Abstract
Purpose
To investigate possible mutations in the carbohydrate sulfotransferase 6 (CHST6) gene of two unrelated cases of macular corneal dystrophy (MCD) and to report atypical stromal deposits in one of them.
Methods
Corneal tissues were stained with anti-sulfated keratan sulfate (KS), anti-transforming growth factor beta 1-induced protein (TGFBIp), thioflavin-T, alcian blue, and Masson trichrome. Sequencing was performed to identify potential mutations in the CHST6 gene and the fourth and twelfth exons of the TGFBI gene.
Results
Alcian blue staining revealed the presence of multiple subepithelial and intra-stromal mucopolysaccharide deposits, confirming the diagnosis of MCD in both cases. Immunofluorescence staining in case 1 revealed the presence of sulfated KS only in the keratocytes and select endothelial cells, consistent with MCD type IA. Preferential expression of sulfated KS was observed in keratocytes and extracellular stromal matrix in case 2, consistent with MCD type II. Atypical sub-epithelial and superficial stromal deposits were observed in case 1, which stained positively with alcian blue, eosin, Masson trichrome and thioflavin-T indicating the presence of hyaline and amyloid materials. CHST6 gene sequencing revealed two heterozygous mutations in case 1 (a p.Arg211Gln and a novel mutation of p.Arg177Gly) and a novel homozygous mutation of p.Pro186Arg in case 2. No mutations were found in exons 4 or 12 of the TGFBI gene in case 1.
Conclusions
Secondary hyalinosis and amyloidosis occur in a case of MCD type IA with a novel p.Arg177Gly mutation in CHST6. A novel p.Pro186Arg mutation in CHST6 is associated with MCD type II in an African American.
Keywords: Macular corneal dystrophy, keratan sulfate, carbohydrate sulfotransferase 6 (CHST6), alcian blue
INTRODUCTION
Macular corneal dystrophy (MCD) is an autosomal recessive stromal dystrophy, which was previously known as Groenouw corneal dystrophy type II. The condition is characterized by bilateral corneal clouding and central corneal thinning1. Signs of disease include irregularly shaped whitish stromal deposits that appear progressively elevated with diffuse stromal haze extending to the limbus. The corneal endothelium can be affected at advanced stages with formation of guttae and stromal thickening. Visual impairment due to stromal opacities usually starts in the second to third decades of life and occasionally patients may suffer from recurrent epithelial erosions or photophobia.
The carbohydrate sulfotransferase 6 (CHST6) gene on chromosome 16q22, which encodes N-acetylglucosamine 6-O-sulfotransferase, has been identified as the disease-causing gene for MCD2. Mutations in CHST6 gene result in improper sulfation of keratan sulfate (KS), a major glycosaminoglycan of the cornea3. Based on the immuno-reactivity of the macular deposits with a monoclonal antibody (5D4 anti-KS antibody) specific for the sulfated epitopes of KS, MCD has been classified into three sub-types4, 5. In MCD type I, there is no immuno-reactivity seen in the cornea and very little to no reactivity seen in serum. In MCD type IA, only the keratocytes are stained by 5D4 antibody and there is very little or no reactivity seen in serum and corneal stroma. In MCD type II, normal to sub-normal sulfated KS levels in the serum are found, along with immuno-reactivity with sulfated KS in stromal deposits, keratocytes, Descemet’s membrane and endothelial cells. However, these three sub-types are clinically indistinguishable from one another. Herein, we describe two unique and unrelated cases of MCD, each with a novel mutation in the CHST6 gene.
PATIENT AND METHODS
Clinical History
Case 1
A 40-year-old Vietnamese man with a history of bilateral decreased vision and painful recurrent corneal erosions was seen at Ho Chi Minh City Eye Hospital, Vietnam. Both corneas showed indiscrete stromal aggregates and diffusely dense corneal haze throughout the stroma. There were no known systemic diseases or history of corneal disease in the immediate family members. Under a presumptive diagnosis of corneal stroma dystrophy, he received penetrating keratoplasty in the left eye for visual rehabilitation.
