Abstract
Ear development requires interactions of transcription factors for proliferation and differentiation. The proto-oncogene N-Myc is a member of the Myc family that regulate proliferation. To investigate the function of N-Myc, we conditionally knocked out N-Myc in the ear using Tg(Pax2-Cre) and Foxg 1KiCre. N-Myc CKOs had reduced growth of the ear, abnormal morphology including fused sensory epithelia, disrupted histology, and disorganized neuronal innervation. Using Thin-Sheet Laser Imaging Microscopy (TSLIM), 3D reconstruction and quantification of the cochlea revealed a greater than fifty percent size reduction. Immunochemistry and in situ hybridization showed a gravistatic organ-cochlear fusion and a “circularized” apex with no clear inner and outer hair cells. Furthermore, the abnormally developed cochlea had cross innervation from the vestibular ganglion near the basal tip. These findings are put in the context of the possible functional relationship of N-Myc with a number of other cell proliferative and fate determining genes during ear development.
Keywords: N-Myc, Histogenesis, Morphogenesis, Inner Ear, Cell Cycle
Introduction
During ear development, the flat otic ectoderm undergoes proliferation and a complex morphogenesis to form a three dimensional (3D) inner ear. Proliferating precursors give rise to neurosensory (hair cells, neurons, and supporting cells) and non-sensory cells. The five resulting vestibular epithelia allow sensation of angular acceleration as well as gravistatic and other linear acceleration forces while the cochlea is responsible for the perception of sound. The adult mammalian auditory and vestibular systems are apparently incapable of continued proliferation or replacement of lost cells. Thus, loss of hair cells in the cochlea renders an individual irreversibly deaf. Cochlear implants are the only current option for neurosensory hearing loss. However, gene therapy promises eventual long term treatment and a possible cure through reconstitution of lost hair cells, preferentially in vivo (Pauley et al., 2008; Brigande and Heller, 2009; Kesser and Lalwani, 2009; Groves, 2010; Oshima et al., 2010). Ultimately, hair cell restoration will depend on reactivating proliferation and re-specification of post-mitotic cells into hair cells in the correct topology of the organ of Corti (Fritzsch et al., 2011).
Past research has predominantly focused on cell specification; here we will discuss a new approach to regulate proliferation of neurosensory precursor cells. Hair cell formation depends on proliferation of variably committed pro-sensory progenitor cell populations with differentiation following terminal mitosis. In the cochlea, hair cell precursors permanently exit the cell cycle beginning at embryonic day 12.5 (E12.5) in the apex and ending at E14.5 in the base (Ruben, 1967; Matei et al., 2005), with prohibition of further proliferation driven by a wave of cell cycle inhibitors including p27kip1 (Lee et al., 2006). A well characterized bHLH transcription factor, Atoh1 is necessary to differentiate hair cells (Bermingham et al., 1999; Dabdoub et al., 2008; Roybon et al., 2009; Fritzsch et al., 2010; Jahan et al., 2010). Subsequent DELTA/NOTCH signaling stabilizes the cell fate of supporting cells (Brooker et al., 2006; Kiernan et al., 2006). This wave of differentiation occurs from the middle-base portion of the cochlea and is driven bi-directionally (Chen et al., 2002) such that cells at the apex are quiescent for the longest period of time and differentiate last (Matei et al., 2005). If proliferation is curtailed due to the removal of a proto-oncogene such as Foxg1 (Pauley et al., 2006), an unusual morphology of cells and sensory epithelia will develop with a truncated cochlea with up to 16 rows of hair cells. This timing and correct quantification is a direct result of cell cycle regulation through the tumor suppressor retinoblastoma (pRB) and proto-oncogene E2F interaction (Rocha-Sanchez and Beisel, 2007) which can be manipulated to result in extra rows of hair cells and supporting cells (Mantela et al., 2005).
Regulation of the tumor suppressor pRB is extensive due to its importance in controlling the cell cycle during normal growth and tumorigenesis. Among the factors affecting pRB function is the proto-oncogene family of three bHLH genes, the Myc family (Lasorella et al., 2000; Lasorella et al., 2002; Knoepfler and Kenney, 2006; Eilers and Eisenman, 2008; Ruggero, 2009; Modak and Cheung, 2010; Mott et al., 2010). The highly conserved Myc family: C-Myc, L-Myc, and N-Myc, (Srivastava et al., 2010) can directly alter the phosphorylation state of pRB and thus regulate the cell cycle. MYCs form heterodimers with MAX to regulate downstream effectors (Foley and Eisenman, 1999; Hurlin et al., 1999; Shen-Li et al., 2000; Hurlin, 2005; Perini et al., 2005; Walker et al., 2005; Hooker and Hurlin, 2006) by binding to E-boxes in the promoter region of a gene (Grandori et al., 2000; Perini et al., 2005; Lebel et al., 2007). MYC is competitively repressed by MNT-MAX (Walker et al., 2005; Dezfouli et al., 2006; Hooker and Hurlin, 2006), MGA-MAX (Hurlin et al., 1999; Hurlin and Huang, 2006), and MAD-MAX (MAD1, MXI1, MAD3, MAD4 (related to the MXD1-4 family)) (Foley and Eisenman, 1999; Shen-Li et al., 2000; Hooker and Hurlin, 2006; Hurlin and Huang, 2006). MYCS regulate the cell cycle through their interaction with Inhibitors of Differentiation and DNA binding (IDs), through E-proteins (Lasorella et al., 2000; Lasorella et al., 2002; Sikder et al., 2003; Rothschild et al., 2006) or through MAX independent mechanisms such as the MYC-GROUCHO/TLE complex (Orian et al., 2007; Jennings and Ish-Horowicz, 2008). IDs are thought to regulate Atoh1 during hair cell development and support the NOTCH1 pathway to form supporting cells (Jones et al., 2006). MYCs have been shown to positively regulate a number of downstream effectors including CYCLIND2 (Bouchard et al., 1999; Knoepfler et al., 2002; Eilers and Eisenman, 2008), CYCLINE (Knoepfler et al., 2002), cyclin-dependent kinase CDK4 (Hermeking et al., 2000) or negatively regulate genes that stimulate proliferation such as CDKIs (Gartel et al., 2001; Staller et al., 2001; Eilers and Eisenman, 2008), some of which have been shown to be important in ear development (Laine et al., 2007). Some microRNAs, controlled by MYC, deplete E2Fs thus regulating the cell cycle (Aguda et al., 2008; Mott et al., 2010). Aside from intracellular regulation, activation of Myc is downstream of Shh (Knoepfler and Kenney, 2006), the Wnt downstream effector β-CATENIN (Shu et al., 2005; De Langhe et al., 2008; Kuwahara et al., 2010), Fgf10 (Taniguchi et al., 2003; De Langhe et al., 2008), Tgf-β/SMAD (Seoane et al., 2001; Seoane et al., 2004), and Foxg1/FoxGO (Seoane et al., 2004). As such, MYC is a nodal point in a complex regulatory network ultimately controlling the cell cycle and proliferation.
Therefore, understanding the function of N-Myc is pivotal to understanding the balance between proliferation, differentiation and morphogenesis during development of the ear. In this paper we describe for the first time the defects in the developing ear caused by absence of one of the three Myc family members: N-Myc. Our data concludes that N-Myc plays an important role in ear proliferation and differentiation.
Results
Loss of N-Myc stunts overall ear growth
The loss of N-Myc resulted in a dramatic reduction in ear size disproportionate to the overall growth of the animal (Fig. 1A and 1A’, Table 1). Other tissues expressing Pax2, including the cerebellum (Fig. 1B) and kidney (Fig. 1C) were significantly reduced in size (Table 1, N=6; p≪0.01). Body height, width and the size of organs not expressing Pax2 such as the heart (Fig. 1D) and the brain (Fig. 1E) tended to be smaller (Table1) but did not reach our criteria for statistical significance (N=6; p>0.01). While the CKOs were often the smallest in their litters, this decrease in overall size was not proportional to the decrease in size of the Cre recombining regions (Table 1). Thus, the reductions in ear, cerebellum, kidney, and forebrain size (Foxg1-Cre N-Myc CKO) were a direct result of the loss of N-Myc.
Figure 1. N-Myc controls growth of the inner ear.
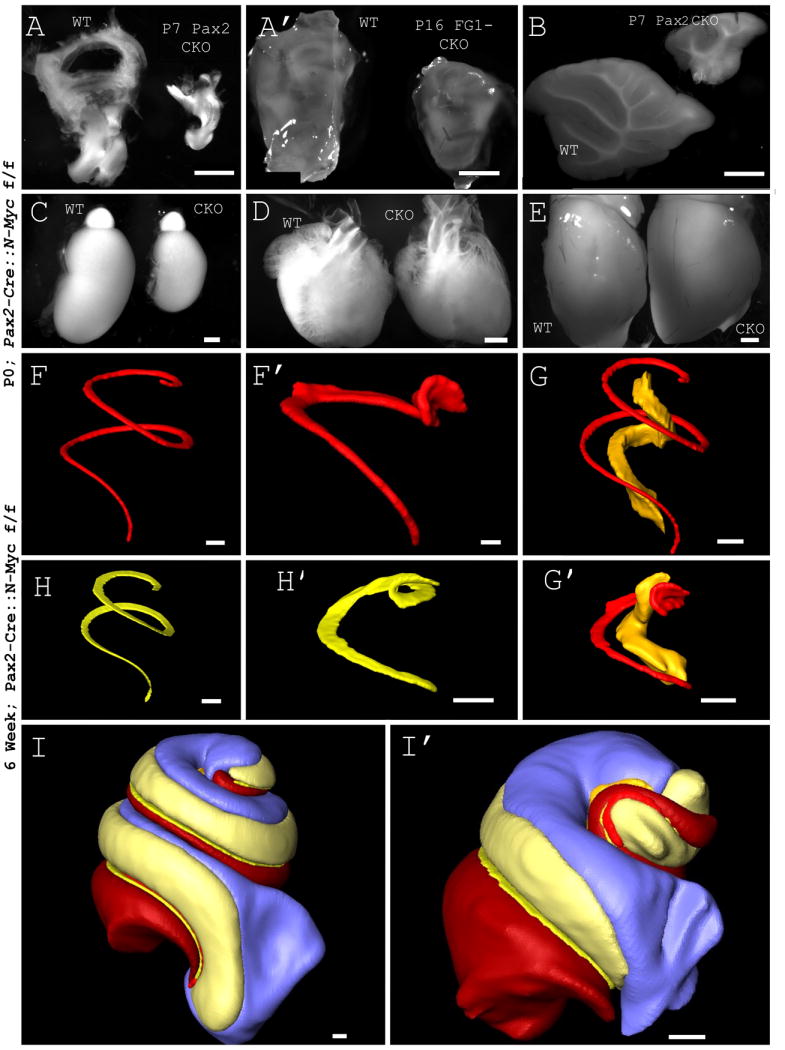
N-Myc CKO reduces the gross size of the inner ear. Pax2-Cre (A) and Foxg1-Cre (A’) are expressed in the ear where we have focused our analysis. Pax2 is expressed in other structures and CKOs of N-Myc have reduced size, including the cerebellum (B) and kidney (C). N-Myc CKO does not affect the heart (D) or forebrain (E). Foxg1 is expressed in and CKOs out N-Myc in the forebrain which is reduced in size (not shown). While overall body size in the CKO is reduced (Table 1), this minimal reduction is not seen in individual organs (unaffected heart and forebrain), and pales in comparison to Cre-expressing regions (Table 1). 3D reconstructions are performed by manually segmenting a collected stack of images acquired through TSLIM. The Pax2-Cre N-Myc CKO displays abnormalities throughout the inner ear, the organ of Corti (F and F’), Rosenthal’s canal (shown in yellow in G and G’), which houses the spiral ganglion neurons, and basilar membrane (H and H’) including a reduction in volume, a shortened cochlea and circularized apex. After 3D reconstructions, quantification showed a reduction in length and volume of the inner ear and is summarized in table 1. Side by side comparisons of WT and CKO illustrate the abnormal morphology and quantitative differences (I and I’). Note that due to the abnormality in the apex of the CKO compared to WT, a helicotrema could not be defined. Furthermore, there is no distinction between the scala vestibuli and scala tympani at the most apical tip. An arbitrary, yet consistent, demarcation is used. This may help account for differences in the scala vestibuli and scala tympani volumes not otherwise noted. FG1 CKO= Foxg1-Cre N-Myc f/f, Pax2 CKO= Pax2-Cre N-Myc f/f. Scale Bars: A-E= 1mm, F-I’=100μm.
