrain level; sc, spinal cord level. Arrows in d.1, d.2, d.3, f1, f.2, and f.3 indicate expressing areas of endoderm (and in some regions, also overlying tissue).
G, FGFR3, H, FGFR3b, and I, FGFR3c. Lines in G indicate section planes shown in g.1-g.4, h.1-h.4, and i.1-i.4. fb, forebrain level; mb, midbrain level; hb, hindbrain level; sc, spinal cord level; s, somite. Arrows in g.1, g.2, i1 and i.2 indicate expressing areas of ectoderm and/or underlying lateral plate mesoderm.
Similarly, in mouse at comparable (i.e., gastrula/neurula) stages, considerable overlap occurs in the expression patterns of Fgfr2 and its ‘b’ and ‘c’ isoforms (Orr-Urtreger al., 1991, 1993). Thus, in both mouse and chick, expression patterns of the generic and isoform-specific Fgf receptors at these stages are less unique.
EXPERIMENTAL PROCEDURES
Cloning of FGF receptor isoforms
Primers were designed (Table 1) using the Gallus gallus reference genome sequence (May 2006; WUGSC 2.1/galGal3) to amplify each of the FGF receptor isoforms by reverse transcription PCR from total RNA of whole chick embryos at HH stage 6–7 (total RNA isolation: QIAGEN RNeasy Micro kit, RT-PCR: Invitrogen SuperScript One-Step RT-PCR with Platinum Taq). The resulting DNA sequences, containing both isoform-specific and flanking regions of each FGF receptor, were ligated into pCRII-TOPO vector (Invitrogen) and were sequenced. New primers (Table 2) were designed based on this sequencing and were used to amplify only the smaller isoform-specific regions of each FGF receptor, which were cloned into pCRII-TOPO vectors. These plasmids were used as template DNA for both dot blot hybridization and for digoxigenin (DIG)-labeled RNA probe synthesis.
Table 1.
Primers for cloning of FGF receptor isoforms.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| FGFR1b | TCCCCGCCAACAAAACGGTG | TCTGGCAAAAGCTACTGAGC |
| FGFR1c | TCCCCGCCAACAAAACGGTG | AGTTCTCGAAGCTACTGAGC |
| FGFR2b | CAAACGCCTCGGCTGTAGTC | GAGTTTACAAGCTCCTGAAA |
| FGFR2c | CAAACGCCTCGGCTGTAGTC | AGTTCTGCCAGCTCCTGAAA |
| FGFR3b | CAATCAGACTGTGGTGGTCG | CAAACCAAAAGCAGAGGAGC |
| FGFR3c | CAATCAGACTGTGGTGGTCG | GGTGCTACCAGCAGAGGAGC |
Table 2.
Primers for preparation of generic and isoform-specific templates and riboprobes.
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| FGFR1 | ACGCAGGACGGGCCCCTCTAT | CCATCAGAGTGATGTTTGGT |
| FGFR1b | GATCCTGAAGCACTCGGGAA | TCTGGCAAAAGCTACTGAGC |
| FGFR1c | GATCCTGAAGACGGCTGGCG | AGTTCTCGAAGCTACTGAGC |
| FGFR2 | GAGGCATGGAGTACTTGGCT | AAAGATTCTGCTTGTACATA |
| FGFR2b | GGTTTTAAAGCATTCGGGGA | GAGTTTACAAGCTCCTGAAA |
| FGFR2c | GGTTTTAAAGGCTGCCGGTG | AGTTCTGCCAGCTCCTGAAA |
| FGFR3 | TCGGTGCTTGCACGCAGGAC | CAGAGCGATGTCTGGTCTTT |
| FGFR3b | GCTGAAGACGTCTTGGATCA | CAAACCAAAAGCAGAGGAGC |
| FGFR3c | GCTGAAGACGGCAGGTGTTA | GGTGCTACCAGCAGAGGAGC |
During preparation of this manuscript, a paper appeared online describing the cloning of 4 chicken FGF receptor isoforms and their expression, along with the remaining two isoforms, by in situ hybridization in the developing wing bud (Sheeba et al., 2010).
Standardized chicken gene nomenclature was used throughout the text, as described previously (Burt et al., 2009).
Dot blot hybridization
To minimize contamination of bacterial sequence derived from pCRII-TOPO vector, template DNA for dot blot hybridization was prepared by amplifying the specific region of each isoform by PCR (primers shown in Table 2). Each PCR product was purified by QIAquick Gel Extraction (QIAGEN). DIG-labeled RNA probe synthesis was done using a Digoxigenin RNA labeling kit (Roche) according to the manufacturer’s instructions.
