Abstract
Orally administered recombinant attenuated Salmonella vaccines (RASV) elicit humoral and mucosal immune responses against the immunizing antigen. The challenge in developing an effective vaccine against a virus or an intracellular bacterium delivered by RASVs is to introduce the protective antigen inside the host cell cytoplasm for presentation to MHC-I molecules for an efficient cell mediated immune response. To target the influenza nucleoprotein (NP) into the host cell cytosol, we constructed a regulated delayed lysis in vivo RASV strain χ11246(pYA4858) encoding influenza NP with a chromosomal deletion of the sifA gene to enable it to escape from the endosome prior to lysis. Oral immunization of mice with χ11246(pYA4858) (SifA−) with 3 booster immunizations resulted in complete protection (100%) against a lethal influenza virus (rWSN) challenge (100 LD50) compared to 25% survival of mice immunized with the isogenic χ11017(pYA4858) (SifA+) strain. Reducing the number of booster immunizations with χ11246(pYA4858) from 3 to 2 resulted in 66% survival of mice challenged with rWSN (100 LD50). Immunization with χ11246(pYA4858) via different routes provided protection in 80% orally, 100% intranasally and 100% intraperitoneally immunized mice against rWSN (100 LD50). A Th1 type immune response was elicited against influenza NP in all experiments. IFN-γ secreting NP147–155 specific T cells were not found to be correlated with protection. The role of antigen-specific CD8+ T cells remains to be determined. To conclude, we showed that Salmonella can be designed to deliver antigen(s) to the host cell cytosol for presumably class I presentation for the induction of protective immune responses.
Keywords: Salmonella, sifA, RASV, influenza, nucleoprotein
1. Introduction
Influenza is one of the most significant diseases worldwide causing acute respiratory illnesses and accounts for 25% of infections that exacerbate chronic lung infections [1]. Several epidemics and four major pandemics have been reported. Influenza infections are primarily and effectively controlled by vaccines that elicit neutralizing antibodies against the surface proteins hemagglutinin (HA) and neuraminidase (NA). However, influenza vaccines are frequently reformulated to match the circulating strains. Therefore, the development of a vaccine that elicits broad based protection against different strains is desirable. Influenza nucleoprotein (NP) is over 90 % conserved between influenza A strains and generates cellular immunity [2]. CD8+ and CD4+ T-cell NP epitopes are well defined [3] and induced T cells afford NP specific protection [4]. NP or the NP-encoding gene have been delivered to mice and pigs using the purified NP [5, 6], adenovirus [7], vaccinia virus [8], or a DNA vaccine [9] to induce NP-specific T cells but moderate to low protection against challenge. DNA vaccines provide the best protection against homologous and low dose heterologous challenges in mice [9–11].
Orally delivered vaccines induce mucosal as well as systemic immune responses to antigens as compared to vaccines delivered via the parenteral routes [12], are cost effective since they eliminate the use of needles and syringes and thus are an affordable choice for mass vaccination. Attenuated Salmonella vaccines have successfully been used as carriers for several bacterial, viral and parasite antigens [12]. Live attenuated Salmonella vaccines following oral inoculation initially colonize the gut-associated lymphoid tissue (GALT) through the M cells of Peyer’s patches, invade the intestinal epithelium [13] and subsequently colonize the deeper tissues like the liver and the spleen. The bacteria reach the mesenteric lymph nodes and blood within 1–3 hours after inoculation [14]. In phagocytes, the bacteria remain in the structure called the Salmonella containing vacuole (SCV). The SCVs do not mature into phagocytic vesicles and the bacteria are sometimes killed and processed via the endolysosomal pathway and presented in the context of MHC-II molecules [15] provoking CD4+ Th1 and Th2 responses [16].
Salmonella, unlike Shigella, do not reach the cytoplasm of invaded cells efficiently [17] and generates a strong CD4+ response [18, 19] but is less likely to elicit a CD8+ cytotoxic T-cell (CTL) response against the heterologous antigens. Orally delivered recombinant Salmonella expressing circumsporozoite protein induced low levels of antigen-specific CD8+ T cells and a low protective immunity against malarial challenge [20, 21]. NP delivered via a Salmonella Typhimurium aroA mutant induced antigen-specific CD4+ T cells in the immunized mice without any induction of CD8+ T cells or protection from viral challenge [18]. Success of a RASV delivering a gene from a pathogen requiring a cell-mediated immune response for protection, depends on its ability to inject or to secrete the protective antigen into the host cell cytosol for proteosomal degradation and presentation in the context of MHC-I molecules.