Table 1.
Size comparison of anatomical structures
| Body Measurements (N=6,P0) | Control Littermate | Pax2-Cre∷N-Myc CKO (mm) | P-Value*** | % Red |
|---|---|---|---|---|
| Trunk Width (shoulders) | 8.04+/-0.37** | 7.61+/-0.43 | 0.097 | 5.53% |
| Trunk Length (shoulder to hip) | 8.24+/-0.66 | 7.45+/-0.40 | 0.035 | 9.6% |
| *Inner Ear (Height) | 3.55+/-0.12 | 2.55+/-0.20 | 5.07×10-06 | 28.10% |
| *Inner Ear (Width) | 2.28+/-0.21 | 1.74+/-0.06 | 0.0013 | 23.54% |
| * Kidney (Height) | 3.55+/-0.38 | 2.37+/-0.07 | 0.00053 | 33.21% |
| * Kidney (Width) | 2.36+/-0.15 | 1.55+/-0.10 | 2.22×10-06 | 34.33% |
| Brain (Height) | 4.31+/-0.07 | 4.43+/-0.07 | 0.011 | (2.80%) |
| Brain (Width) | 7.95+/-0.38 | 7.69+/-0.34 | 0.24 | 3.30% |
| Heart (Height) | 3.94+/-0.26 | 3.93+/-0.12 | 0.96 | 0.01% |
| Heart (Width) | 3.77+/-0.80 | 3.65+/-0.79 | 0.79 | 3.3% |
| Inner Ear Measurements (N=1, 6 wk)* | % Red | |||
| Length BM | 6.73mm | 2.63mm | 61% | |
| WT (cubic μl) | MT (cubic μl) | |||
| Organ of Corti | 0.0101 | 0.00607 | 34% | |
| Basilar Membrane (BM) | 0.00728 | 0.00294 | 61% | |
| Tectorial Membrane | 0.0155 | 0.00423 | 73% | |
| Scala Vestibuli | 0.535 | 0.112 | 79% | |
| Scala Media | 0.348 | 0.0746 | 78% | |
| Scala Tympani | 0.437 | 0.189 | 57% | |
| SGN | 0.0448 | 0.0171 | 61% | |
Indicates area of Pax2 expression
Mean +/- Standard Deviation
Two-tailed Student T-Test
In addition, these size truncations were compounded by both abnormal histogenesis and altered morphogenesis. We define histogenesis as the differentiation of sensory cells from precursor populations within a sensory epithelium and morphogenesis as the acquisition of the 3D structure of the ear as a whole. 3D reconstructions have allowed for qualification of these defects as well as quantification of the size reduction (Table 1). Instead of more than two turns of a tightly coiled cochlea, CKOs failed to complete a single complete turn (Fig. 1F and 1F’) and displayed an abnormal organ of Corti with a shortened and circularized apex (Fig. 1F and 1F’). The basilar membrane of a six week old N-Myc CKO (Fig. 1H and 1H’) was less than half the length of the WT littermate (Table 1). Rosenthal’s canal which contains spiral ganglion neurons was also reduced (Fig. 1G and 1G’). The volume of Rosenthal’s canal and the basilar membrane were reduced by over sixty percent compared to the control littermate (Table 1). The scala tympani showed a sixty percent volume reduction while the scalae vestibuli and media were reduced by nearly eighty percent. TSLIM enabled visualization of some or all portions of the inner ear to provide comparison of WT and N-Myc CKO (Fig. 1I and 1I’) and showed not only the abnormal morphological development but also the magnitude of size reduction (Table 1). These effects may reflect changes in proliferation as previously suggested in a different mouse model (Pauley et al., 2006).
N-Myc CKOs show a reduction of EdU positive cells in a shortened cochlea
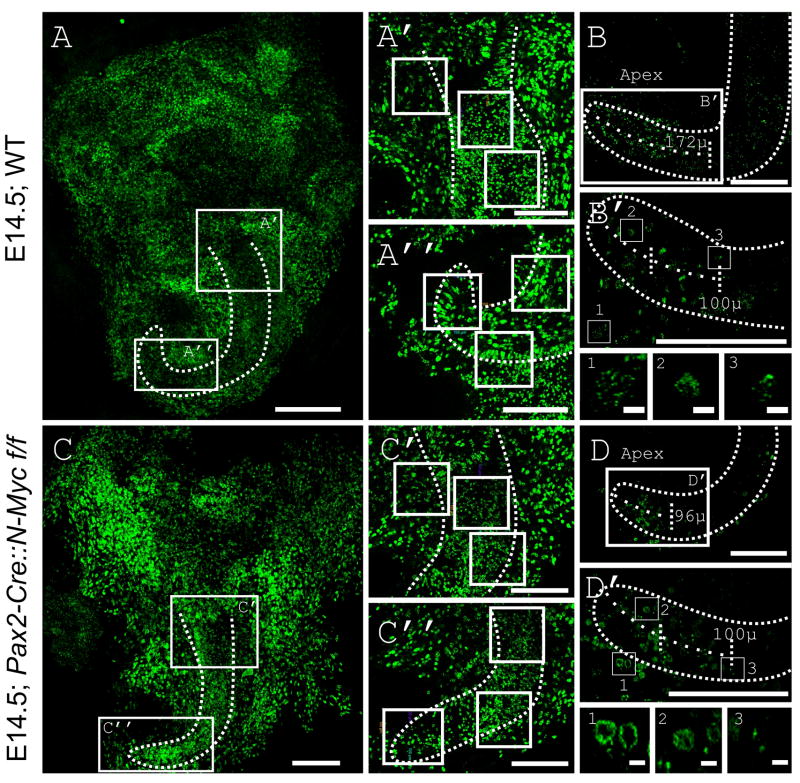
5-ethynyl-2′-deoxyuridine (EdU) incorporates stably into DNA during the S-phase of the cell cycle (Diermeier-Daucher and Brockhoff, 2010) allowing direct assessment of proliferation. Following injection, EdU will be incorporated during a two hour time frame until it is metabolized. We injected pregnant dams at E12.5 and collected three wild type and three N-Myc CKO mutant pups at E14.5. We imaged and counted the number of cells which incorporated EdU in both our control (Fig. 2A) and CKO (Fig. 2C). Six regions from the base (Fig. 2A’) and apex (Fig. 2A”) of the control were matched with similar regions from the CKO (Fig. 2C’ and 2C”). The CKO had statistically significant lower total number of EdU positive cells (202+/-16 vs. 402+/- 200; p=0.007). [EdU positive cells were manually counted if they were present above a set threshold (criteria remained the same throughout all counts).]
Figure 2. N-Myc CKO result in less EdU positive cells.
Pregnant dams were injected intraperitoneally at E12.5 with EdU and the DNA incorporated EdU was allowed to dilute for 48 hours. Overview of the EdU reacted WT (A) and CKO (C) ears shows reaction throughout the ear in both prosensory and non-sensory domains. EdU quantification was performed by selecting a 100 μm square box along the prosensory region. Three regions were selected in the WT base (A’) and apex (A”) and compared with CKO base (C’) and apex (C”) (squares shown are collapsed region where EdU positive cells were counted in each individual section). Direct EdU count showed a significant reduction of EdU positive cells in N-Myc CKO. The distance from the apical tip to where EdU could no longer be detected was measured (B and D). Enlarged images show an apical to base gradient within this apical tip (B’ and D’). Selected EdU positive nuclei were selected from outside the cochlear duct (B’ box 1 and D’ box 1), approximately 25μm from the apical tip (B’ box 2 and D’ box 2), and approximately 100μm from the apical tip (B’ box 3 and D’ box 3). Note that cells outside the cochlear duct had larger nuclei (one of criterion for identifying cochlear duct) and that as cells progressed further medial along the cochlear duct, EdU in cell nuclei was diluted; indicating that additional cell cycles had occurred after EdU incorporation. Outline of cochlear duct based on collapsed stack but shown on single image. Orientation differences seen are result of remounting. Scale Bars: A-B’ and C-D’= 100μm; B’ boxes 1-3 and D’ boxes 1-3= 10μm.
Since much of the ear is proliferating at E12.5, EdU will be incorporated into the DNA of those proliferating cells. However, upon completion of each subsequent cell cycle, the EdU is diluted by a factor of two; eventually cells that continue proliferation will no longer contain detectable levels of EdU. Cells that exited the cell cycle shortly after administration of EdU or cells that are slowly dividing (and have not been fully diluted out) are detectable (Fig. 2A and 2C). Thus, cells positive for EdU after two days had either exited the cell cycle shortly after EdU administration or had a longer cell cycle. Assuming a cell cycle length of 8 hours, six additional divisions would reduce the EdU labeling from 100% to 1.6 %, most likely below the detection threshold (Pauley et al., 2005). Shorter cell cycles will reduce EdU concentration even further over the same period. Cells in the apex undergo terminal mitosis around E12.5 (Ruben, 1967; Matei et al., 2005; Lee et al., 2006). Therefore, most apical cells maintain high levels of EdU expression in the organ of Corti as they have stopped proliferating while cells more basal lose their EdU (Fig. 2B and 2D) as previously described (Matei et al., 2005).
We also measured the length of the cochlear duct of one N-Myc CKO mutant and one wild-type prepared to avoid undue stretching. The length from the apex to the base in the E14.5 WT was 578 μm while the length in the E14.5 N-Myc CKO was 325 μm (N=1). This reduction in size of the cochlear duct (325μm/578μm= 0.56) is similar to the size reductions we see in the ear as a whole and through TSLIM, suggesting that reduction of cochlear duct growth is happening uniformly across all developmental stages. Using all the same settings to compare the two, the apical EdU was maintained for 172 μm in the control (172μm/578μm=0.30) (Fig. 2B and 2B ’) and 96 μm in the mutant (96μm/325μm=0.30) (Fig. 2D and 2D’). Our data support the idea that lack of N-Myc reduces the number of EdU positive cells proportional to the shrinking of the cochlea. Furthermore, we confirm that the apex to base entry into terminal mitosis is consistent between control and N-Myc CKO. To our knowledge, this is the first time that such a correlation has been shown directly in the many mutants with a shortened cochlear duct, also suggestions of the importance of proliferation for cochlear duct elongation have been raised before (Grimsley-Myers et al., 2009).
N-Myc CKOs have reduced cochlear length and number of cochlear hair cells, but do not have reduced number of canal cristae hair cells compared to control littermates
Our adult data suggest that the gross sizes of Cre expressing regions are significantly decreased in N-Myc CKO mice (Fig. 1 and Table 1). We also have seen that the reduction of N-Myc leads to an early reduction in size and total number of cells exiting the cell cycle (Fig. 2). In order to quantify the effect of N-Myc on the number of hair cells, we imaged twelve ears and manually counted MYO7A positive hair cells at P0 (Table 2). The length (4.97+/-0.36mm vs. 1.73+/-0.38mm, p=3.02×10-8) and number of hair cells (2760+/-186 vs. 1261+/-398, p=6.46×10-5) in the cochlea were significantly reduced. There was no statistically significant difference in the number of hair cells in the canal cristae.
Table 2.