Case 2
A 38-year-old African-American woman presented to Washington University Eye Center with decreased vision and severe photophobia in both eyes. In addition to clinical symptoms of recurrent corneal erosions in both eyes, the patient was noted to have mild central corneal thinning with central corneal thickness of 474 μm and 495 μm for the right and left eye, respectively. Multiple nummular stromal deposits at various depths of the corneal stroma were noted in both corneas. Moderate stromal haze extending to the limbus was noted in the intervening spaces between the deposits (Figure 1). There were scattered and subtle endothelial guttae without evident corneal edema. There was no known history of corneal disease or known systemic hereditary diseases in the immediate family members. Under the working clinical diagnosis of macular corneal dystrophy, the patient underwent deep anterior lamellar keratoplasty (DALK) in the left eye for visual rehabilitation.
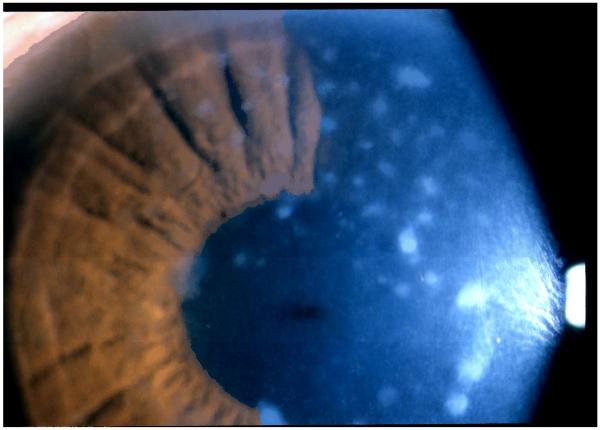
Figure 1.
A slit lamp photo of the left eye of case 2. Multiple nummular stromal opacities that extending to the limbus were observed at various depths of the corneal stoma with diffuse stromal haze in the intervening spaces between the deposits. The central corneal thickness was 495 μm.
The study adheres to the tenets of the Declaration of Helsinki. The retrospective review was approved by Institutional Review Boards of Washington University and Eye Hospital of Ho Chi Minh City, Vietnam, respectively. An informed consent was also obtained from each patient for further gene analysis and histopathological examination. Both excised corneal specimens after keratoplasty were fixed in 10% buffered formalin and submitted to the Department of Pathology, Washington University in Saint Louis, MO for pathological examination. Corneal specimens were subsequently embedded in paraffin and tissue sections of 5μm thickness were then stained with various histochemical dyes.
Laboratory Studies
Staining for sulfated KS and gene sequencing of CHST6 was conducted in the Cornea Research Lab of Washington University. Because case 1 was initially diagnosed with stromal dystrophy, a blood sample was submitted to the John and Marcia Carver Nonprofit Genetic Testing Laboratory at the University of Iowa for genotyping for possible mutations in transforming growth factor beta 1-induced protein (TGFBIp) coding gene.
To detect the presence of sulfated KS, immunofluorescence staining was carried out on de-paraffinized and hydrated tissue sections by incubation with a mouse monoclonal antibody against sulfated KS (5D4, Seikagaku Corporation, Tokyo, Japan) and an AlexaFluor 633-conjugated goat anti-mouse IgG secondary antibody (Invitrogen, Carlsbad, CA). To detect TGFBIp, a rabbit anti-human TGFBIp antibody (Proteintech Group, Chicago, IL) and an AlexaFluor 633 goat anti-rabbit IgG secondary antibody (Invitrogen, Carlsbad, CA) were used. Thioflavin-T (ThT) was used to detect amyloid deposits.