S. Typhimurium strains engineered to express p60 or Hly from Listeria monocytogenes secreted the expressed antigen to the cytosol, induced efficient CD8+ T cell responses and protected mice against listeriosis [22]. S. Typhimurium strains expressing E. coli hemolysin delivered a DNA vaccine vector to the cytosol of macrophages [23]. However, these bacteria lacked a cell lysis feature for efficient delivery of the DNA vaccine to the host cell cytosol to enable uptake into the nucleus for expression.
Gram-negative bacteria use a type III secretion system (T3SS) for injection of effector proteins into the host cell cytosol [24]. Heterologous epitopes fused to T3SS effectors are secreted to the cytosol of the host cell and are presented by MHC-I molecules[25–27] generating efficient CD8+ T-cell responses but induced none to moderate protection for different pathogens [26, 28]. In addition, some proteins require deletion of regions of the gene for secretion through the T3SS [29].
Our laboratory has developed RASVs against several pathogens including Streptococcus pneumoniae, Yersinia, and Eimeria [26, 30–32]. These RASVs are genetically modified for attenuation and rely on an Asd+ balanced-lethal host-vector system for plasmid maintenance eliminating the need for a plasmid antibiotic-resistance marker [33]. Deletion of the asdA gene puts an obligate requirement for diaminopimelic acid (DAP), an essential constituent of peptidoglycan, so the bacterium cannot survive in vivo. Also, a regulated delayed lysis in vivo system based on a ΔasdA mutation and insertion of an arabinose-regulated expression of the chromosomal murA gene coupled with a plasmid vector encoding arabinose-regulatable expression of asdA and murA genes conferred attenuation and biological containment. Vaccination with such RASV resulted in induced antibody responses to a released bolus of pneumococcal antigen and protective immunity [34].
We designed S. Typhimurium to escape the endosome after invasion by deleting the sifA gene (SifA−) in the chromosome. The sifA gene is a Salmonella pathogenicity island 2 (SPI-2) encoded, T3SS secreted effector protein that governs conversion of the SCV into filaments. Its deletion releases Salmonella into the cytosol [35]. Replication of S. Typhimurium alters vacuole processing by the usual endocytic pathway [36] and leads to the production of Salmonella induced filaments (Sifs) that connect the SCVs. SCVs containing SifA− mutants loose the integrity of the vacuolar membranes. S. Typhimurium SifA− strains are attenuated but replicate more efficiently than the wild-type bacteria in epithelial cells [36]. However, S. Enteritidis SifA− were virulent for mice [37]. Salmonella SifA− strain (CD12) was more invasive and survived longer both in vivo and in vitro than SifA+ strains WT05 and SL7202 [38]. RASV regulated delayed lysis strain (χ11246) (SifA−) should exit the endosome soon after invasion of a host cell, multiply in the cytoplasm and deliver by lysis the synthesized NP protein from the plasmid (pYA4858) to the cell cytosol to induce cellular immunity. We report here the results of experiments to test these hypotheses.
2. Materials and Methods
2.1. Bacterial strains and plasmids
Bacterial strains and plasmids used in this study are listed in Table 1. S. Typhimurium strains were derived from the highly virulent strain UK-1 [39]. Bacteriophage P22HTint was used for generalized transduction [40]. Escherichia coli and S. Typhimurium cultures were grown in LB broth [41] or on LB agar plates at 37°C. LB agar without NaCl and with 5% sucrose was used for sacB gene-based counter-selection in allelic exchange experiments. Diaminopimelic acid (DAP) was added at the concentration of 50 µg/ml for the growth of Asd− strains [33]. For host-regulated delayed lysis vector combinations, LB was supplemented with 0.2% arabinose [34].
Table 1.