Hair cell quantification between WT and N-Myc CKO
| Hair Cell Quantification (N=6,P0) | Control Littermate | Pax2-Cre∷N-Myc CKO | P-Value** |
|---|---|---|---|
| Cochlea Length | 4.97+/-0.36mm* | 1.73+/-0.38mm | 3.08×10-8 |
| Cochlear Hair Cells | 2760+/-186 | 1261+/-398 | 6.46×10-5 |
| Horizontal Crista Hair Cells | 245+/-69 | 223+/-42 | 0.523 |
| Anterior Crista Hair Cells | 372+/-38 | 320+/-37 | 0.036 |
| Posterior Crista Hair Cells | 279+/-59 | 365+/-36 | 0.054 |
Mean +/- Standard Deviation
Two-tailed Student T-Test
N-Myc CKO disrupts proper adult histogenesis
We next investigated overall histologic effects through plastic sectioning of the adult organ of Corti. The WT cochlea had a readily identifiable histology of one row of inner hair cells plus three rows of outer hair cells with an overlying tectorial membrane and was sitting on a basilar membrane (Fig. 3A). The inner and outer pillar cells forming the tunnel of Corti above the basilar membrane were very conspicuous. In contrast, the CKO cochlea illustrated considerable disruption to both hair cells, supporting cells and surrounding structures compared to WT in the middle turn (Fig. 3A and 3A’), base (Fig. 3B) and most notably in the apex (Fig. 3C and 3D). No hair cells could be identified in any section of the adult mutant cochlea. The pillar cells and surrounding structures of spiral limbus, spiral sulcus, basilar membrane, and tectorial membrane were vaguely identifiable in the middle turn of the cochlea but were not oriented properly (Fig. 3A’). A greater reduction in identifiable structures at the base was noted with only a possible tectorial membrane, pillar cells, and tunnel of Corti (Fig. 3B). Histologic disruption was greatest at the apex where no organ of Corti or pillar cells were discernable (Fig. 3C). Higher magnification of a potential basilar membrane-like structure showed pillar-like cells but with no definitive cellular characterization possible (Fig. 3D). Importantly, hair cells in the horizontal canal crista were easily identifiable and appeared normal, despite the absence of a canal (Fig. 3E). Our hair cell quantification data of MYO7A hair cells supports these conclusions (Table 2).
Figure 3. N-Myc is important for the survival of hair cells and the stability of the organ of Corti.
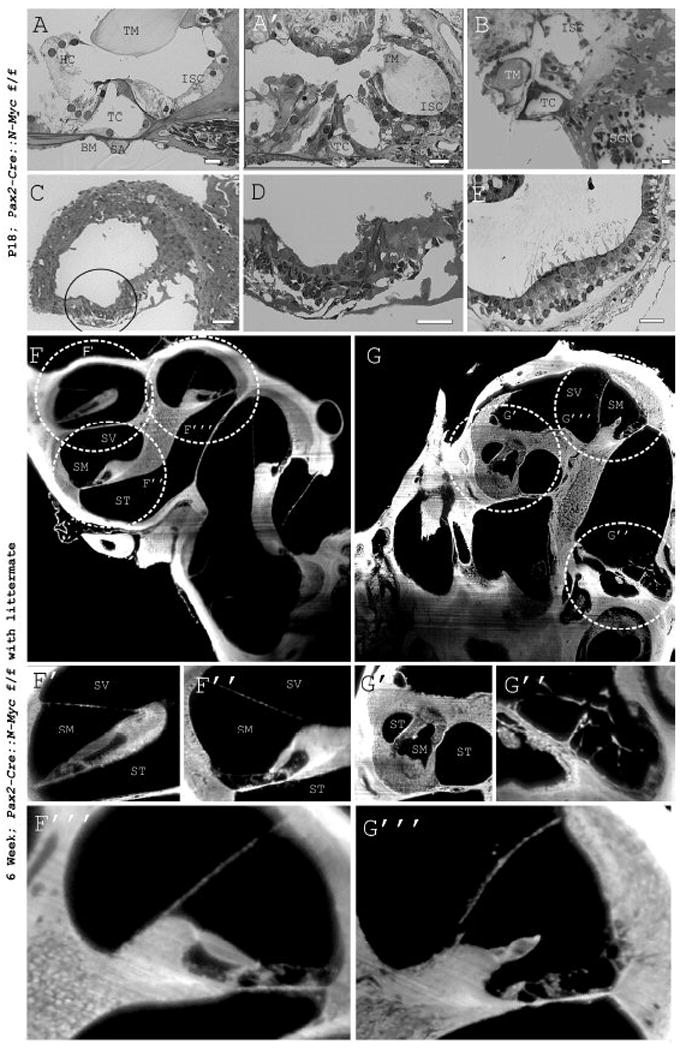
Plastic embedded sections provide high resolution images of the cochlea. Of note, the tunnel of Corti (TC), one row of inner hair cells, three rows of outer hair cells, pillar cells, tectorial membrane (TM), basilar membrane (BM), inner sulcus cells (ISC), Hensen cells (HC), radial fibers (RF), and spiral artery (SA) are seen in WT littermate (A). These structures are poorly defined in the mutant middle turn (A’), base (B), and most poorly so in the apex (C and D). In contrast, despite the lack of a horizontal canal, the canal crista appears normal, including hair cells (E). TSLIM imaging allows well-registered cross sections of the whole inner ear (F and G). The helicotrema and organ of Corti is compared to the N-Myc CKO (F’ and G’). Notably, the helicotrema in the WT is matched with the circularized apex in the mutant while the organized organ of Corti of the WT in the base is matched with the degenerated base of the N-Myc CKO (F” and G”). The mutant middle turn (G”’) is most similar histologically to that of the WT (F”’) compared to the apex or base as shown by both plastic sections and TSLIM. Dashed circles correspond to magnified regions below. SV=scala vestibuli, SM= scala media, ST= scala tympani. Scale Bars: A-E= 10μm.
Using TSLIM imaging, it was possible to produce well-registered serial cross sections of the whole inner ear without disruption due to embedding and sectioning. This not only provided for more accurate data analysis as compression stresses were not a factor, but allowed for a wider field of view of the specimen at high resolution. Comparing six week N-Myc CKO with a WT littermate showed the truncation of the cochlea (Fig. 3F and 3G) as well as the fusion of the utricle, saccule, and cochlea (data not shown). It also showed the increased disorganization of the organ of Corti near the apex and base compared to the middle turn consistent with plastic sections. The CKO apex did not contain a helicotrema (Fig. 3F’ and 3G’) where the scala vestibuli becomes confluent with the scala tympani at the tip of the scala media. Instead the scala tympani wrapped around the distorted tip of the scala media (Fig. 3G’). Additionally, high power images highlighted the disrupted histology of the organ of Corti in the N-Myc CKO (Fig. 3F” and 3G”) similar to serial cross sections (Fig. 3B). Individual hair cells and a properly formed organ of Corti were present in the WT middle turn but were disrupted in the middle turn of CKO (Fig. 3F”’ and 3G”’). In summary, absence of N-Myc resulted in a truncated cochlea and disorganized organ of Corti with abnormalities greatest at the apex and the base.
Absence of N-Myc alters neural growth
The inner ear is densely innervated and highly patterned (Fig. 4A). Efferent neurons project from the hindbrain (rhombomere 4) to the inner ear (Fritzsch and Nichols, 1993) while afferents of the cochlea project from the spiral ganglion to the cochlear nucleus (Rubel and Fritzsch, 2002) and afferents from the vestibular system originate from both the inferior and superior vestibular ganglia and project to the vestibular nuclei and the cerebellum (Maklad et al., 2010). The fibers from the inferior vestibular ganglion innervate the saccule and posterior canal crista while the superior vestibular ganglion neurons innervate the utricle, anterior canal crista and horizontal canal crista (Maklad and Fritzsch, 2003) (Fig. 4A’).
Figure 4. Neural path-finding is dependent in part on N-Myc.

Lipophilic dyes placed in the cochlear nucleus and cerebellum allow for fluorescent visualization of cochlear and vestibular afferents, respectively. Dye placement in rhombomere 4 allows for efferent labeling. Dye tracing imaged by confocal microscopy shows several innervation defects in the inner ear of N-Myc CKO mice compared to WT (A-A”). Normally, the vestibular ganglion receives inputs from the vestibular epithelia and the cochlear (spiral) ganglion receives innervations from the cochlear hair cells (WT control). In the N-Myc CKO there is cross innervations between vestibular and cochlear fibers with opposing sensory epithelia. The vestibular ganglion innervates not only vestibular epithelia, but also portions of the cochlea. Likewise, the cochlear fibers are innervating portions of the saccule (B). Additionally, an ectopic patch of hair cells near the base of the cochlea is innervated with what appears to be vestibular fibers (B’ with insert). Furthermore, the malformed apex shows pathfinding defects of fibers (B and E with MYO7A insert). These patterning defects were supported by osmium tetroxide (OsO4) staining and further show that innervation to the posterior canal is absent (C and C’). Innervation to the horizontal canal crista remains in the absence of a horizontal canal in the Pax2-Cre N-Myc CKO (C’ and D). Innervation to the anterior and horizontal canal cristae remain in the Foxg1-Cre N-Myc CKO despite the absence of all three canals (D’). Lastly, cross innervations are seen in the Foxg1-Cre N-Myc CKO at P0 as well. Merged innervations patterning of cochlear and vestibular fibers labeled from the cochlear nucleus and cerebellum, respectively, show cross innervations to both the saccule and base of the cochlea. In summary, N-Myc CKOs lack innervation to the posterior canal, have cross innervations to both the saccule and base of the cochlea, and have an abnormal apical innervation that is both disorganized and reduced. AC= anterior canal crista, Co=cochlea, G=ganglion, HC= horizontal canal crista, PC=posterior canal crista, S= saccule, U= utricle. Scale Bars: 100μm.
N-Myc CKOs showed abnormal innervation patterns (Fig. 4A” and 4B) in all mutants analyzed for innervation defects (N=5), regardless of age. Dye tracing suggested that vestibular fibers not only innervated vestibular epithelia, but also the base of the cochlea. Cochlear fibers innervated not only the cochlea but also the saccule (Fig. 4B). Furthermore, innervation defects were seen in the apex where fibers appeared disorganized and sparse (Fig. 4B and 4E) compared to a WT littermate (Fig. 4A). An ectopic patch of MYO7A (Fig. 4B’ insert) staining cells near the base of the cochlea were innervated by fibers originating in the vestibular ganglion (Fig. 4B and 4B’). Osmium tetroxide staining of myelinated nerve fibers confirmed this finding for adults (data not shown). Furthermore, the posterior canal lacked innervation entirely in both Pax2-Cre CKO (Fig. 4C-4D) and Foxg1-Cre CKO (Fig. 4D’). The lack of innervation was unrelated to the presence of a canal as the Pax2-Cre CKO and Foxg1-Cre CKO have innervations to the horizontal and anterior canal cristae despite the absence of either the horizontal canal only or horizontal and anterior canals, respectively (Fig. 4D and 4D’). Dual labeling of cochlear and vestibular fibers (Fig. 4F) with vestibular fibers only (Fig. 4F’) showed cochlear innervations in the saccule and vestibular fibers in the base of the cochlea (Fig. 4F”). In summary, N-Myc CKOs had cross innervations of vestibular and cochlear fibers, lacked innervations to the posterior canal, innervated an ectopic patch of hair cells near the base of the cochlea, and had sparse and disorganized innervations at the apex (Fig. 4A’ and 4A”).
