Abstract
Neuromodulators have complex effects on both the presynaptic release and postsynaptic detection of neurotransmitters. Here we describe recent advances in our understanding of synaptic modulation by metabotropic GABAB receptors. By inhibiting multivesicular release from the presynaptic terminal, these receptors decrease the synaptic glutamate signal. GABAB receptors also inhibit the Ca2+ permeability of NMDA receptors to decrease Ca2+ signals in postsynaptic spines. These new findings highlight the importance of GABAB receptors in regulating many aspects of synaptic transmission. They also point to novel questions about the spatiotemporal dynamics and sources of synaptic modulation in the brain.
Keywords: GABAB receptor, NMDA receptor, multivesicular release, calcium signaling, dendrite, spine, two-photon microscopy, two-photon uncaging
Introduction
Neurons throughout the brain communicate via the release and detection of chemical neurotransmitters. Release involves the fusion of vesicles at the presynaptic terminal and detection involves the activation of receptors in the postsynaptic membrane. Both processes are constantly changing, allowing synaptic transmission to be highly plastic over many time scales. These changes can reflect either the intrinsic properties of synapses or the influence of extrinsic chemical neuromodulators. In this review, we describe recent advances in our understanding of the impact of these neuromodulators at the level of individual synapses. We focus on regulation by GABAB receptors (GABAB–Rs), drawing comparisons when possible to other neuromodulators working via similar mechanisms. Finally, we discuss important questions that remain about synaptic modulation and the technologies that may help provide answers.
Receptor diversity
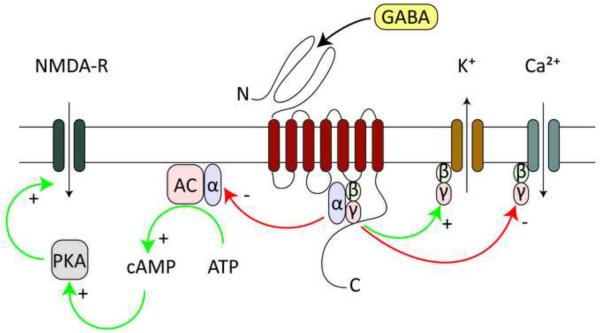
Gamma aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the brain and acts via ionotropic and metabotropic receptors to control the electrical and biochemical properties of neurons [1]. GABAB-Rs are metabotropic G-protein coupled receptors found at both excitatory and inhibitory synapses in almost all regions of the brain [2]. These receptors are usually thought to release Gβγ subunits that inhibit Ca2+ channels [3] and activate K+ channels [4]. They also release Gαi/Gαo subunits that inhibit adenylyl cyclase (AC) to reduce cAMP levels and decrease protein kinase A (PKA) activity [5]. By interacting with multiple downstream signaling cascades, it is likely that GABAB-Rs have many physiological roles that we have only begun to characterize (Figure 1).
Figure 1. Post-synaptic intracellular GABAB-R signaling.
GABA binding to GABAB-R heterodimers releases Gβγ subunits that locally diffuse to open K+ channels and close Ca2+ channels. In addition, released Gαi/Gαo subunits inhibit adenylyl cyclase (AC), which constitutively produces cAMP to activate PKA, with potentially many downstream targets including NMDA-Rs.
In order to function, GABAB-Rs require two distinct subunits known as GABAB1 and GABAB2 [6–8]. GABAB1 is needed for activation by external agonists and GABAB2 is responsible for both signaling and membrane targeting [9,10]. Gene splicing divides GABAB1 into two isoforms known as GABAB1a and GABAB1b, which differ in their N-terminal regions, where only GABAB1a contains a pair of sushi domains [11]. These domains preferentially target GABAB1a to the presynaptic terminals of excitatory synapses, where it modulates glutamate release [*12]. On the postsynaptic side, both isoforms are found in the dendrites, but only GABAB1b is located in spines [*12]. This isoform seems to provide the majority of coupling to K+ channels, as knocking out GABAB1b reduces postsynaptic K+ currents, whereas knocking out GABAB1a has no effect [*12]. GABAB1b is also responsible for inhibition of dendritic Ca2+ spikes [13], possibly via a direct effect on voltage-sensitive Ca2+ channels (VSCCs) [*14].