Bacterial strains and plasmids used in this study.
| Strain or plasmid | Genotype or relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli K-12 strains | ||
| TOP 10 | Invitrogen | |
| χ6212 | Ф80d lacZΔM15 deoR Δ(lacZYA-argF)-U169 glnV44 λ− gyrA96 recA1 endA1 ΔasdA4 Δzhf-2∷Tn10 hsdR17 (R−M+) | Lab collection |
| χ7213 | thi-1 thr-1 leuB6 glnV44 fhuA21 lacY1 recA1 RP4-2-Tc ∷Mu[λ pir] ΔasdA4 (Δzhf-2∷Tn10) | [65] |
| S. enterica serovar Typhimurium UK-1 strains | ||
| χ8926 | ΔsifA26 | Lab collection |
| χ11017 | ΔasdA27∷TT araC PBAD c2ΔaraBAD23 Δ(gmd-fcl)-26 Δpmi-2426ΔrelA198∷araC PBAD lacITT ΔPmurA25∷TT araC PBAD murA | Lab collection |
| χ11246 | ΔasdA27∷TT araC PBAD c2ΔaraBAD23Δ(gmd-fcl)-26 Δpmi-2426ΔrelA198∷araC PBAD lacITT ΔPmurA25∷TT araC PBAD murA ΔsifA26 | χ11017 This study |
| Plasmids | ||
| pCAGGS-NP | Vector containing nucleoprotein (NP) gene from A/WSN/33 | Provided by Dr. Andrew Pekosz |
| pUC57-WSN-NP | Commercial vector pUC-57 containing codon optimized A/WSN/33 NP | Genscript |
| pYA3681 | Lysis vector containing Ptrc promoter | [34] |
| pYA4702 | NP gene expressed from Ptrc promoter cloned into lysis vector pYA3681 with pBR ori | pYA3681. This study |
| pYA4858 | Codon optimized NP gene expressed from Ptrc promoter cloned into lysis vector pYA3681 with pBR ori | pYA3681. This study |
| pYA4651 | ply gene from S. pneumonia expressed from Ptrc promoter cloned into lysis vector pYA3681 with pBR ori | Provided by Dr. Wei Xin |
| Suicide Vector | ||
| pYA3716 | ΔsifA26 | pRE112 |
2.2. Strain construction and characterization
The ΔsifA26 mutation is a defined in-frame deletion of the sifA gene. It was introduced into strain χ11017 by phage P22 transduction [40] from (χ8926∷pYA3716) to generate strain χ11246. The presence of the mutation was verified by PCR. The presence of the ΔasdA27 mutation in Salmonella was confirmed by its dependence on DAP for growth [33]. The presence of the ΔPmurA25∷TT araC PBAD murA mutation (Table 1) was verified by its dependence on arabinose for growth. LPS profiles were examined as previously described [42].
The lysis phenotype of the bacterial strains was confirmed by diluting overnight cultures 10−3 and 10−4 and plating 100 µl samples on LB plates with or without 0.2% arabinose followed by incubation at 37°C. Strains displaying regulated delayed lysis were grown on LB agar containing arabinose only, depicting complete dependence on the presence of arabinose for survival [34].
2.3. General DNA procedures
DNA manipulations were carried out as described by Sambrook et al. [43]. Transformations of E. coli and S. Typhimurium strains were done by electroporation (Bio-Rad, Hercules, CA). Transformants containing Asd+ plasmids were selected on LB agar plates without DAP. Plasmid stability was determined as described before [26]. The primer pairs used in this study are listed in Table 2. Vent DNA polymerase was used for PCR reactions with dNTPs (Invitrogen).
Table 2. Primers used in this study.
Underlined sequence = Restriction enzyme site.
| Primer name | Sequence |
|---|---|
| RDLP-2 | 5’-tcccccccgggttactatttatcgtcgtcatctttgtagtcgatatcatgatctttataatcaccgtcatggtctttgtagtcattgtcgtactcctctgcattgtctccgaa |
| RDLF-3 | 5’- catgccatggcgaccaaaggcaccaaacga |
| RDLF-5 | 5’- atgccatggcgatggcgacca |
| RDLRP-7 | 5’- ctattaccatgggttatcatattcttccgcg |
| Ptrc-F | 5’- attctgaaatgagctgtt |
| Ptrc-R | 5’-tctcatccgccaaaacagcc |
2.4. Synthesis of peptide
Synthetic peptide NP147–155 (T YQRTRALV) was obtained from Biosynthesis Inc. (Lewisville, TX). It was dissolved in water according to the manufacturer’s instruction, aliquoted, and stored at −20°C until used.