N-Myc loss affects morphogenesis of the ear
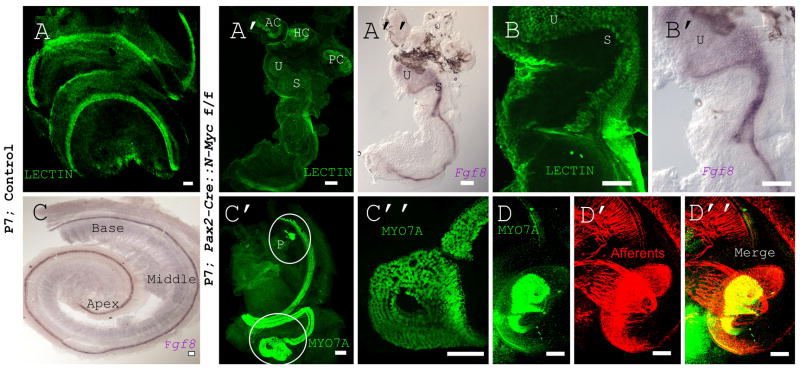
N-Myc CKOs had a number of morphogenetic defects including a failure of sensory epithelia to segregate, a circularized apex with up to twenty rows of hair cells, and the lack of formation of one or more semicircular canals. The inner ear consists of six distinct sensory epithelia, a cochlea and five vestibular epithelia including the three canal cristae, the utricle, and the saccule. Importantly, the utricle, saccule, and cochlea are segregated by non-sensory areas. The ductus reuniens starts to form at E13.5 to separate the cochlea from the saccule to form two distinct endorgans (Fig. 5A and 5C). Because the cochlea and saccule perform distinct functions (vertical linear movement sensation vs. hearing) this sensory separation allows segregated stimulus presentation. The utricle and saccule, both gravistatic sensing organs, align perpendicular to each other and reside in two distinct recesses separated by the utriculo-saccular foramen. This foramen forms five days after placodal thickening and separates the inferior and superior halves of the inner ear (Cantos et al., 2000). In both these developmental steps, non-sensory epithelia form constrictions between the two separate sensory epithelia. Neither the utriculo-saccular foramen nor the ductus reuniens formed in the N-Myc CKO. N-Myc CKOs not only failed to undergo these morphogenetic steps but also had a fusion of the utricular and saccular epithelium with the basal portion of the cochlea (Fig. 5A’ and 5A”). High magnification showed the failure of the utriculo-saccular foramen and ductus reuniens to form and the fusion of the sensory epithelia (Fig. 5B and 5B’). In the absence of N-Myc, sensory epithelia were unable to segregate and appropriate non-sensory constrictions did not form suggesting that N-Myc plays a role in both sensory and non-sensory development.
Figure 5. N-Myc is necessary for proper morphogenesis and CKOs display fused sensory epithelia.
IHC and ISH allows for the visualization of proteins and mRNA in tissue, respectively. LECTIN IHC (A) and Fgf8 ISH (C) of WT cochlea show normal cochlear morphology with three rows of outer hair cells lateral to a single row of inner hair cells. Additionally, WT inner ears have six distinct sensory epithelia: the cochlea and five vestibular epithelia (three canal cristae, the utricle, and the saccule). Pax2-Cre N-Myc CKO whole mount imaging shows a confluence of sensory epithelia from the cochlea to the saccule and utricle with both LECTIN IHC (A’) and Fgf8 ISH (A”). High magnifications of these regions show the lack of distinct recesses and the absence of both the utriculo-saccular foramen and the ductus reuniens (B and B’). Furthermore, a truncated and circularized apex is seen with an ectopic patch of MYO7A positive cells near the base (C’-C”). Consistent with innervation data, the abnormal apical tip which stains positive for MYO7A (D) is also innervated (D’) leading to a disorganized innervation at the apex (D”). AC= anterior canal crista, HC= horizontal canal crista, P= patch of hair cells, PC= posterior canal crista, S= saccule, U= utricle. Scale Bars: A,C”=100μm, C’=10μm, D-D”= 100μm.
The cochlea extends two turns (Fig. 5A and 5C) to provide a tonotopic map ranging between 10-90% of the cochlea from 7-61kHz (Muller et al., 2005). N-Myc CKOs did not have the full cochlear extension and had a truncated apex with an unusual coil near the apical tip that contained MYO7A positive cells (Fig. 5C’ and 5C”). Neither the hearing range nor the frequency distribution in our mutants is known. Two other obvious defects were found in the cochlea. Near the base, an ectopic patch of hair cells stained MYO7A positive (circled in Fig. 5C’). These hair cells were not in the same plane as the organ of Corti and were innervated by what appeared to be vestibular fibers labeled from the cerebellum (Fig. 4B’ insert). Additionally, up to 22 rows of hair cells were noted at the apex (Fig. 5C” and 5D). This shows that although morphologically abnormal, a P7 apex stained positive for both MYO7A (a marker of hair cells) and β-TUBULIN (a marker for neuronal processes) (Fig. 5D”). This is important to note given the observation of a histologically abnormal organ of Corti in adults. Lastly, Foxg1-Cre CKOs lacked all three non-sensory canals but retained the canal cristae (Fig. 4D’). To our knowledge, this is the first mutant in which all three canal cristae form near normal without partial or complete non-sensory canal formation.
All members of the Myc family are expressed in the adult inner ear but only L-Myc and N-Myc are expressed embryonically
Using in situ hybridization we detected the presence of C-Myc, L-Myc and N-Myc mRNA in the inner ear. N-Myc was ubiquitously expressed in both E10.5 (Fig. 6A) and E11.5 (Fig. 6B) WT ears. N-Myc became restricted to prosensory domains by E12.5 (Fig. 6C) where it formed a base to apex gradient (Fig. 6C’). It was strongly expressed in the vestibular system, including the utricle and saccule (Fig. 6C”). N-Myc was expressed until E13.5 in the semicircular canal cristae but might persist in the ventral half of the ear (Fig. 6D and 6D’). At E14.5, N-Myc was expressed in the entire cochlea as well as the saccule and some non-sensory epithelia such as the ductus reuniens (Fig. 6E-E” insert) while L-Myc was expressed in all sensory epithelia, including strong expression in the semicircular canal cristae (Fig. F-F”’). N-Myc and L-Myc were thus co-expressed in the saccule and cochlea (Fig. 6E” and 6F”). Both L-Myc and N-Myc were expressed in the inner ear embryonically (Fig. 6E and 6F) while C-Myc was not expressed in the embryonic inner ear (Fig. 6G) as previously reported (Romand et al., 1994). Patterns of expressions during development differed from those at P0. At P0, L-Myc and N-Myc were expressed strongly in all sensory epithelia (Fig. 6H-6I’) including the organ of Corti (Fig. 6H’ and 6I’ with insert), C-Myc expression was only seen in the basal portions of the cochlea (Fig. 6J and 6J’). Interestingly, this expression was found in differentiated hair cells instead of areas of known proliferation. A summary diagram illustrates where the Mycs were expressed between E10.5 and P0 (Fig. 6O-T). C-Myc, L-Myc, and N-Myc were all expressed differentially in the brain (Fig. 6K-M). To determine the success of our N-Myc CKO, we investigated the N-Myc expression after recombination. After the recombination with Pax2-Cre, N-Myc was not detected in the inferior colliculus, rhombic lip, or cochlear nucleus (Fig. 6N) and the ear, indicating appropriate recombination in areas of known Foxg1 expression (Grimsley-Myers et al., 2009; Kersigo et al., 2011).
Figure 6. Myc expression in both embryonic and postnatal mouse inner ear sensory epithelia.
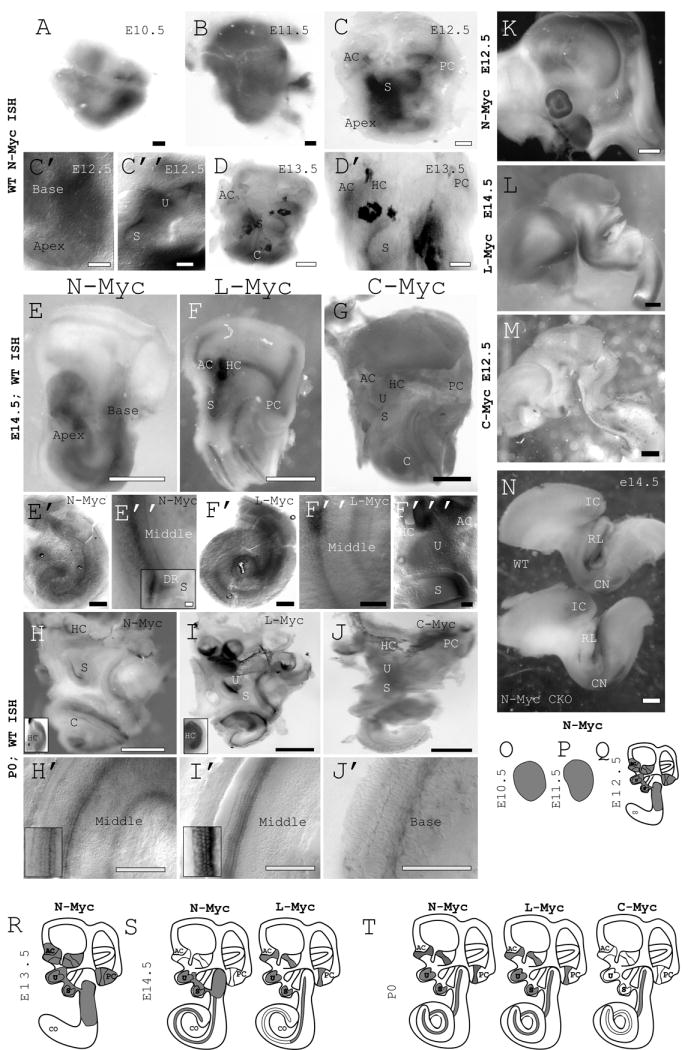
N-Myc is expressed ubiquitously in the ear at E10.5 (A) and E11.5 (B). Beginning at E12.5, N-Myc expression is restricted to all sensory epithelia (C) including the cochlea (C’), utricle and saccule (C”). At E13.5 N-Myc expression is also seen in the canal cristae (D and D’). At E14.5 N-Myc is expressed strongly in the cochlea (E’ and E”) as well as the saccule and the ductus reuniens (see insert on E”) while L-Myc is expressed strongly in all sensory epithelia in the inner ear (F-F”’). Notably, they appear co-expressed in the cochlea and saccule at this stage. C-Myc is not detected at E14.5 (G). All three Mycs are detected in WT ears at P0 in sensory epithelia. N-Myc and L-myc are detected by in situ hybridization in the organ of Corti as well as the saccule, utricle, and canal cristae (H-I’)). C-Myc is barely detectable in portions of the cochlea but absent elsewhere in the inner ear (J and J’). Interestingly, the presence of Mycs at P0 does not correlate to areas of proliferation. Rather, the Mycs are expressed in differentiated cells such as shown in the cochlea (inserts on H’ and I’) and horizontal canal crista (inserts on H and I). It appears that the Mycs play a different role in maturation and early development. All three Mycs are expressed selectively in the brain (K,L,M,N). Furthermore, expression of N-Myc is drastically reduced in the Pax2-Cre CKO in the rhombic lip (RL), inferior colliculus (IC), and cochlear nucleus (CN) in E14.5 mice (J). A summary of Myc expressions is shown (O-T). Note that at E12.5 and E14.5, no C-Myc expression was detected whereas at E12.5, L-Myc ISH was not performed. AC= anterior canal crista, C/Co= cochlea, DR= ductus reuniens, HC= horizontal canal crista, PC= posterior canal crista, S= saccule, U= utricle. Scale Bars: A-D’=100μm, E-G=1mm, E’-F”’=100μm, H-J=1mm, H’-J’=100μm, K-N=1mm.
Summary of N-Myc CKO Defects
N-Myc CKOs had a smaller inner ear compared to matched controls. This decrease in size is likely the result of early changes in the cell cycle length leading to defects such as a fused saccule, utricle, and cochlea, an abnormal (and shortened) apex, and a lack of either a horizontal canal (Pax2-Cre N-Myc CKO) or all three semicircular canals (Foxg1-Cre N-Myc CKO) without disrupting the canal cristae. There were up to 22 rows of hair cells near the apex in the neonate (and four to five rows of hair cells elsewhere), and an apparent loss of hair cells throughout the cochlea in the adult. Additionally, N-Myc was important in neuronal path-finding as N-Myc CKOs had vestibular fibers innervating the cochlea and cochlear fibers innervating the saccule. The posterior canal was uninnervated but an ectopic patch of MYO7A positive cells near the base of the cochlea received possibly vestibular nerve fibers. All three Mycs were expressed in differentiated hair cells at P0 but only L-Myc and N-Myc were expressed during development in proliferating cells. In portions of the cochlea, saccule, and utricle, there was co-expression of both L-Myc and N-Myc.