Before the advent of gene cloning, pharmacological studies predicted a wide range of GABAB-R isoforms with different functional properties [15,16]. It was thus surprising when only two isoforms were ultimately discovered [17], which have similar agonist binding and signaling properties [2]. This discrepancy was recently resolved by the discovery of auxiliary binding proteins including KCTD (potassium channel tetramerization domain-containing) proteins [18,19], Mupp1 [20], and GISP [21], which together help confer the diversity observed in earlier studies. Understanding the roles of different GABAB-R isoforms and auxiliary binding proteins in synaptic modulation remains an exciting topic for future study.
Presynaptic release
GABAB-Rs are one of many neuromodulatory receptors that can powerfully influence the release of neurotransmitters. Release requires a presynaptic action potential to open VSCCs and allow Ca2+ influx to activate the molecular machinery of vesicle fusion. GABAB-Rs inhibit VSCCs to decrease Ca2+ influx and reduce release at both excitatory and inhibitory synapses [22,23]. GABAB-Rs can also inhibit release by activating K+ channels, which shunt the presynaptic action potential and indirectly limit Ca2+ influx [24]. Moreover, GABAB-Rs can reduce vesicle priming by decreasing cAMP concentrations in the presynaptic terminal [25]. Results from other neuromodulators suggest that Gβγ subunits can also interact with the fusion machinery to change the mode of release [26]. Thus, GABA and other neuromodulators can act through multiple targets to tightly regulate presynaptic release.
Measuring presynaptic modulation at single synapses is challenging but can be accomplished with a variety of imaging probes. For example, presynaptic Ca2+ signals can be imaged with Ca2+-sensitive dyes [22,23], vesicle sorting probed with styryl dyes [27], and GABAB-R subunit interactions studied with FRET measurements [27]. In some cases, modulation of glutamate release can be detected with two-photon optical quantal analysis [28,29]. With this approach, release properties are measured using large NMDA-R Ca2+ signals evoked in dendritic spines, where successful events are clearly separated from failures. The influence of different modulators is then assessed by the impact of pharmacological agonists on release probability. This approach has now been used to demonstrate that GABAB [**30] muscarinic [*31], and adenosine receptors [32] all inhibit presynaptic release onto postsynaptic spines.
Multivesicular release
Until recently, each presynaptic action potential was usually thought to release only a single vesicle from the presynaptic terminal. However, it is now clear that many different synapses have the ability to release multiple vesicles in response to a single action potential [33]. Multivesicular release (MVR) is pronounced at high release probabilities and is dynamically regulated by activity [32,34]. The ability to release multiple vesicles at individual boutons shifts the synaptic glutamate concentration from a binary to a graded signal. Because postsynaptic glutamate receptors are often not saturated [29,35], this increases the information capacity by extending the dynamic range of synaptic communication.
Recent evidence shows that presynaptic GABAB-Rs suppress MVR to modulate glutamate signals at synapses [**30]. As predicted, GABAB-Rs increase the number of failures detected by optical quantal analysis, suggesting a decrease in release probability. Surprisingly, GABAB-Rs also decrease the amplitude of postsynaptic Ca2+ signals evoked by successful release events. Although inhibition of these Ca2+ signals could reflect direct modulation of NMDA-Rs, blocking postsynaptic G-protein signaling has no effect on this attenuation. Instead, decreasing the extracellular Ca2+ concentration to reduce MVR occludes the GABAB-R-evoked decrease in these Ca2+ signals. By inhibiting MVR, GABAB-Rs can decrease the synaptic glutamate concentration and thereby control postsynaptic responses in a graded fashion. Similar results have also been found for muscarinic receptors in the striatum [*31], suggesting that regulation of MVR may be common throughout the brain.