For mutational analyses, DNA was isolated from the patients’ blood using a DNA extraction kit (DNeasy, Qiagen Inc, Valencia, CA), according to the manufacturer’s protocols. The coding region of the CHST6 gene was amplified by polymerase chain reaction (PCR). Each PCR reaction was carried out in a 40 μl reaction mixture containing genomic DNA (25-40 ng), primers (2 ng/μl each), Taq DNA polymerase (0.1 μl, Invitrogen, Carlsbad, CA), MgCl2 (1.5 mM), dNTPs (0.2 mM each), and 1x PCR buffer (provided with Taq polymerase). Amplification reactions were performed under the following conditions: 2 min of denaturation at 96°C, followed by 35 cycles of denaturation at 96°C, annealing at 55 °C or 57°C, and extension at 72°C, for 30 sec each, followed by further extension at 72°C for 5 min. The following primer sets, designed by Akama et al.2, were used: for the 5′-coding region, CK71h-intrn (5′-GCCCCTAACCGCTGCGCTCTC-3′) and CK71h-R1180 (5′-GGCTTGCACACGGCCTCGCT-3′); for the middle coding region, CK71h-F1041 (5′-GACGTGTTTGATGCCTATCTGCCTTG-3′) and CK71h-R1674 (5′-CGGCGCGCACCAGGTCCA-3′); for the 3′-coding region, CK71h-F1355 (5′-CTCCCGGGAGCAGACAGCCAA-3′) and CK71h-R1953 (5′-CTCCCGGGCCTAGCGCCT-3′). Another pair of primers designed by El-Ashry et al.6 was also used for amplification: CK71M-F1 (5′-GACATGGACGTGTTTGATGC-3′) and CK71M-R2 (5′-ATCCGTGGGTGATGTTATGG-3′). For direct sequencing, PCR products were purified from a 1% agarose gel using the Qiaquick purification kit (Qiagen Inc, Valencia, CA), and sequencing reactions were carried out with the same primers as those used for amplification, along with two other primers designed by El-Ashry et al.6: CK71M-R1 (5′-GCACGATGCCGTTGTCAC-3′) and CK71M-F2 (5′-GCTCAACCTACGCATCGTG-3′)6. Sequencing reactions were carried out in forward and reverse directions for 60 cycles at 95°C for 10 sec for denaturation, at 50°C for 5 sec for annealing and at 60°C for 4 min for extension, with the Terminator BigDye 3.1 sequencing reagent (Applied Biosystems, Foster City, CA). Automated DNA sequencing was then performed on the reaction products using the Applied Biosystems 3730 DNA sequencer (Foster City, CA) by the Protein and Nucleic Acid Chemistry Laboratory (PNACL) at Washington University School of Medicine.
RESULTS
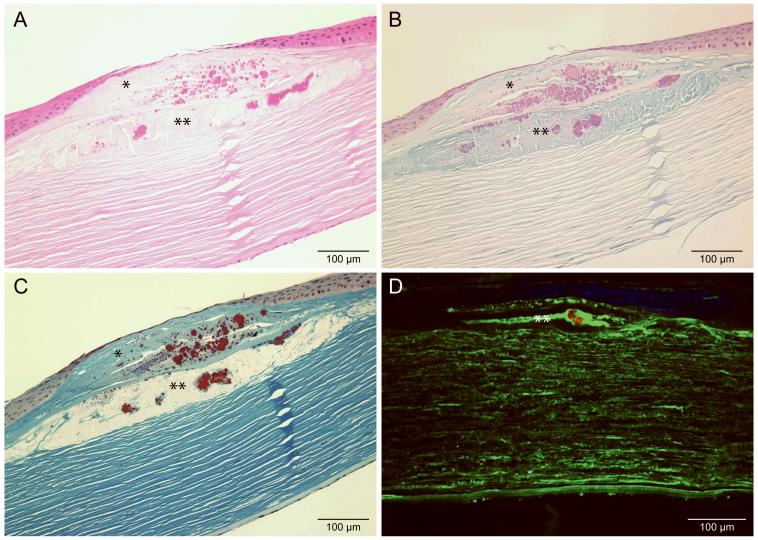
Histochemical staining of tissue sections from case 1 are shown in Figure 2. In the section stained with hematoxylin and eosin, both the stromal and epithelial regions of these lesions contain deposits that stain with eosin (Figure 2A). Staining with alcian blue revealed the presence of classic mucopolysaccharide accumulations in the stroma, supporting a diagnosis of MCD (Figure 2B). In addition to these small and evenly distributed stromal deposits, we observed large alcian blue-stained deposits in some areas of the anterior stroma (Figure 2B). Most interestingly, in other regions of the central cornea, we also observed some deposits in the anterior stroma extending profusely into the epithelial layer of the cornea. While most of the superficial stromal component of this lesion stained with alcian blue (Figure 2B), there were a few deposits that stained with Masson trichrome in the sub-epithelial region (Figure 2C) and within the large alcian blue-positive aggregates (Figure 2B). TGFBIp was found to be present in the larger aggregates in the superficial stroma (Figure 2D, in green), as well as throughout the lamellae of the stroma which is also found in the normal cornea (data not shown). However, we also consistently observed some amyloid deposits by ThT within the larger anterior stromal aggregates in multiple tissue sections (Figure 2D, in red).