Dot blot hybridization was done using standard methods. Briefly, nitrocellulose membranes (GE Healthcare) were presoaked in sterilized water and briefly dried at room temperature. Template DNA containing the sequence of each individual FGF receptor isoform was serially diluted with sterilized water and 1μl of each diluted template was applied onto the membrane and dried at room temperature. Template DNA was denatured in a solution of 1.5M NaCl and 0.5N NaOH, neutralized in a solution of 1.5M NaCl and 1.5M Tris-HCl (pH 7.5), and then rinsed with 2xSSC. Membranes were heated at 70 for 2 hours to crosslink the spotted DNA. The membranes were then prehybridized with DIG Easy hyb solution (Roche) at 55°C for 30 min, the prehybridization solution was decanted, and the preheated DIG Easy hyb solution containing the appropriate RNA probe (at 0.5μg/ml) was added to the membrane and then hybridized at 55°C for 3 hours. After hybridization, the membranes were rinsed with a solution containing 2xSSC and 0.1% SDS at room temperature and washed with a solution containing 0.1xSSC and 0.1% SDS at 68°C. After stringency washes, the membranes were briefly washed with washing buffer containing 0.1M Maleic acid, 0.15M NaCl, and 0.3% Tween 20 (pH 7.5) at room temperature. The membranes were incubated for 30 min in blocking solution containing 1.0% Blocking reagent (Roche), and they were then incubated in blocking solution containing a 1:10000 dilution of anti-DIG-AP antibody. The membranes were washed with washing buffer and equilibrated for 15 min in detection buffer containing 0.1M Tris-HCl (pH 9.5) and 0.1M NaCl. Finally, the membranes were soaked in a 1:200 dilution of CSPD solution (Roche) and exposed to X-ray film to detect the signals.
In situ hybridization for gene expression analysis
Fertilized White Leghorn chicken eggs were purchased from Merrill’s Poultry Farm (Paul, Idaho, USA) and incubated at 38.5°C until embryos reached the desired stages according to the criteria of Hamburger and Hamilton (1951; HH stages; reprinted as Hamburger and Hamilton, 1992). Embryos were fixed with 4% PFA/PBS overnight at 4 and processed for in situ hybridization and subsequent vibratome sectioning (Chapman et al., 2002). For in situ hybridization, the following modifications were made from the published protocol: hybridization was done at 65°C over night, with a probe concentration of 0.5μg/ml; the use of proteinase K was omitted; 2–30 min washes were done with Solution X; and the color reaction was allowed to occur for 7–10 days. Finally, embryos in each group of stages were hybridized in parallel under identical conditions using the three generic and six isoform-specific FGFR probes.
Image levels, brightness, and contrast were adjusted uniformly across each photograph using Photoshop.
Supplementary Material
The alignment of several vertebrate FGF receptor isoforms at the alternative spliced region (Exon IIIb/c) is shown. Solid bar above each sequence marks the isoform-specific region. Asterisks indicate nucleotide identity among all six vertebrates.
Acknowledgments
We gratefully acknowledge the essential financial support of this project from grant no. DC004185 from the NIH, and the helpful discussion of our colleagues.
References
- Bell GW, Yatskievych TA, Antin PB. GEISHA, a whole-mount in situ hybridization gene expression screen in chicken embryos. Dev Dyn. 2004;229:677–687. doi: 10.1002/dvdy.10503. [DOI] [PubMed] [Google Scholar]
- Böttcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Burt DW, Carrë W, Fell M, Law AS, Antin PB, Maglott DR, Weber JA, Schmidt CJ, Burgess SC, McCarthy FM. The Chicken Gene Nomenclature Committee report. BMC Genomics. 2009;10(Suppl 2):S5. doi: 10.1186/1471-2164-10-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Schubert FR, Schoenwolf GC, Lumsden A. Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev Biol. 2002;245:187–199. doi: 10.1006/dbio.2002.0641. [DOI] [PubMed] [Google Scholar]
- Darnell DK, Kaur S, Stanislaw S, Davey S, Konieczka JH, Yatskievych TA, Antin PB. GEISHA: an in situ hybridization gene expression resource for the chicken embryo. Cytogenet Genome Res. 2007;117:30–35. doi: 10.1159/000103162. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Evertsz EM, Au-Young J, Ruvolo MV, Lim AC, Reynolds MA. Hybridization cross-reactivity within homologous gene families on glass cDNA microarrays. BioTechniques. 2001;31:1182, 1184, 1186. doi: 10.2144/01315dd03. passim. [DOI] [PubMed] [Google Scholar]
- Givol D, Yayon A. Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. FASEB J. 1992;6:3362–3369. [PubMed] [Google Scholar]