Postsynaptic conductances
The rapid detection of neurotransmitter is accomplished at synapses throughout the brain by ionotropic receptors. At excitatory synapses, these include a variety of both AMPA and NMDA receptors [36]. It is well known that these receptors possess multiple sites for post-translational modifications [37]. Phosphorylation is often considered in terms of receptor trafficking, especially during synaptic plasticity [38]. However, this modification can also change open times, agonist affinity and ion selectivity of channels. For example, the Ca2+ permeability of NMDA-Rs is usually under tonic up-regulation by constitutive PKA activity [*39]. By targeting different signaling cascades, GABAB-Rs have the potential to change many properties of postsynaptic transmission.
It has been difficult to detect postsynaptic modulation of ionotropic glutamate receptor function by GABAB-Rs. One complication is the widespread prevalence of presynaptic inhibition, which is difficult to avoid in most experiments. Two-photon glutamate uncaging bypasses the presynaptic terminal and allows direct study of modulation at single spines throughout the dendritic arbor (Figure 2A) [40,41]. Given that GABAB-Rs are located in close proximity to glutamate receptors [42], it was initially predicted that GABAB-Rs would modulate glutamate receptors. Surprisingly, however, GABAB–Rs do not impact either AMPA-R or NMDA-R EPSCs at pyramidal neuron spines in the prefrontal cortex [**30]. This is also true for D2-R modulation at striatal synapses [**43], despite the clear role these and other neuromodulatory receptors play in synaptic plasticity.
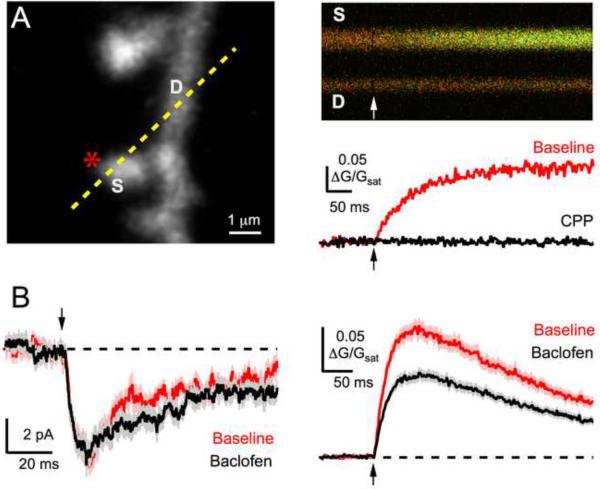
Figure 2. Modulation of postsynaptic NMDA-R Ca signals.
A, Left, Two-photon image of dendrite and spines, showing uncaging location (asterisk) and line-scan position (dashed yellow line). Right, Line-scans (top) show a change in green Ca2+ signal after two-photon uncaging, quantified (bottom) before (red) and after (black) wash-in of the NMDA-R antagonist CPP. B, Average NMDA-R currents (left) and Ca2+ signals (right) before (red) and after wash-in of the GABAB-R agonist baclofen (black) (adapted from Chalifoux & Carter, 2010).
Postsynaptic calcium signals
In addition to generating postsynaptic conductances, NMDA-Rs are the predominant source of Ca2+ signals in the spines of many neurons throughout the brain [29,44]. These signals are particularly important for initiating the physiological and morphological changes that occur during synaptic plasticity [38]. GABAB-Rs are usually thought to inhibit these signals by opening a variety of K+ channels [45] found in both dendrites and spines [42]. The resulting hyperpolarization enhances Mg2+ blockade of NMDA-Rs to reduce their overall current and thus Ca2+ influx [46,47]. However, the impact of this GABAB-R-evoked hyperpolarization on postsynaptic Ca2+ signals remains unexplored at the level of individual spines.