Discussion
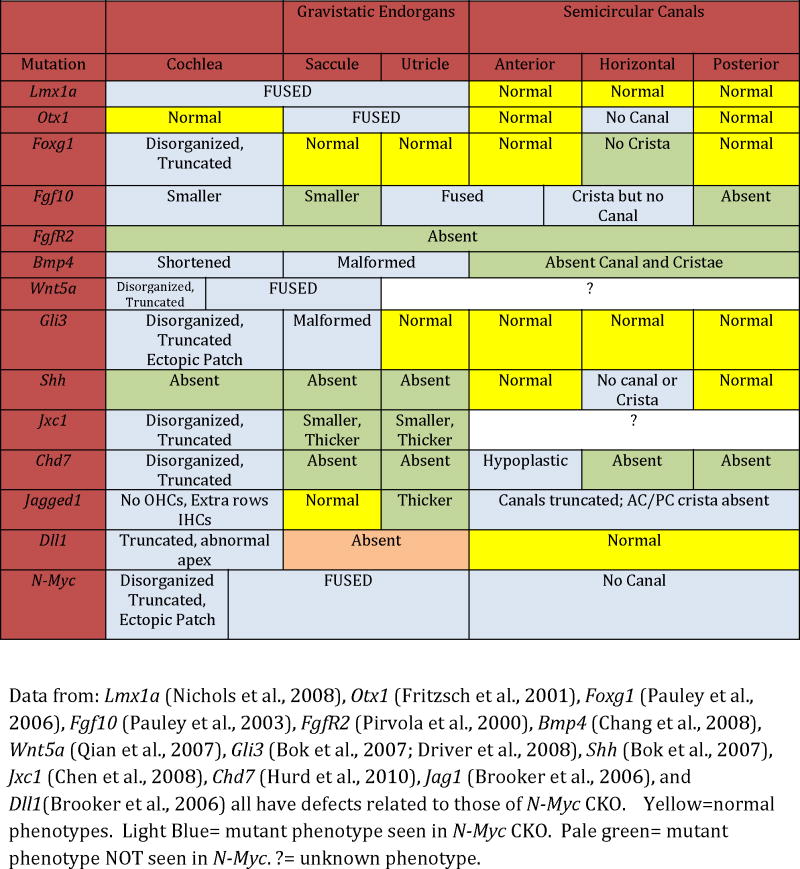
Aside from its implications in development and cancer, understanding the Myc bHLH transcription factor family may help elucidate its continued importance in stem cell biology (Takahashi and Yamanaka, 2006; Beisel et al., 2008; Yamanaka, 2008; Boland et al., 2009) which has recently made progress in the development of hair cells in vitro (Oshima et al., 2010). N-Myc is a critical hub in controlling the cell cycle and, as such, is highly integrated among a number of upstream as well as downstream pathways (Supplemental Fig. 1A and 1B). Many mouse knockouts of genes that interact with Myc family members (Supplemental Fig. 1B asterisks) show phenotypes reminiscent of what we and others have described (Table 3). N-Myc has not been thoroughly studied in these knockouts nor has analysis of these various genes been studied in the N-Myc CKO. Despite this, the observed phenotypes of both our N-Myc CKO in comparison to mutations of other genes in interactive pathways, allows us to speculate on some of the interactions and possible mechanisms through which N-MYC might act.
Table 3.
Phenotypic comparisons between N-Myc CKO and implicated pathway genes
 |
Mycs are co-expressed in the ear and may play redundant functions
The Mycs share the same DNA target sequences (Henriksson and Luscher, 1996; Dang et al., 1999; Grandori et al., 2000; Gostissa et al., 2009) and genetic studies swapping C-Myc coding exon with the N-Myc gene (Malynn et al., 2000) have suggested high levels of functional redundancy. We are in the process of looking at the effects that L-Myc CKO and L-Myc/N-Myc double CKO have in the ear (Tg(Pax2-Cre)L-Myc f/+ N-Myc f/+ x L-Myc f/f x N-Myc f/f). Ideally, we have to look at the binding partner of the MYCs, MAX. MAX null mice are embryonic lethal (Shen-Li et al., 2000). A conditional knockout of MAX would allow for complete analysis of the effects of all MYC proteins in the ear and several laboratories are in the process of generating such a mouse.
Non-sensory constrictions did not form in the absence of N-Myc suggesting a possible cooperation between N-Myc and Otx1 and Lmx1a
In the absence of proper molecular signals, sensory specifications will not occur (Fritzsch et al., 2001; Daudet and Lewis, 2005; Fritzsch et al., 2006; Nichols et al., 2008). Both Lmx1a (Nichols et al., 2008; Hwang et al., 2009) and Otx1 (Morsli et al., 1999; Fritzsch et al., 2001) are important in the formation of the utriculo-saccular foramen and the ductus reuniens. Both form from non-sensory constrictions. Lmx1a and Otx1 null mice do not form these constrictions and therefore there is a lack of segregation of the epithelia in these mutants similar to the lack of separation between the utricle, saccule, and cochlea that is present in the N-Myc CKO (Table 3). Lmx1a and Otx1 have no known oncogene function and Lmx1a is restricted to non-sensory epithelia, yet reduced levels of N-Myc provided a convergence of phenotype with the Otx1 and Lmx1a null suggesting a convergence or cooperation between these genes or their signaling pathways.
In the N-Myc CKO, the non-sensory compartment was not capable of forming the ductus reuniens or utriculo-saccular foramen closely akin to data on Lmx1a null mice (Nichols et al., 2008; Koo et al., 2009). It is possible that N-Myc is essential for the proliferation in the non-sensory compartment. Thus, the non-sensory tissue would not be able to proliferate sufficiently to respond to molecular cues to expand a duct or to form the constrictions. As such, reducing levels of N-Myc would have no effect on the expression of Lmx1a or Otx1. Furthermore, ISH shows that N-Myc is expressed in the presumptive utriculo-saccular foramen and ductus reuniens (Fig. 6E” insert). Thus, the topology of N-Myc expression in the WT correlates with defects in the mutant. Similarity of Lmx1a, Otx1 and N-Myc phenotype would reflect convergence through non-identical processes.
An alternative explanation would suggest a direct molecular interaction between N-Myc. Lmx1a, and Otx1. Otx2 regulates Fgf10 (an upstream activator of N-Myc) expression in the otic placode of chicken (Bok et al., 2005; Miyazaki et al., 2006) and a link between increased levels of Otx2 and N-Myc was shown in meduloblastomas, suggesting that Otx2 and N-Myc may form a feedback loop (Adamson et al., 2010). Otx2 is found in the cochlea while Otx1 is present in the vestibular epithelia (Morsli et al., 1999) and while there appears to be a possible connection between Otx2 and N-Myc, the interaction between Otx1 and N-Myc remains unstudied. We are looking into this interaction with Otx-/- Pax2-Cre N-Myc f/f double null mice.
N-Myc may act as a proto-oncogene and as a node for proliferation control
N-Myc appears to be important for proliferation and growth of the organ of Corti (Fig. 2). In the absence of N-Myc, the growth of the organ of Corti is reduced proportional to reduced numbers of organ of Corti cells that exit the cell cycle and presumptive cochlear hair cells enter terminal mitosis progressively from apex to base. Our EdU studies show that the number of cells positive for EdU after 48 hours (and thus had very likely exited the cell cycle at E12.5) are reduced in the N-Myc CKO compared to the control littermate. Longer time delays are needed as used by others (Matei et al., 2005) to conclusively show that all cells positive for EdU at E14.5 have terminally exited the cell cycle at E12.5. Additionally, we are unable to directly quantify the change in length of the cell cycle in N-Myc CKOs. What one can conclude is that cells positive for EdU either exited the cell cycle shortly after administration of EdU or these cells have undergone a limited number of additional mitoses. Further experiments are needed to fully quantify the change of the cell cycle in N-Myc CKOs. Alternative hypotheses other than a reduction in proliferation due to the loss of N-Myc could include a differential increase in proliferation (only some EdU cells would quickly dilute out EdU, resulting in less EdU positive cells) or increased cell death (otherwise EdU positive cells undergo premature/increased apoptosis and results in less EdU positive cells). These alternative explanations are more complicated compared to our conclusion that N-Myc CKO have a reduction in proliferation which is in agreement with other work regarding the cell cycle exit of hair cells (Ruben, 1967; Matei et al., 2005; Lee et al., 2006), other work on the effects of N-MYC on the cell cycle (Kenney et al., 2003; Pession et al., 2004; Okubo et al., 2005) and recent studies suggesting the impact of proliferation on overall cochlear growth (Grimsley-Myers et al., 2009) whereas the influence of cell death has recently been shown to be negligible in this early stage even in the complete absence of hair cell differentiation (Pan et al., 2010a; Fritzsch et al., 2011). N-Myc, like most proto-oncogenes, drives proliferation through its direct and indirect interactions with pRB (Supplemental Fig. 1A and 1B). N-Myc CKO show a dramatic reduction in size in all areas where Cre is expressed (Fig. 1 and Table 1). When N-MYC protein levels are reduced or are absent, downstream effectors are likely reduced (Supplemental Fig. 1C). This may truncate overall proliferation to reduce the overall size of the ear (Fig. 1 and Supplemental Fig. 1C-C”). Our system shows what is observed when N-Myc is knocked out and the downstream effects that occur. However, in order to fully understand proliferation acting through N-Myc, we must look upstream of N-MYC and factors known to act through Myc.
A number of proto-oncogenes are thought to act upstream of, or in combination with, N-Myc (Supplemental Fig. 1B). If these genes act through N-Myc in the ear as they do elsewhere in the body, then we would expect the N-Myc CKO phenotype to be representative of these previously described mutants. Knockouts of Foxg1 [an upstream gene to N-Myc and coincident in meduloblastoma, (Pauley et al., 2006; Adesina et al., 2007)], Chd7 [implicated with N-Myc as effectors in CHARGE syndrome (Genevieve et al., 2007; Hurd et al., 2010)], and Fgf10 [upstream activator of Myc and part of the Wnt pathway (Pauley et al., 2003; Rash and Grove, 2007)] each reduce overall proliferation resulting in a truncated cochlea and histologic alterations in hair cells corresponding to N-Myc CKO (Table 3). Hence, reducing an upstream activator of N-Myc curtails overall proliferative capacity of the system and shows that N-Myc, at minimum, integrates the upstream signals in the ear from other proto-oncogenes to likely regulate the intrinsic cell cycle control of pRB.
N-Myc CKOs cause cochlear truncation and extra rows of hair cells in the apex likely by forcing hair cell precursors out of the cell cycle prematurely
The cochlea is thought to elongate by convergent extension (CE) that links proliferation with the post-mitotic differentiation and movements of cells. During CE, a thinning of the sensory epithelia occurs as the cochlea is elongated (Chen et al., 2002). Defects in CE are thought to result in a reduced cochlear elongation (Chen et al., 2008) resulting in a truncated cochlea that may contain excess hair cells with or without polarity defects due to cochlear immaturity. In Foxg1, Ngn1, Wnt5a (Table 2), Vangl2, and the Dvl1/2 mutants, disruptions to convergent extension resulted in shortened cochleae, an apex containing extra rows of hair cells, and hair cell polarity defects (Wang et al., 2005; Montcouquiol et al., 2006; Qian et al., 2007). The Jackson Waltzer mutant (Jxc1) has a shortened cochlea as well as polarity defects, but only at its apex (Chen et al., 2008) (Table 3). In general, the apex is differentially affected in terms of additional hair cells, disorganization, and truncation (Chen et al., 2008).