2.5. Codon optimization of NP gene
The sequence of the nucleoprotein (NP) gene of influenza virus strain A/WSN/33 (NCBI, accession number EU330203) was codon optimized for maximal expression in Salmonella. The gene sequence was commercially codon-optimized and cloned in pUC-57 to yield pUC-57-NP-WSN by Genscript (Piscataway, NJ). The replaced codons are depicted in Table 3. The average G+C content was changed from 46.66 for the non-optimized gene to 54.59 after codon optimization. The stem-loop structures, which impact ribosomal binding and stability of mRNA were disrupted. The codon usage bias for E. coli was increased from a codon adaptation index of 0.57 to 0.98.
Table 3.
Codon-optimized versus original nucleotide and amino acid sequences of A/WSN/33 NP gene.
|
Opt |
ATG |
GCG |
ACC |
AAA |
GGC |
ACC |
AAA |
CGT |
AGC |
TAT |
GAA |
CAG |
ATG |
GAA |
ACC |
45 |
| Ori |
ATG M |
GCG A |
ACC T |
AAA K |
GGC G |
ACC T |
AAA K |
CGA R |
TCT S |
TAC Y |
GAA E |
CAG Q |
ATG M |
GAG E |
ACT T |
|
|
Opt |
GAT |
GGC |
GAA |
CGT |
CAG |
AAC |
GCG |
ACC |
GAA |
ATT |
CGT |
GCG |
AGC |
GTG |
GGC |
90 |
| Ori |
GAT D |
GGA G |
GAA E |
CGC R |
CAG Q |
AAT N |
GCC A |
ACT T |
GAA E |
ATC I |
AGA R |
GCA A |
TCT S |
GTC V |
GGA G |
|
|
Opt |
AAA |
ATG |
ATT |
GAT |
GGC |
ATT |
GGC |
CGT |
TTT |
TAT |
ATT |
CAG |
ATG |
TGC |
ACC |
135 |
| Ori |
AAA K |
ATG M |
ATT I |
GAT D |
GGA G |
ATT I |
GGA G |
CGA R |
TTC F |
TAC Y |
ATC I |
CAA Q |
ATG M |
TGC C |
ACC T |
|
|
Opt |
GAA |
CTG |
AAA |
CTG |
AGC |
GAT |
TAT |
GAA |
GGC |
CGT |
CTG |
ATT |
CAG |
AAC |
AGC |
180 |
| Ori |
GAA E |
CTT L |
AAA K |
CTC L |
AGT S |
GAT D |
TAT Y |
GAG E |
GGA G |
CGG R |
CTG L |
ATT I |
CAG Q |
AAC N |
AGC S |
|
|
Opt |
CTG |
ACC |
ATT |
GAA |
CGT |
ATG |
GTG |
CTG |
AGC |
GCG |
TTT |
GAT |
GAA |
CGT |
CGT |
225 |
| Ori |
TTA L |
ACA T |
ATA I |
GAG E |
AGA R |
ATG M |
GTG V |
CTC L |
TCT S |
GCT A |
TTT F |
GAC D |
GAG E |
AGG R |
AGG R |
|
|
Opt |
AAC |
AAA |
TAT |
CTG |
GAA |
GAA |
CAT |
CCG |
AGC |
GCG |
GGC |
AAA |
GAT |
CCA |
AAG |
270 |
| Ori |
AAT N |
AAA K |
TAT Y |
CTA L |
GAA E |
GAA E |
CAT H |
CCC P |
AGT S |
GCG A |
GGG G |
AAA K |
GAT D |
CCT P |
AAG K |
|
|
Opt |
AAA |
ACC |
GGC |
GGC |
CCG |
ATT |