Recent results demonstrate that GABAB-Rs exert direct and powerful inhibition of Ca2+ influx through NMDA-Rs (Figure 2B) [**30]. Even though GABAB-Rs do not inhibit NMDA-R EPSCs, they can reduce postsynaptic Ca2+ signals by approximately half. This effect is independent of Gβγ subunits, K+ channel activation, VSCC activation and internal Ca2+ stores. Instead, it is mediated by Gαi/Gαo subunits, which inhibit AC to decrease cAMP levels and suppress PKA activity. Because PKA normally enhances Ca2+ influx through NMDA-Rs, this enables GABAB-Rs to inhibit Ca2+ signals in spines. Similar results have been found for D2-R modulation in the striatum [**43], suggesting this may be a widespread function of neuromodulators that signal via Gαi/Gαo subunits. However, the molecular mechanisms for the selective reduction of NMDA-R Ca2+ permeability still need to be resolved. In addition, it will be interesting to determine the roles of auxiliary proteins including AKAPs (A-kinase anchoring proteins) in this new form of synaptic modulation [48].
Future directions
We now know a great deal about how GABAB-Rs and other neuromodulatory receptors regulate synaptic transmission, but there are many important questions remaining. To finish, we briefly explore three future directions in the study of synaptic modulation, addressing new technologies that may help provide answers.
Targets of modulation
Both excitatory post-synaptic potentials (EPSPs) and synaptic Ca2+ signals are shaped by interactions between channels and receptors in spines. For example, opening R-type VSCCs generates a Ca2+ signal that activates Ca2+-sensitive K+ Channels and generates a hyperpolarization to block NMDA-Rs [49]. How do GABAB-Rs regulate voltage- and Ca2+-sensitive ion channels to influence these local feedback loops? Recent results indicate that GABAB-Rs can inhibit VSCCs in spines and dendrites throughout the dendritic arbor of cortical pyramidal cells [*14]. It will be interesting to determine if this inhibition leads to any changes in EPSPs and synaptic Ca2+ signals, as seen for D2-Rs in the striatum [**43]. GABAB-Rs may also regulate Ca2+-sensitive K+ channels themselves, as recently discovered for muscarinic receptors in the hippocampus [50], leading to an entirely new kind of synaptic modulation.
Timing of modulation
GABAB-Rs initiate multiple signaling cascades to influence ion channels and glutamate receptors in pre- and postsynaptic structures. What is the temporal profile over which these different cascades regulate the release and detection of glutamate? Answering this question is difficult with classical pharmacology involving the tonic application of specific agonists. Fortunately, a range of caged compounds is now available that are rapidly released with either one- or two-photon excitation. Local uncaging generates a brief pulse of GABA whose effects on EPSCs and Ca signals can be studied over time [*14,51]. FRET probes can also be used to study how this time-locked GABAB-R activation influences protein-protein interactions in different subcellular compartments [27]. These approaches may help reveal different kinetic profiles for GABAB-R modulation via both Gβγ and Gαi/Gαo subunits [52].
Sources of modulation
Anatomical studies show that a variety of inhibitory interneurons innervate distinct subcellular domains in pyramidal neurons [53]. For example, parvalbumin-positive neurons synapse near the cell body, while somatostatin-expressing neurons target dendrites. Which interneurons are responsible for supplying the GABA that modulates the release and detection of glutamate at excitatory synapses? In some cases, paired recordings can be used to target individual interneurons and assess their modulatory impact. For example, neurogliaform cells release clouds of GABA that can activate GABAB-Rs on dendritic spines [52,54]. Novel optogenetic tools can also be used to target different populations of interneurons and control their firing properties [55,56]. These approaches may ultimately help define the activity patterns needed to activate presynaptic and postsynaptic GABAB-Rs and trigger synaptic modulation [57].