The propensity for apical disorganization and immaturity is possibly linked to early exit from the cell cycle and an extended period of time until expression of proper differentiation cues. The Jag1 CKO, known to have a reduction in the cell cycle inhibitor p27Kip1 (a protein that is inhibited by N-Myc) (Brooker et al., 2006; Kiernan et al., 2006; Pan et al., 2010b), indicates that by altering the cell cycle, CE is affected and the cochlea develops abnormally (Table 3). Exit from the cell cycle is dependent on levels of proto-oncogene and tumor suppressor. The apex is the first portion of the cochlea to exit the cell cycle at E12.5 and is the last to differentiate. The apex has the longest “waiting” period of quiescence between cell cycle exit and onset of Atoh1 mediated differentiation. In mutants that curtail proliferation, i.e. Dll1, Foxg1, Neurog1, and N-Myc CKOs, we notice an increased susceptibility of cells at the apex to be abnormal, which is possibly due to an even earlier exit from the cell cycle (presumably due to reduced proto-oncogene levels) and the resulting extended period of time until differentiation (Matei et al., 2005; Brooker et al., 2006) (Table 3). Thus, it is possible that the N-Myc CKO cochlear phenotype is directly related to N-MYC’s function as a proto-oncogene and N-MYC’s control of the cell cycle. While at this time we are unable to conclusively prove that N-Myc CKOs have a reduction in the proliferating sensory precursors, our data and the data of others suggest that by knocking out N-Myc, precursor populations exit cell cycle prematurely, and, as a result, are unable to reach the nominal number of cochlear duct cells, and the cochlea is thus proportionally shortened.
N-Myc CKO organ of Corti was severely disrupted
N-Myc CKO has both an increase in hair cells towards the apex as well as the abnormal organ of Corti formation noted above. It appears that the absence of N-Myc results in a shortened cochlea (possibly due to changes in the proliferation) and the reduced extension and coiling at the apex may be in part related to this. In addition, hair cells appear to be lost in adults in the cochlea, but not the vestibular sensory epithelia. Therefore, Mycs play a role in either the proper development of hair cells, the long term maintenance of the hair cells, or both.
Our ISH data show that the Mycs are expressed to at least P0 in differentiated hair cells suggesting that they may play a role beyond proliferation. In the N-Myc CKO, hair cells appear normal until P7 but by P18 the organ of Corti is disrupted and by six weeks of age, the organ of Corti is entirely abnormal, missing nearly all recognizable hair cells. This, combined with the fact that N-Myc regulates a number of microRNAs that inhibit apoptosis (Mott et al., 2010) may suggest that the Mycs play a role in the stability and long term retention of hair cells.
N-Myc is important in neuronal path-finding and may be linked with neurotrophic regulation
Inferior vestibular ganglion neurons delaminate and project to the posterior canal crista and saccule under the guidance of BDNF (Tessarollo et al., 2004). There is no cross innervations between vestibular neurons and cochlear epithelia unless a neurotrophin is misexpressed. Our N-Myc CKO show cross innervation of saccule and basal turn of the cochlea, a complete lack of innervation of the posterior canal, innervation of an ectopic patch of hair cells near the base of the cochlea, and disorganized innervation of the apex. It is possible that posterior canal fibers from the inferior vestibular ganglion project to the base of the cochlea. N-Myc CKO misexpression of BDNF possibly related to the cross innervation patterning remains to be shown.
N-Myc has been associated with altered levels of BDNF in neuroblastomas (Edsjo et al., 2004; Nakamura et al., 2006) as well as interacting with FGFR2 to regulate levels of NTF-3 (Hossain et al., 2008). BDNF misexpression has been shown to disrupt neuronal path-finding (Tessarollo et al., 2004; Hossain et al., 2008; Feng et al., 2010; Hoshino et al., 2010) but a direct correlation with Myc and the expression of neurotrophic factors has not been studied in the ear. Our lab is currently working with Bdnf and Ntf-3 transgenic mice to better understand the effects of altering neurotrophin expression on neuronal path-finding beyond what is already known (Fritzsch et al., 2004). Logically, N-Myc CKO must be studied for Bdnf and Ntf-3 expression profiles.
Conclusion
N-Myc and other members of the Myc family have long been implicated in a number of human diseases. All three Mycs (C-Myc, L-Myc, N-Myc) are correlated with tumor formation (Albihn et al., 2010). N-Myc expression is increased in neuroblastomas (Lasorella et al., 2000; Lasorella et al., 2002; Rothschild et al., 2006). A number of genes known to be related to N-Myc are affected in humans as parts of the VACTERL syndrome. N-Myc (Feingold syndrome), Chd7 (CHARGE syndrome), Gli3 (Pallister-Hall), and Sox2 are all genes implicated in the group of birth defects which include vertebral, anal, cardiac, esophageal (TE fistulas), renal and kidney malformations. These mutants also have ear defects and need to be analyzed for the expression changes in L-Myc and N-Myc.
N-Myc acts in a complex and integrated manner to control the highly conserved pRB/E2F cell cycle regulator. Conditional knockouts of N-Myc allow for the analysis of N-Myc in the development of the inner ear. N-Myc CKOs have highlighted the possible interaction of a number of pathways as well as provided an ample foundation for continued exploration of cell cycle regulation, tumorigenesis, stem cell therapy, and inner ear development. N-Myc CKOs must be analyzed for the expression levels of possible interactors (Table 3 and Supplemental Fig. 1) through both ISH and qRT-PCR. We conclude that N-Myc acts as a nodal point and regulates the growth of the ear as a whole as well as differentially regulating certain sensory and non-sensory components. N-Myc is also essential to the proper 3D formation of the ear due to its interactions with not only cell proliferative factors but also cell differentiation cues. Lastly, N-Myc is necessary for sensory neuron pathfinding.
Experimental Procedures
Mice and Genotyping
N-Myc null mice are embryonic lethal (Knoepfler et al., 2002). To study postnatal time points, two conditional lines were generated by crossing Foxg1-Cre (Foxg1KiCre)or Pax2-Cre (Tg(Pax2-Cre) )with N-Myc floxed mice (Jackson Labs B6.129-N-Myctm1Psk/J. Either Foxg1-Cre (Jackson Labs129(Cg)-Foxg1tm1(cre)Skm/J) or Pax2-Cre (Ohyama and Groves, 2004) were crossed with N-Myc f/f. Both lines are viable; however, they appear to have an increased postnatal mortality rate. Pax2-Cre expressed in the inner ear, cerebellum, and kidney, as well as Foxg1-Cre, expressed in the inner ear and forebrain, have allowed later embryonic and postnatal analysis (including an ongoing behavioral analysis) of N-Myc as our CKOs have survived for over eight months. While CKO mice looked morphologically normal, they were stunted in growth and displayed circling behavior and disorientation. Tail biopsies were used for genomic DNA and polymerase chain reaction for genotyping using the following primers (N-Myc: IMR6727 5’gtcgcgctagtaagagctgagatc 3’ IMR6729 5’ cacagctctggaaggtgggagaaagttgagcgtctcc 3’ Cre: 1 5’ cctgttttgcacgttcaccg 3’ 2 5’ atgcttctgtccgtttgccg 3’ IMR42 5’ ctaggccacagaattgaaagatct 3’ IMR43 5’ gtaggtggaaattctagcatcatcc 3’). Animal care and usage was in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines for the use of laboratory animals in biological research and approved (ACURF # 0804066).
Perfusion
Pups were injected intraperitoneally with 0.025mL/g of the anesthetic Tribromoethanol (Avertin®) and after a surgical level of anesthesia was induced, as assessed by ocular and pedal reflexes, 4% paraformaldehyde (PFA) was pumped continuously with a 30 gauge needle into the left ventricle until blood emptied and peripheral veins appeared clear. The right ventricle was opened to facilitate clearing. After fixation, heads were hemi-dissected and placed in 4% PFA for long-term storage.
Thin-Sheet Laser Imaging Microscopy (TSLIM)
Fixed six week old Pax2-Cre N-Myc CKO and control littermate ears were dissected from the skull. Ears were decalcified with 10% EDTA for three days and the solution was changed daily. Decalcified ears were dehydrated in a graded ascending ethanol series and cleared with Spalteholz solution (5:3 methyl salicylate: benzyl benzoate). Specimens were stained in Rhodamine B isothiocyanate (1mg/200mL in Spalteholz for one day) by immersion and imaged with TSLIM at the University of Minnesota (Santi et al., 2009). Whole inner ears were nondestructively, serially sectioned in a mid-modiolar plane at 5μm thickness. A complete Z-stack of optical sections containing the full dimension of the inner ear resulted in 270 well-registered images using a Retiga 2000 digital camera on TSLIM. Image voxel size was 1.5×l.5×5μm. Images were adjusted for brightness and contrast and saved as .tif files.
3D reconstruction
The Z-stack for each inner ear was loaded into Amira ver. 5.2 (Visage Imaging, San Diego, CA) for 3D reconstruction of inner ear structures. In order to isolate different inner ear structures and compute their morphometric parameters, a process called segmentation was used. Using Amira’s semi-automated tools, the borders of each structure of interest were outlined in a different color in every section of the stack. We defined the scala media to include the endolymphatic space within the scala but it did not include the tectorial membrane or the inner sulcus. After segmentation, Amira provided isosurface volume reconstructions of individual inner ear structures as well as an estimate of their volume based on voxel size. Structure centroids were determined by the centerline tree module in Amira. To compute the spiral length of each structure, a smooth B-spline curve fit was computed from each structure’s centroid.
Plastic Sectioning
Dissected ears were incubated overnight with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and were fixed in 1% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4) for approximately one hour or until nerve fibers were dark. Samples were imaged with Nikon Eclipse 800 or dehydrated with an ethanol and propylene oxide mixture. Sections were orientated and embedded with epoxy resin and placed in an incubator for two days at 60°C. Ears were sectioned at 2 μm with an Ultratome (Ultracut, Reichert-Jung), stained with Stevenel’s Blue for one minute and imaged (Nichols et al., 2008). Briefly, sections were retrieved from water bath and placed on heated slides in sequential order. They were allowed to bake onto the glass slide and stained for one minute with Stevenel’s Blue. Stained slides were rinsed with distilled water and allowed to dry before imaging. Chosen sections were coverslipped using DMX mounting solution and allowed to dry overnight. Samples were imaged with Nikon Eclipse 800 microscope and captured with Image-Pro.
Lipophilic Dye Tracing
Flattened pieces of filter paper soaked in lipophilic dye were inserted in the cochlear nucleus, rhombomere 4, or cerebellum of hemisected heads (Fritzsch et al., 2005). The dye was allowed to diffuse for three days at 60°C in 4%PFA. Imaging by confocal microscopy was performed shortly after filter paper removal to prevent transcellular diffusion. Embryos were mounted on a slide in glycerol and images were captured with a Leica TCS SPE confocal microscope or TCS SP5 multiphoton confocal microscope.
Immunochemistry
Ears were dissected in 0.4% PFA and defatted overnight in 70% ethanol. They were removed from ethanol and blocked for two hours in blocking solution (5% normal goat serum (NGS), 0.1% Triton X-100, in PBS). The following primary antibodies were diluted in the blocking solution at the given dilution ratio (primary antibody: blocking solution) and incubated for three days at room temperature. MYO7A (1:200) (Proteus Biosciences, 25-6790), LECTIN (GS IB4) Isoconjugated (1:400) (Santa Cruz Biotechnology), and β-TUBULIN (1:800) (Sigma, T7451). Samples were washed with PBS and secondary antibodies were diluted in blocking buffer and incubated for two days, covered with aluminum foil, in 4°C. Secondary antibodies were used as follows: Alexa Fluor 532 anti-rabbit (1:400) and Alexa Fluor 633 anti-mouse (1:400) (both from Sigma). Embryos were mounted on a slide in glycerol and images were captured with a Leica TCS SPE confocal microscope or TCS SP5 multiphoton confocal microscope, as previously described (Pauley et al., 2006; Pan et al., 2009).
In situ Hybridization
Fixed embryos were prepared to avoid RNAse. RNA probes labeled with digoxigenin were N-Myc, L-Myc, C-Myc, and Fgf8. The protocol was previously described (Pauley et al., 2006; Pan et al., 2009). Briefly, ears were dissected in 0.4%PFA, washed and digested with 10μg/mL Proteinase K (Ambion, Austin, Tex. USA). The samples were incubated overnight with riboprobe in 60°C in hybridization solution (50% formamide, 2X saline sodium citrate, and 6% dextran sulfate). Samples were washed and incubated overnight with anti-digoxigenin antibody (Roche Diagnostics, Mannhein, Germany). Unbound anti-digoxigenin antibody was washed off with PBS and samples were reacted with BM Purple substrate to cause a purple reaction product in places of bound mRNA. Samples were dissected, mounted, and imaged with Nikon Eclipse 800 microscope and captured with Image-Pro.