Figure 2.
Histological studies of paraffin sections from the cornea of case 1 stained with A, hematoxylin-eosin, B, alcian blue, C, Masson trichrome, and D, immunofluorescent image of cornea stained with TGFBI antibody (green), ThT for amyloid (red) and nuclear stain SYTOX Orange (blue). Alcian blue staining shows the presence of acid mucopolysaccharides in the large superficial stromal aggregate (depicted by ** in B). Masson trichrome staining shows presence of hyaline material in both the sub-epithelial (depicted by * in C) and superficial stromal regions (depicted by ** in C). ThT staining shows the presence of amyloid in the large superficial stromal aggregate (depicted by ** in D). TGFBI is noted throughout the stroma, similar to normal tissue (not shown).
Histochemical staining of corneal sections from case 2 with alcian blue also revealed the presence of multiple subepithelial and intra-stromal mucopolysaccharide deposits. This data confirms the diagnosis of MCD (Figures 3C and 3D).
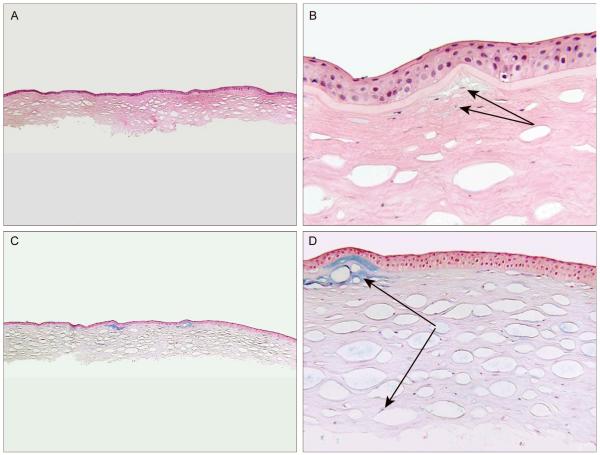
Figure 3.
A representative section of left corneal button from case 2 from deep anterior lamellar keratoplasty (A and B, H&E staining; C and D, alcian blue staining). A, Note numerous round to oval vacuoles in corneal stroma, consistent with air injection used for lamellar dissection (magnification, X40). B, Note slightly basophilic deposits beneath Bowman’s layer in anterior stroma (arrows, magnification, X400). C, Deposits stain positive for alcian blue throughout corneal stroma, confirming the diagnosis of MCD (magnification, X40). D, Higher magnification demonstrates that prominent alcian blue-positive deposits are present in the subepithelial layer and anterior stroma (top arrow), but less conspicuous interlamellar deposits can also be seen throughout the stroma (lower arrow, magnification, X200).
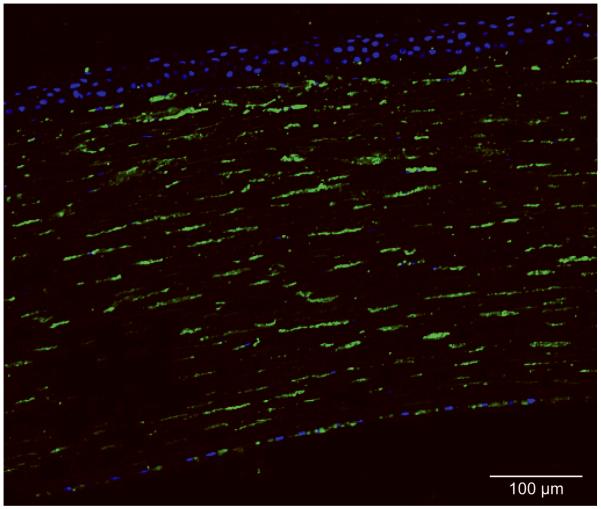
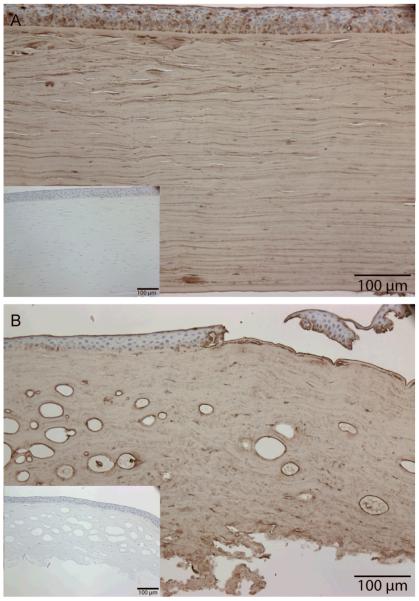
Upon staining the corneal sections of case 1 with the 5D4 anti-KS antibody, only the keratocytes in the stroma showed presence of sulfated KS (Figure 4, in green), along with minor staining of select endothelial cells. The extracellular stromal matrix and Descemet’s membrane, however, did not stain for sulfated KS. These findings suggest that case 1 is consistent with MCD type IA.