Summary
GABAB-R modulation plays a central role in the ability of neurons to function in circuits. This is highlighted by the consequences of disrupted modulation in the prefrontal cortex in neuropsychiatric diseases [58]. Recent studies have revealed new ways in which GABAB-Rs can control synaptic responses. Thus, GABAB-Rs can suppress MWR to decrease the synaptic glutamate concentration. Unexpectedly, GABAB-Rs can also act via the PKA pathway to decrease postsynaptic NMDA-R Ca2+ signals. By also inhibiting VSCCs in spines and dendrites, GABAB-Rs are thus poised to potently regulate Ca2+ -mediated plasticity. In addition to GABAB-Rs, these effects are also found with other modulators like acetylcholine and dopamine, suggesting that these processes are occurring at diverse synapses throughout the brain. However, many questions remain about the spatial, temporal and cell-type specific effects of neuromodulators. A variety of new technologies will allow us to better understand the properties of synaptic modulation in normal physiology and disease states.
Acknowledgements
We thank members of the Carter lab for helpful comments on the manuscript. This work was supported by NIH (F30MH087409) to JRC and the Klingenstein Fund and NIH (1R01MH085974-01A1) to AGC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no financial conflicts of interests.
References
- 1.Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich D, Bettler B. GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol. 2007;17:298–303. doi: 10.1016/j.conb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- 4.Gähwiler BH, Brown DA. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci USA. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Wojcik WJ. Gamma aminobutyric acid B receptor-mediated inhibition of adenylate cyclase in cultured cerebellar granule cells: blockade by islet-activating protein. J Pharmacol Exp Ther. 1986;239:568–573. [PubMed] [Google Scholar]
- 6.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 7.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 8.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 9.Robbins MJ, Calver AR, Filippov AK, Hirst WD, Russell RB, Wood MD, Nasir S, Couve A, Brown DA, Moss SJ, et al. GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calver AR, Robbins MJ, Cosio C, Rice SQ, Babbs AJ, Hirst WD, Boyfield I, Wood MD, Russell RB, Price GW. The C-terminal domains of the GABA(b) receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J Neurosci. 2001;21:1203–1210. doi: 10.1523/JNEUROSCI.21-04-01203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biermann B, Ivankova-Susankova K, Bradaia A, Abdel Aziz S, Besseyrias V, Kapfhammer JP, Missler M, Gassmann M, Bettler B. The Sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci. 2010;30:1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Vigot R, Barbieri S, Bräuner-Osborne H, Turecek R, Shigemoto R, Zhang Y-P, Luján R, Jacobson LH, Biermann B, Fritschy J-M. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors use knockout mice to investigate the anatomical and functional roles of GABAB1a and GABAB1b receptors. They use electron microscopy and electrophysiology to study these isoforms in pre- and post-synaptic structures. They also examine the consequences of losing these GABAB receptor isoforms on synaptic plasticity and behavior.
- 13.Pérez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- *14.Chalifoux JR, Carter AG. GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.4561-10.2011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors use two-photon imaging in cortical pyramidal neurons to show that GABAB receptors inhibit voltage-sensitive Ca channels in apical and basal spines and dendrites.