Hair Cell Quantification
Using Leica TCS SPE confocal microscope or TCS SP5 multiphoton confocal microscope, stacks of MYO7A IHC ears were taken. For length measurements, LAS AF Lite software measurement tools were used on collapsed Z-stacks. For cochlear hair cell quantification, inner and outer hair cells positive for MYO7A were manually counted after collapsing the Z-stack. For canal cristae hair cells counts, a Z-stack was taken in six μm steps (about the size of a hair cell nucleus). Hair cells were manually counted and great effort was taken to avoid double counting.
EdU Staining
Pregnant dams were injected intraperitoneally with 80 μg EdU/gram mouse. After 48 hours, the mothers were sacrificed and pups were fixed and stored in 4%PFA. Dissected ears were then reacted with Click-iT reaction cocktail according to manufacturer’s instructions (Invitrogen C35002). The ears were imaged using TCS SP5 multiphoton confocal microscope.
Supplementary Material
MAX forms a heterodimer with MYC but is also capable of forming a heterodimer with one of three antagonists, MGA, MNT, or MXD. Either the MYC-MAX or MNT/MGA/MXD (MMM)-MAX pairs will bind to the E-box sequence on target genes. Along with accessory binding partners, MYC-MAX will allow for the transcription of target genes responsible for leading to proliferation. Contrarily, MMM-MAX will inhibit the transcription of these target genes, leading to differentiation. Target genes of MYC-MAX include CYCLINs, IDs, E2F, and various microRNAs. Both the CYCLINs and IDs remove the pRB from E2F through phosphorylation and allow E2F to bind to S-phase promoting genes and allow subsequent proliferation. IDs are also able to bind bHLH transcription factors but since they lack a basic motif, are unable to bind E-Boxes, inhibiting differentiation. In the absence of IDs or CYCLINs, bHLH transcription factors are free to bind to E-proteins and the E-box, promoting differentiation (A).
In WT controls, upon activation of NOTCH1 by JAG1, the Notch IntraCellular Domain (NICD) is cleaved by γ-SECRETASE. NICD translocates to the nucleus (shown by gray dotted arrow) to form a complex with RBPJ to positively regulate the transcription of the Hes family of bHLH transcription factors (Doetzlhofer et al., 2009). MYC is regulated by a number of pathways, including the Wnt/β-CATENIN pathway, TGF-β or BMP/SMAD, Fgf/Erk1-2, and the Shh/Smo pathways. Once activated, MYC interacts with partner proteins (A) to regulate a number of downstream effectors, including the CYCLINs, E2F, IDs, CDKIs, and various microRNAs. This regulation ultimately leads to the inhibition of activator bHLH transcription factors from binding to the E-proteins which then allows the HES to occupy the E-proteins. It also leads to the removal of pRB from E2F and allows E2F to bind S-phase promoting genes. When E2F binds with pRB, proliferation cannot commence and the cell enters a state of quiescence where differentiation may occur (B). To the right, a WT control cartoon shows the phenotype of a WT inner ear (B’). In the absence of Myc, Myc downstream effectors have a dosage related decrease and growth is reduced while onset of differentiation may be accelerated (C). To the right are the resulting phenotypes of knocking out N-Myc in the inner ear using both Pax2-Cre (C’) and Foxg1-Cre (C”). Asterisks mark genes whose phenotypes have similar characteristics as seen in N-Myc CKO and are qualified in table 3. The larger circle represents the cell as a whole while the smaller circle is the nucleus. Arrows indicate upregulation while blunted lines represent blocking. Dashed lines represent a reduction to endogenous protein levels. Red indicates pathways favoring differentiation, blue indicates pathways favoring proliferation. Modified after (Fritzsch et al., 2006). Note: given the conservation and functional redundancy of the Mycs from yeast to man, it is reasonable to assume that the possible interactions for a Myc revealed in the literature apply to the ear.
After 3D reconstruction of TSLIM images using Amira software, we were able to rotate the six week WT littermate to our N-Myc CKO. Translucent structures are the three scalae while the basilar membrane, organ of Corti, and Rosenthal’s canal are opaque.
3D reconstruction and animation allows for a unique perspective to analyzing mutants with grossly abnormal phenotypes. Provided here is a 3D reconstruction and video of our six week N-Myc CKO. Translucent structures are the three scalae while the basilar membrane, organ of Corti, and Rosenthal’s canal are opaque.
Acknowledgments
We like to thank Drs. Ohyama and Groves for providing the Pax2-Cre line. We thank Jackson Lab and Dr. Knoepfler providing the Foxg1-Cre and N-Myc flox mice. We also thank Dr. Eisenman for personal communications regarding the N-Myc and L-Myc CKOs. The Leica TCS SP5 confocal microscope was purchased in part with a grant from the Roy. J. Carver foundation. Grant funding was provided through the NIH and NIDCD RO1-DC055095590 to Dr. Fritzsch. TSLIM imaging for the mouse cochleae was provided by funding from the Capita Foundation and the NIDCD (RO1-DC007588 and DC007588-03S1) to Dr. Santi. We wish to thank the following people for providing the plasmids used in our in situ hybridization experiments: Dr. Pirvola (Fgf8), Dr. Moses (C-Myc), Dr. Yu (L-Myc), and Dr. Beisel/Jason Pecka (N-Myc). We thank the University of Iowa Office of the Vice President for Research and the College of Liberal Arts and Sciences for support. We thank Jennifer Kersigo for her technical expertise and Chris Donahue for assistance with genotyping.
References
- Adamson DC, Shi Q, Wortham M, Northcott PA, Di C, Duncan CG, Li J, McLendon RE, Bigner DD, Taylor MD, Yan H. OTX2 is critical for the maintenance and progression of Shh-independent medulloblastomas. Cancer Res. 2010;70:181–191. doi: 10.1158/0008-5472.CAN-09-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesina AM, Nguyen Y, Mehta V, Takei H, Stangeby P, Crabtree S, Chintagumpala M, Gumerlock MK. FOXG1 dysregulation is a frequent event in medulloblastoma. J Neurooncol. 2007;85:111–122. doi: 10.1007/s11060-007-9394-3. [DOI] [PubMed] [Google Scholar]
- Aguda BD, Kim Y, Piper-Hunter MG, Friedman A, Marsh CB. MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proc Natl Acad Sci U S A. 2008;105:19678–19683. doi: 10.1073/pnas.0811166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albihn A, Johnsen JI, Henriksson MA. MYC in oncogenesis and as a target for cancer therapies. Adv Cancer Res. 2010;107:163–224. doi: 10.1016/S0065-230X(10)07006-5. [DOI] [PubMed] [Google Scholar]
- Beisel K, Hansen L, Soukup G, Fritzsch B. Regenerating cochlear hair cells: quo vadis stem cell. Cell Tissue Res. 2008;333:373–379. doi: 10.1007/s00441-008-0639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132:2115–2124. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- Bok J, Dolson DK, Hill P, Ruther U, Epstein DJ, Wu DK. Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development. 2007;134:1713–1722. doi: 10.1242/dev.000760. [DOI] [PubMed] [Google Scholar]
- Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, Kupriyanov S, Baldwin KK. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, Reed S, Sicinski P, Bartek J, Eilers M. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigande JV, Heller S. Quo vadis, hair cell regeneration? Nat Neurosci. 2009;12:679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Cantos R, Cole LK, Acampora D, Simeone A, Wu DK. Patterning of the mammalian cochlea. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11707–11713. doi: 10.1073/pnas.97.22.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Lin Z, Kulessa H, Hebert J, Hogan BL, Wu DK. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008;4:e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen Z, Montcouquiol M, Calderon R, Jenkins NA, Copeland NG, Kelley MW, Noben-Trauth K. Jxc1/Sobp, encoding a nuclear zinc finger protein, is critical for cochlear growth, cell fate, and patterning of the organ of corti. J Neurosci. 2008;28:6633–6641. doi: 10.1523/JNEUROSCI.1280-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Resar LM, Emison E, Kim S, Li Q, Prescott JE, Wonsey D, Zeller K. Function of the c-Myc oncogenic transcription factor. Exp Cell Res. 1999;253:63–77. doi: 10.1006/excr.1999.4686. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One. 2008;3:e1516. doi: 10.1371/journal.pone.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfouli S, Bakke A, Huang J, Wynshaw-Boris A, Hurlin PJ. Inflammatory disease and lymphomagenesis caused by deletion of the Myc antagonist Mnt in T cells. Mol Cell Biol. 2006;26:2080–2092. doi: 10.1128/MCB.26.6.2080-2092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diermeier-Daucher S, Brockhoff G. Dynamic proliferation assessment in flow cytometry. Curr Protoc Cell Biol. 2010;Chapter 8(Unit 8 6):1–23. doi: 10.1002/0471143030.cb0806s48. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver EC, Pryor SP, Hill P, Turner J, Ruther U, Biesecker LG, Griffith AJ, Kelley MW. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28:7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsjo A, Nilsson H, Vandesompele J, Karlsson J, Pattyn F, Culp LA, Speleman F, Pahlman S. Neuroblastoma cells with overexpressed MYCN retain their capacity to undergo neuronal differentiation. Lab Invest. 2004;84:406–417. doi: 10.1038/labinvest.3700061. [DOI] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Bendiske J, Morest DK. Postnatal development of NT3 and TrkC in mouse ventral cochlear nucleus. J Neurosci Res. 2010;88:86–94. doi: 10.1002/jnr.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KP, Eisenman RN. Two MAD tails: what the recent knockouts of Mad1 and Mxi1 tell us about the MYC/MAX/MAD network. Biochim Biophys Acta. 1999;1423:M37–47. doi: 10.1016/s0304-419x(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006;28:1181–1193. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Eberl DF, Beisel KW. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Jahan I, Pan N, Kersigo J, Duncan J, Kopecky B. Dissecting the molecular basis of organ of Corti development: Where are we now? Hear Res. 2011 doi: 10.1016/j.heares.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue Maroon, NeuroVue Red and NeuroVue Green and their use for double and triple labeling of neuronal profile. Brain Res Bull. 2005;66:249–258. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Nichols DH. DiI reveals a prenatal arrival of efferents at the differentiating otocyst of mice. Hear Res. 1993;65:51–60. doi: 10.1016/0378-5955(93)90200-k. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001;211:388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Ye X, Goufman E, Shianov P, Hay N, Najmabadi F, Tyner AL. Myc represses the p21(WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc Natl Acad Sci U S A. 2001;98:4510–4515. doi: 10.1073/pnas.081074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevieve D, de Pontual L, Amiel J, Sarnacki S, Lyonnet S. An overview of isolated and syndromic oesophageal atresia. Clin Genet. 2007;71:392–399. doi: 10.1111/j.1399-0004.2007.00798.x. [DOI] [PubMed] [Google Scholar]
- Gostissa M, Ranganath S, Bianco JM, Alt FW. Chromosomal location targets different MYC family gene members for oncogenic translocations. Proc Natl Acad Sci U S A. 2009;106:2265–2270. doi: 10.1073/pnas.0812763106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci. 2009;29:15859–15869. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AK. The challenge of hair cell regeneration. Exp Biol Med (Maywood) 2010;235:434–446. doi: 10.1258/ebm.2009.009281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson M, Luscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, O’Connell BC, Mateyak MK, Tam W, Kohlhuber F, Dang CV, Sedivy JM, Eick D, Vogelstein B, Kinzler KW. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci U S A. 2000;97:2229–2234. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CW, Hurlin PJ. Of Myc and Mnt. J Cell Sci. 2006;119:208–216. doi: 10.1242/jcs.02815. [DOI] [PubMed] [Google Scholar]
- Hoshino N, Vatterott P, Egwiekhor A, Rochlin MW. Brain-derived neurotrophic factor attracts geniculate ganglion neurites during embryonic targeting. Dev Neurosci. 2010;32:184–196. doi: 10.1159/000313902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain WA, D’Sa C, Morest DK. Interactive roles of fibroblast growth factor 2 and neurotrophin 3 in the sequence of migration, process outgrowth, and axonal differentiation of mouse cochlear ganglion cells. J Neurosci Res. 2008;86:2376–2391. doi: 10.1002/jnr.21685. [DOI] [PubMed] [Google Scholar]