Figure 4.
Positive immunofluorescent staining of keratocytes and select endothelial cells by 5D4 anti-sulfated KS antibody (green) in case 1. Extracellular matrix of stroma does not show reactivity with the antibody. Nuclei were stained by SYTOX Orange dye (Invitrogen, Carlsbad, CA) (blue). The findings indicate that case 1 is a MCD type IA.
Control normal corneal section from an unaffected individual showed 5D4 reactivity in corneal epithelium and stroma, but not in keratocyes upon staining with 5D4 anti-KS antibody (Figure 5A), whereas preferential expression of sulfated KS was observed in keratocytes and extracellular stromal matrix in case 2 (Figure 5B), indicating a diagnosis of MCD type II.
Figure 5.
Corneal sections stained with anti-KS 5D4 antibody. Normal control corneal section showed 5D4 reactivity in corneal epithelium and stroma, but not in keratocyes (Figure 5A), whereas preferential expression of sulfated KS was observed in keratocytes and extracellular stromal matrix in case 2 (Figure 5B), consistent with MCD type II. Insets show staining without primary anti-KS 5D4 antibody.
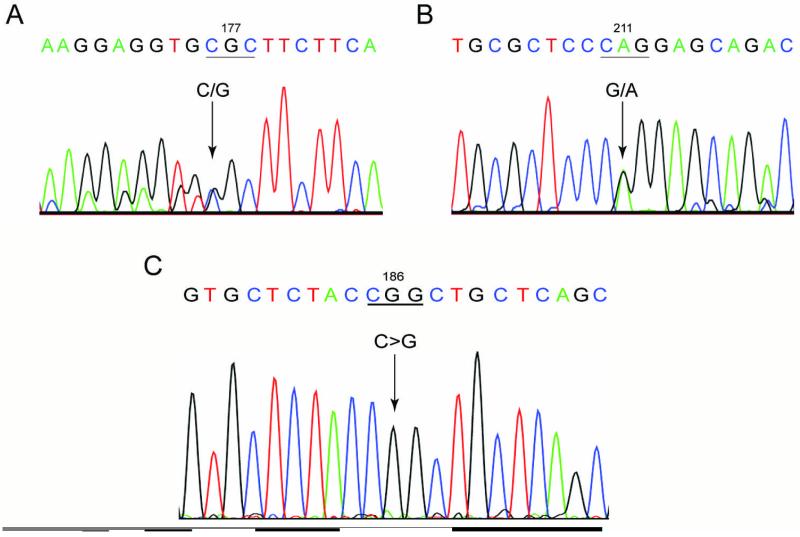
DNA sequencing data from multiple reads of case 1 revealed two heterozygous missense mutations in the coding region of the CHST6 gene (Figures 6A and 6B). The patient was found to have compound heterozygous mutations in c.529C>G (encoding an amino acid substitution of p.Arg177Gly) (Figure 6A), and in c.632G>A (encoding an amino acid substitution of p.Arg211Gln) (Figure 6B). The p.Arg177Gly mutation has never been reported, whereas the Arg211Gln mutation has been previously linked to MCD7. Since hyaline and amyloid materials were found in the sub-epithelial and superficial stromal deposits in case 1, blood samples were analyzed for possible mutations in the mutation hotspots (fourth and twelfth exons) of the TGFBI gene. No mutations were found in these two exons of the TGFBI gene.