- 15.Dutar P, Nicoll RA. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988;1:585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- 16.Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- 17.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 18.Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, et al. Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature. 2010;465:231–235. doi: 10.1038/nature08964. [DOI] [PubMed] [Google Scholar]
- 19.Bartoi T, Rigbolt KT, Du D, Kohr G, Blagoev B, Kornau HC. GABAB receptor constituents revealed by tandem affinity purification from transgenic mice. J Biol Chem. 2010;285:20625–20633. doi: 10.1074/jbc.M109.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasubramanian S, Fam SR, Hall RA. GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J Biol Chem. 2007;282:4162–4171. doi: 10.1074/jbc.M607695200. [DOI] [PubMed] [Google Scholar]
- 21.Kantamneni S, Correa SA, Hodgkinson GK, Meyer G, Vinh NN, Henley JM, Nishimune A. GISP: a novel brain-specific protein that promotes surface expression and function of GABA(B) receptors. J Neurochem. 2007;100:1003–1017. doi: 10.1111/j.1471-4159.2006.04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu LG, Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. The Journal of Physiology. 1995;485(Pt 3):649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson SM, Gahwiler BH. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 26.Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- 27.Laviv T, Riven I, Dolev I, Vertkin I, Balana B, Slesinger PA, Slutsky I. Basal GABA regulates GABA(B)R conformation and release probability at single hippocampal synapses. Neuron. 2010;67:253–267. doi: 10.1016/j.neuron.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Yuste R, Majewska A, Cash SS, Denk W. Mechanisms of calcium influx into hippocampal spines: heterogeneity among spines, coincidence detection by NMDA receptors, and optical quantal analysis. J Neurosci. 1999;19:1976–1987. doi: 10.1523/JNEUROSCI.19-06-01976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature. 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- **30.Chalifoux JR, Carter AG. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors use optical quantal analysis and two-photon glutamate uncaging to probe GABAB receptor modulation of synaptic transmission. They show that GABAB receptors can modulate multivesicular release at cortical synapses. This is the first study to show that neuromodulators can selectively inhibit NMDA-R Ca influx.
- *31.Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci. 2009;12:1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors use optical quantal analysis to investigate synaptic modulation in striatal medium spiny neurons. They show that muscarinic receptors decrease multivesicular release, which in turn affects the temporal summation of inputs.
- 32.Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K. Facilitation at single synapses probed with optical quantal analysis. Nat Neurosci. 2002;5:657–664. doi: 10.1038/nn867. [DOI] [PubMed] [Google Scholar]
- 33.Tong G, Jahr CE. Multivesicular release from excitatory synapses of cultured hippocampal neurons. Neuron. 1994;12:51–59. doi: 10.1016/0896-6273(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 34.Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- 35.McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:6173–6178. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 37.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 38.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- *39.Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]; In this study, the authors use electrophysiology and imaging techniques to show that PKA can modulate NMDA receptor Ca influx. This study motivated subsequent work on neuromodulation of postsynaptic Ca signals through NMDA receptors.
- 40.Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulik A, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors showed that D2 dopamine receptors inhibit corticostriatal glutamate release at striatopallidal medium spiny neurons. They find that D2 receptors inhibit Ca influx through both NMDA receptors and R-type VSCCs. This is the first study to show bidirectional modulation of NMDA receptor Ca influx via the PKA pathway.
- 44.Kovalchuk Y, Eilers J, Lisman J, Konnerth A. NMDA receptor-mediated subthreshold Ca(2+) signals in spines of hippocampal neurons. J Neurosci. 2000;20:1791–1799. doi: 10.1523/JNEUROSCI.20-05-01791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng P-Y, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S. GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron. 2009;63:230–243. doi: 10.1016/j.neuron.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrisett RA, Mott DD, Lewis DV, Swartzwelder HS, Wilson WA. GABAB-receptor-mediated inhibition of the N-methyl-D-aspartate component of synaptic transmission in the rat hippocampus. J Neurosci. 1991;11:203–209. doi: 10.1523/JNEUROSCI.11-01-00203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. Journal of Neurophysiology. 2004;92:2027–2039. doi: 10.1152/jn.00427.2004. [DOI] [PubMed] [Google Scholar]
- 48.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 49.Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Giessel AJ, Sabatini BL. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron. 2010;68:936–947. doi: 10.1016/j.neuron.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dittman JS, Regehr WG. Mechanism and kinetics of heterosynaptic depression at a cerebellar synapse. J Neurosci. 1997;17:9048–9059. doi: 10.1523/JNEUROSCI.17-23-09048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 54.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 55.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 58.Bowery NG. GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol. 2006;6:37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]