- Goldfarb M. Functions of fibroblast growth factors in vertebrate development. Cytokine Growth Factor Rev. 1996;7:311–325. doi: 10.1016/s1359-6101(96)00039-1. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Havens BA, Rodgers B, Mina M. Tissue-specific expression of Fgfr2b and Fgfr2c isoforms, Fgf10 and Fgf9 in the developing chick mandible. Arch Oral Biol. 2006;51:134–145. doi: 10.1016/j.archoralbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2010 doi: 10.1093/jb/mvq121. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabagli H, Karabagli P, Ladher RK, Schoenwolf GC. Comparison of the expression patterns of several fibroblast growth factors during chick gastrulation and neurulation. Anat Embryol. 2002a;205:365–370. doi: 10.1007/s00429-002-0264-7. [DOI] [PubMed] [Google Scholar]
- Karabagli H, Karabagli P, Ladher RK, Schoenwolf GC. Survey of fibroblast growth factor expression during chick organogenesis. Anat Rec. 2002b;268:1–6. doi: 10.1002/ar.10129. [DOI] [PubMed] [Google Scholar]
- Lunn JS, Fishwick KJ, Halley PA, Storey KG. A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Dev Biol. 2007;302:536–552. doi: 10.1016/j.ydbio.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, Chan AM, Aaronson SA. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci USA. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Honegger AM, Rotin D, Fischer R, Bellot F, Li W, Dionne CA, Jaye M, Rubinstein M, Schlessinger J. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-gamma 1. Mol Cell Biol. 1991;11:5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A, Bedford MT, Burakova T, Arman E, Zimmer Y, Yayon A, Givol D, Lonai P. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2) Dev Biol. 1993;158:475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Givol D, Yayon A, Yarden Y, Lonai P. Developmental expression of two murine fibroblast growth factor receptors, flg and bek. Development. 1991;113:1419–1434. doi: 10.1242/dev.113.4.1419. [DOI] [PubMed] [Google Scholar]
- Paxton CN, Bleyl SB, Chapman SC, Schoenwolf GC. Identification of differentially expressed genes in early inner ear development. Gene Expr Patterns. 2010;10:31–43. doi: 10.1016/j.gep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotet E, Houssaint E. The choice between alternative IIIb and IIIc exons of the FGFR-3 gene is not strictly tissue-specific. Biochim Biophys Acta. 1995;1264:238–242. doi: 10.1016/0167-4781(95)00156-b. [DOI] [PubMed] [Google Scholar]
- Sheeba CJ, Andrade RP, Duprez D, Palmeirim I. Comprehensive analysis of fibroblast growth factor receptor expression patterns during chick forelimb development. Int J Dev Biol. 2010;54:1517–1526. doi: 10.1387/ijdb.092887cs. [DOI] [PubMed] [Google Scholar]
- Shin M, Watanuki K, Yasugi S. Expression of Fgf10 and Fgf receptors during development of the embryonic chicken stomach. Gene Expr Patterns. 2005;5:511–516. doi: 10.1016/j.modgep.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Spivak-Kroizman T, Lemmon MA, Dikic I, Ladbury JE, Pinchasi D, Huang J, Jaye M, Crumley G, Schlessinger J, Lax I. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell. 1994;79:1015–1024. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Walshe J, Mason I. Expression of FGFR1, FGFR2 and FGFR3 during early neural development in the chick embryo. Mech Dev. 2000;90:103–110. doi: 10.1016/s0925-4773(99)00225-7. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Hatch EP, Karabagli H, Karabagli P, Schoenwolf GC, Mansour SL. Expression of mouse fibroblast growth factor and fibroblast growth factor receptor genes during early inner ear development. Dev Dyn. 2003;228:267–272. doi: 10.1002/dvdy.10362. [DOI] [PubMed] [Google Scholar]
- Xu W, Bak S, Decker A, Paquette SM, Feyereisen R, Galbraith DW. Microarray-based analysis of gene expression in very large gene families: the cytochrome P450 gene superfamily of Arabidopsis thaliana. Gene. 2001;272:61–74. doi: 10.1016/s0378-1119(01)00516-9. [DOI] [PubMed] [Google Scholar]
- Yayon A, Zimmer Y, Shen GH, Avivi A, Yarden Y, Givol D. A confined variable region confers ligand specificity on fibroblast growth factor receptors: implications for the origin of the immunoglobulin fold. EMBO J. 1992;11:1885–1890. doi: 10.1002/j.1460-2075.1992.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The alignment of several vertebrate FGF receptor isoforms at the alternative spliced region (Exon IIIb/c) is shown. Solid bar above each sequence marks the isoform-specific region. Asterisks indicate nucleotide identity among all six vertebrates.