Figure 6.
Sequence analysis of the coding region of CHST6. Case 1 shows compound heterozygous mutations (A, B). A, a heterozygous single base pair transition of c.529C>G with resultant p.Arg177Gly mutation. B, a heterozygous single base pair transition of c.632G>A with resultant p.Arg211Gln mutation. C, Case 2 shows a homozygous single nucleotide transition of c.557C>G, resulting in p.Pro186Arg mutation.
DNA sequencing results from multiple reads of case 2 revealed two sequence variants in the coding region of the CHST6 gene. A single nucleotide polymorphism (SNP), c.258A>C, did not result in a protein mutation. The other homozygous missense mutation, c.557C>G, encoded a p.Pro186Arg amino acid substitution (Figure 6C), which has not been previously reported.
DISCUSSION
Since MCD is a disease caused by improper sulfation of KS by abnormal carbohydrate sulfotransferase encoded by the mutated CHST6 gene, blue mucopolysaccharide deposits within the stroma by alcian blue (a copper-containing phthalocyanic dye) staining were noted in both patients as expected. In case 1, only keratocytes along with few endothelial cells stained with 5D4 antibody. As classified by 5D4 anti-sulfated KS staining patterns, this case represents MCD type IA5. As shown in Figure 1, our findings are consistent with a previous report that sulfated KS in select endothelial cells and the anterior aspect of the posterior Descemet’s membrane can be seen in patients with MCD type IA 4. In contrast, multiple subepithelial and stromal deposits and extracellular matrix, but not the keratocytes, stained with 5D4 antibody in case 2. These findings thus suggest a diagnosis of MCD type II the latter case.
In addition to discrete stromal opacities, corneas with MCD also present with a diffuse stromal haze that extends to the limbus. In contrast, the intervening spaces between stromal opacities are usually clear in granular corneal dystrophy (GCD)1. MCD can be easily distinguished from GCD histopathologically. Mucopolyscaccharide deposits of MCD stain with Alcian blue, whereas hyaline deposits of GCD do not. Instead, hyaline deposits of GCD stain bright red with Masson trichrome. In addition to classic MCD findings by anti-sulfated KS and Alcian blue staining, case 1 showed sub-epithelial and superficial stromal Masson trichrome-positive hyaline deposits that are atypical for MCD. There were also some amyloid deposits detected by ThT in the superficial stroma. However, no mutations in the hotspots of the TGFBI gene were found in this case, suggesting that the hyalinosis and amyloidosis were secondary in nature. Secondary amyloidosis has previously been reported in Fuchs endothelial dystrophy8 and a variety of corneal degenerations9, 10, but not in MCD. Taken together, this clinicopathological report is noteworthy as case 1 represents MCD with degenerative hyalinosis and amyloidosis. To the best of our knowledge, amyloid or hyaline deposits in MCD have not been previously reported. Our findings also suggest that secondary amyloidosis and hyalinosis can occur in severe MCD as a result of recurrent corneal erosions.
A plethora of disease-causing mutations in the CHST6 gene has been linked to MCD1 in various ethnic groups. Compound heterozygous missense mutations of CHST6 (p.Arg211Gln and p.Arg177Gly) responsible for MCD were identified in case 1. The nucleotide transition c.632G>A, causing an amino acid change p.Arg211Gln, has been reported previously in the Vietnamese population7. Although other substitutions at amino acid 177, i.e. pArg177Cys11 and p.Arg177His12, associated with nucleic acid mutations c.529C>T and c.530G>A, respectively, have been reported, the heterozygous mutation p.Arg177Gly found in our case 1 is novel. To the best of our knowledge, the homozygous mutation p.Pro186Arg found in our case 2 has never been reported previously. Moreover, this may be the first reported case of MCD in a patient of African-American descent.
ACKNOWLEDGEMENTS
We thank Drs. Yael Alevy and Morton Smith at Washington University for lending their expertise in genotyping and corneal histopathology, respectively. We also thank Dr. Diep Huu Thang at the Eye Hospital of Ho Chi Minh City, Vietnam, and Dr. Hunter Cherwek of Orbis International for coordinating tissue collection and clinical information of case 1.