- Hurd EA, Poucher HK, Cheng K, Raphael Y, Martin DM. The ATP-dependent chromatin remodeling enzyme CHD7 regulates pro-neural gene expression and neurogenesis in the inner ear. Development. 2010;137:3139–3150. doi: 10.1242/dev.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin PJ. N-Myc functions in transcription and development. Birth Defects Res C Embryo Today. 2005;75:340–352. doi: 10.1002/bdrc.20059. [DOI] [PubMed] [Google Scholar]
- Hurlin PJ, Huang J. The MAX-interacting transcription factor network. Semin Cancer Biol. 2006;16:265–274. doi: 10.1016/j.semcancer.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Hurlin PJ, Steingrimsson E, Copeland NG, Jenkins NA, Eisenman RN. Mga, a dual-specificity transcription factor that interacts with Max and contains a T-domain DNA-binding motif. EMBO J. 1999;18:7019–7028. doi: 10.1093/emboj/18.24.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CH, Simeone A, Lai E, Wu DK. Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev Dyn. 2009;238:2725–2734. doi: 10.1002/dvdy.22111. [DOI] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010;5:e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BH, Ish-Horowicz D. The Groucho/TLE/Grg family of transcriptional co-repressors. Genome Biol. 2008;9:205. doi: 10.1186/gb-2008-9-1-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26:550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- Kersigo J, D’Angelo A, Gray B, Soukup GA, Fritzsch B. The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre mediated microRNA loss. Genesis. 2011 doi: 10.1002/dvg.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesser BW, Lalwani AK. Gene therapy and stem cell transplantation: strategies for hearing restoration. Adv Otorhinolaryngol. 2009;66:64–86. doi: 10.1159/000218208. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16:2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS, Kenney AM. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle. 2006;5:47–52. doi: 10.4161/cc.5.1.2292. [DOI] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin ZS, Millen KJ, Wu DK. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev Biol. 2009;333:14–25. doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara A, Hirabayashi Y, Knoepfler PS, Taketo MM, Sakai J, Kodama T, Gotoh Y. Wnt signaling and its downstream target N-myc regulate basal progenitors in the developing neocortex. Development. 2010;137:1035–1044. doi: 10.1242/dev.046417. [DOI] [PubMed] [Google Scholar]
- Laine H, Doetzlhofer A, Mantela J, Ylikoski J, Laiho M, Roussel MF, Segil N, Pirvola U. p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J Neurosci. 2007;27:1434–1444. doi: 10.1523/JNEUROSCI.4956-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasorella A, Boldrini R, Dominici C, Donfrancesco A, Yokota Y, Inserra A, Iavarone A. Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res. 2002;62:301–306. [PubMed] [Google Scholar]
- Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2000;407:592–598. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- Lebel R, McDuff FO, Lavigne P, Grandbois M. Direct visualization of the binding of c-Myc/Max heterodimeric b-HLH-LZ to E-box sequences on the hTERT promoter. Biochemistry. 2007;46:10279–10286. doi: 10.1021/bi700076m. [DOI] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull. 2003;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Kamel S, Wong E, Fritzsch B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010;340:303–321. doi: 10.1007/s00441-010-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn BA, de Alboran IM, O’Hagan RC, Bronson R, Davidson L, DePinho RA, Alt FW. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 2000;14:1390–1399. [PMC free article] [PubMed] [Google Scholar]
- Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377–2388. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H, Kobayashi T, Nakamura H, Funahashi J. Role of Gbx2 and Otx2 in the formation of cochlear ganglion and endolymphatic duct. Dev Growth Differ. 2006;48:429–438. doi: 10.1111/j.1440-169X.2006.00879.x. [DOI] [PubMed] [Google Scholar]
- Modak S, Cheung NK. Neuroblastoma: Therapeutic strategies for a clinical enigma. Cancer Treat Rev. 2010 doi: 10.1016/j.ctrv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126:2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 2010;110:1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–358. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- Orian A, Delrow JJ, Rosales Nieves AE, Abed M, Metzger D, Paroush Z, Eisenman RN, Parkhurst SM. A Myc-Groucho complex integrates EGF and Notch signaling to regulate neural development. Proc Natl Acad Sci U S A. 2007;104:15771–15776. doi: 10.1073/pnas.0707418104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2010a doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Lee JE, Fritzsch B. Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg(Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res. 2009;337:407–428. doi: 10.1007/s00441-009-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A. 2010b;107:15798–15803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Kopecky B, Beisel K, Soukup G, Fritzsch B. Stem cells and molecular strategies to restore hearing. Panminerva Med. 2008;50:41–53. [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Matei VA, Beisel KW, Fritzsch B. Wiring the ear to the brain: the molecular basis of neurosensory development, differentiation and survival. In: Kelley MW, Wu DK, Popper AN, Fay RR, editors. The development of the inner ear. New York: Springer Verlag; 2005. pp. 85–121. [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini G, Diolaiti D, Porro A, Della Valle G. In vivo transcriptional regulation of N-Myc target genes is controlled by E-box methylation. Proc Natl Acad Sci U S A. 2005;102:12117–12122. doi: 10.1073/pnas.0409097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pession A, Tonelli R, Fronza R, Sciamanna E, Corradini R, Sforza S, Tedeschi T, Marchelli R, Montanaro L, Camerin C, Franzoni M, Paolucci G. Targeted inhibition of NMYC by peptide nucleic acid in N-myc amplified human neuroblastoma cells: cell-cycle inhibition with induction of neuronal cell differentiation and apoptosis. Int J Oncol. 2004;24:265–272. [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20:6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Patterning the dorsal telencephalon: a role for sonic hedgehog? J Neurosci. 2007;27:11595–11603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sanchez SM, Beisel KW. Pocket proteins and cell cycle regulation in inner ear development. IntJ Dev Biol. 2007;51:585–595. doi: 10.1387/ijdb.072387sr. [DOI] [PubMed] [Google Scholar]
- Romand R, Hirning-Folz U, Ehret G. N-myc expression in the embryonic cochlea of the mouse. Hear Res. 1994;72:53–58. doi: 10.1016/0378-5955(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Rothschild G, Zhao X, Iavarone A, Lasorella A. E Proteins and Id2 converge on p57Kip2 to regulate cell cycle in neural cells. Mol Cell Biol. 2006;26:4351–4361. doi: 10.1128/MCB.01743-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, Brundin P. Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS One. 2009;4:e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220(Suppl):221–244. [PubMed] [Google Scholar]
- Ruggero D. The role of Myc-induced protein synthesis in cancer. Cancer Res. 2009;69:8839–8843. doi: 10.1158/0008-5472.CAN-09-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi PA, Johnson SB, Hillenbrand M, GrandPre PZ, Glass TJ, Leger JR. Thin-sheet laser imaging microscopy for optical sectioning of thick tissues. Biotechniques. 2009;46:287–294. doi: 10.2144/000113087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J. TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol. 2001;3:400–408. doi: 10.1038/35070086. [DOI] [PubMed] [Google Scholar]
- Shen-Li H, O’Hagan RC, Hou H, Jr, Horner JW, 2nd, Lee HW, DePinho RA. Essential role for Max in early embryonic growth and development. Genes Dev. 2000;14:17–22. [PMC free article] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3:525–530. doi: 10.1016/s1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, Richards GS, Conaco C, Dacre M, Hellsten U, Larroux C, Putnam NH, Stanke M, Adamska M, Darling A, Degnan SM, Oakley TH, Plachetzki DC, Zhai Y, Adamski M, Calcino A, Cummins SF, Goodstein DM, Harris C, Jackson DJ, Leys SP, Shu S, Woodcroft BJ, Vervoort M, Kosik KS, Manning G, Degnan BM, Rokhsar DS. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–726. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Moroy T, Bartek J, Massague J, Hanel F, Eilers M. Repression of p15INK4b expression by Myc through association with Miz-1. Nat Cell Biol. 2001;3:392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Harada T, Sakamoto Y, Yamauchi N, Yoshida S, Iwabe T, Terakawa N. Activation of mitogen-activated protein kinase pathway by keratinocyte growth factor or fibroblast growth factor-10 promotes cell proliferation in human endometrial carcinoma cells. J Clin Endocrinol Metab. 2003;88:773–780. doi: 10.1210/jc.2002-021062. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W, Zhou ZQ, Ota S, Wynshaw-Boris A, Hurlin PJ. Mnt-Max to Myc-Max complex switching regulates cell cycle entry. J Cell Biol. 2005;169:405–413. doi: 10.1083/jcb.200411013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif. 2008;41(Suppl 1):51–56. doi: 10.1111/j.1365-2184.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MAX forms a heterodimer with MYC but is also capable of forming a heterodimer with one of three antagonists, MGA, MNT, or MXD. Either the MYC-MAX or MNT/MGA/MXD (MMM)-MAX pairs will bind to the E-box sequence on target genes. Along with accessory binding partners, MYC-MAX will allow for the transcription of target genes responsible for leading to proliferation. Contrarily, MMM-MAX will inhibit the transcription of these target genes, leading to differentiation. Target genes of MYC-MAX include CYCLINs, IDs, E2F, and various microRNAs. Both the CYCLINs and IDs remove the pRB from E2F through phosphorylation and allow E2F to bind to S-phase promoting genes and allow subsequent proliferation. IDs are also able to bind bHLH transcription factors but since they lack a basic motif, are unable to bind E-Boxes, inhibiting differentiation. In the absence of IDs or CYCLINs, bHLH transcription factors are free to bind to E-proteins and the E-box, promoting differentiation (A).
In WT controls, upon activation of NOTCH1 by JAG1, the Notch IntraCellular Domain (NICD) is cleaved by γ-SECRETASE. NICD translocates to the nucleus (shown by gray dotted arrow) to form a complex with RBPJ to positively regulate the transcription of the Hes family of bHLH transcription factors (Doetzlhofer et al., 2009). MYC is regulated by a number of pathways, including the Wnt/β-CATENIN pathway, TGF-β or BMP/SMAD, Fgf/Erk1-2, and the Shh/Smo pathways. Once activated, MYC interacts with partner proteins (A) to regulate a number of downstream effectors, including the CYCLINs, E2F, IDs, CDKIs, and various microRNAs. This regulation ultimately leads to the inhibition of activator bHLH transcription factors from binding to the E-proteins which then allows the HES to occupy the E-proteins. It also leads to the removal of pRB from E2F and allows E2F to bind S-phase promoting genes. When E2F binds with pRB, proliferation cannot commence and the cell enters a state of quiescence where differentiation may occur (B). To the right, a WT control cartoon shows the phenotype of a WT inner ear (B’). In the absence of Myc, Myc downstream effectors have a dosage related decrease and growth is reduced while onset of differentiation may be accelerated (C). To the right are the resulting phenotypes of knocking out N-Myc in the inner ear using both Pax2-Cre (C’) and Foxg1-Cre (C”). Asterisks mark genes whose phenotypes have similar characteristics as seen in N-Myc CKO and are qualified in table 3. The larger circle represents the cell as a whole while the smaller circle is the nucleus. Arrows indicate upregulation while blunted lines represent blocking. Dashed lines represent a reduction to endogenous protein levels. Red indicates pathways favoring differentiation, blue indicates pathways favoring proliferation. Modified after (Fritzsch et al., 2006). Note: given the conservation and functional redundancy of the Mycs from yeast to man, it is reasonable to assume that the possible interactions for a Myc revealed in the literature apply to the ear.
After 3D reconstruction of TSLIM images using Amira software, we were able to rotate the six week WT littermate to our N-Myc CKO. Translucent structures are the three scalae while the basilar membrane, organ of Corti, and Rosenthal’s canal are opaque.
3D reconstruction and animation allows for a unique perspective to analyzing mutants with grossly abnormal phenotypes. Provided here is a 3D reconstruction and video of our six week N-Myc CKO. Translucent structures are the three scalae while the basilar membrane, organ of Corti, and Rosenthal’s canal are opaque.