Sources of Support that require acknowledgement: This research was supported by grants from National Institutes of Health R01EY017609 (AJWH), NRSA 5-T32-EY13360-09 (DAP), a RPB Physician Scientist Award (AJWH) and an unrestricted grant from Horncrest Foundation, Inc. (AJWH). This work was also supported in part by awards to the Department of Ophthalmology and Visual Sciences at Washington University from a Research to Prevent Blindness, Inc unrestricted grant and a NIH Vision Core Grant P30 EY02687. The authors have no proprietary interest in the information published in this report.
REFERENCES
- 1.Weiss JS, Moller HU, Lisch W, et al. The IC3D Classification of the Corneal Dystrophies. Cornea. 2008;27:S1–S42. doi: 10.1097/ICO.0b013e31817780fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akama TO, Nishida K, Nakayama J, et al. Macular corneal dystrophy type I and type II are caused by distinct mutations in a new sulphotransferase gene. Nat Genet. 2000;26:237–241. doi: 10.1038/79987. [DOI] [PubMed] [Google Scholar]
- 3.Hassell JR, Newsome DA, Krachmer JH, Rodrigues MM. Macular corneal dystrophy: failure to synthesize a mature keratan sulfate proteoglycan. Proc Natl Acad Sci U S A. 1980;77:3705–3709. doi: 10.1073/pnas.77.6.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cursiefen C, Hofmann-Rummelt C, Schlotzer-Schrehardt U, et al. Immunohistochemical classification of primary and recurrent macular corneal dystrophy in Germany: subclassification of immunophenotype I A using a novel keratan sulfate antibody. Exp Eye Res. 2001;73:593–600. doi: 10.1006/exer.2001.1080. [DOI] [PubMed] [Google Scholar]
- 5.Saito T, Nishida K, Nakayama J, et al. Sulfation patterns of keratan sulfate in different macular corneal dystrophy immunophenotypes using three different probes. Br J Ophthalmol. 2008;92:1434–1436. doi: 10.1136/bjo.2008.139527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Ashry MF, Abd El-Aziz MM, Wilkins S, et al. Identification of novel mutations in the carbohydrate sulfotransferase gene (CHST6) causing macular corneal dystrophy. Invest Ophthalmol Vis Sci. 2002;43:377–382. [PubMed] [Google Scholar]
- 7.Ha NT, Chau HM, Cung le X, et al. Identification of novel mutations of the CHST6 gene in Vietnamese families affected with macular corneal dystrophy in two generations. Cornea. 2003;22:508–511. doi: 10.1097/00003226-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Suesskind D, Auw-Haedrich C, Schorderet DF, Munier FL, Loeffler KU. Keratoepithelin in secondary corneal amyloidosis. Graefes Arch Clin Exp Ophthalmol. 2006;244:725–731. doi: 10.1007/s00417-005-0153-x. [DOI] [PubMed] [Google Scholar]
- 9.Hsu HY, Phillips NJ, Harocopos GJ. Secondary amyloidosis in the hydrops lesion of a patient with pellucid marginal degeneration. Cornea. 2007;26:992–994. doi: 10.1097/ICO.0b013e3180950162. [DOI] [PubMed] [Google Scholar]
- 10.Tai TY, Damani MR, Vo R, et al. Keratoconus associated with corneal stromal amyloid deposition containing TGFBIp. Cornea. 2009;28:589–593. doi: 10.1097/ICO.0b013e31818c9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klintworth GK, Smith CF, Bowling BL. CHST6 mutations in North American subjects with macular corneal dystrophy: a comprehensive molecular genetic review. Mol Vis. 2006;12:159–176. [PubMed] [Google Scholar]
- 12.Iida-Hasegawa N, Furuhata A, Hayatsu H, et al. Mutations in the CHST6 gene in patients with macular corneal dystrophy: immunohistochemical evidence of heterogeneity. Invest Ophthalmol Vis Sci. 2003;44:3272–3277. doi: 10.1167/iovs.02-0910. [DOI] [PubMed] [Google Scholar]