TAT |
CGT |
CGT |
GTG |
GAT |
GGC |
AAA |
TGG |
CGT |
315 |
| Ori |
AAA K |
ACT T |
GGA G |
GGA G |
CCT P |
ATA I |
TAC Y |
AGG R |
AGA R |
GTA V |
GAT D |
GGA G |
AAG K |
TGG W |
AGG R |
|
|
Opt |
CGT |
GAA |
CTG |
ATT |
CTG |
TAT |
GAT |
AAA |
GAA |
GAA |
ATT |
CGT |
CGT |
ATT |
TGG |
360 |
| Ori |
AGA R |
GAA E |
CTC L |
ATC I |
CTT L |
TAT Y |
GAC D |
AAA K |
GAA E |
GAA E |
ATA I |
AGA R |
CGA R |
ATC I |
TGG W |
|
|
Opt |
CGT |
CAG |
GCG |
AAC |
AAC |
GGC |
GAT |
GAT |
GCG |
ACC |
GCG |
GGC |
CTG |
ACC |
CAC |
405 |
| Ori |
CGC R |
CAA Q |
GCT A |
AAT N |
AAT N |
GGT G |
GAC D |
GAT D |
GCA A |
ACG T |
GCT A |
GGT G |
CTG L |
ACT T |
CAC H |
|
|
Opt |
ATG |
ATG |
ATT |
TGG |
CAT |
AGC |
AAC |
CTG |
AAC |
GAT |
GCG |
ACC |
TAT |
CAG |
CGT |
450 |
| Ori |
ATG M |
ATG M |
ATC I |
TGG W |
CAC H |
TCC S |
AAT N |
TTG L |
AAT N |
GAT D |
GCA A |
ACT T |
TAC Y |
CAG Q |
AGG R |
|
|
Opt |
ACC |
CGT |
GCG |
CTG |
GTG |
CGT |
ACC |
GGC |
ATG |
GAC |
CCA |
CGT |
ATG |
TGC |
AGC |
495 |
| Ori |
ACA T |
AGA R |
GCT A |
CTT L |
GTT V |
CGC R |
ACA T |
GGA G |
ATG M |
GAT D |
CCC P |
AGG R |
ATG M |
TGC C |
TCA S |
|
|
Opt |
CTG |
ATG |
CAG |
GGC |
AGC |
ACC |
CTG |
CCG |
CGT |
CGT |
AGC |
GGT |
GCA |
GCA |
GGT |
540 |
| Ori |
CTG L |
ATG M |
CAG Q |
GGT G |
TCA S |
ACC T |
CTC L |
CCT P |
AGG R |
AGG R |
TCT S |
GGG G |
GCC A |
GCA A |
GGT G |
|
|
Opt |
GCA |
GCA |
GTG |
AAA |
GGC |
GTG |
GGT |
ACG |
ATG |
GTG |
ATG |
GAA |
CTG |
ATT |
CGT |
585 |
| Ori |
GCT A |
GCA A |
GTC V |
AAA K |
GGA G |
GTT V |
GGA G |
ACA T |
ATG M |
GTG V |
ATG M |
GAA E |
TTG L |
ATC I |
AGA R |
|
|
Opt |
ATG |
ATT |
AAA |
CGT |
GGC |
ATT |
AAC |
GAT |
CGT |
AAC |
TTT |
TGG |
CGT |
GGC |
GAA |
630 |
| Ori |
ATG M |
ATC I |
AAA K |
CGT R |
GGG G |
ATC I |
AAT N |
GAT D |
CGG R |
AAC N |
TTC F |
TGG W |
AGG R |
GGT G |
GAG E |
|
|
Opt |
AAC |
GGC |
CGT |
CGT |
ACC |
CGT |
ATT |
GCG |
TAT |
GAA |
CGT |
ATG |
TGC |
AAC |
ATT |
675 |
| Ori |
AAT N |
GGA G |
CGG R |
AGA R |
ACA T |
AGG R |
ATT I |
GCT A |
TAT Y |
GAA E |
AGA R |
ATG M |
TGC C |
AAC N |
ATT I |
|
|
Opt |
CTG |
AAA |
GGC |
AAA |
TTT |
CAG |
ACC |
GCG |
GCG |
CAG |
CGT |
ACG |
ATG |
GTG |
GAT |
720 |
| Ori |
CTC L |
AAA K |
GGG G |
AAA K |
TTT F |
CAA Q |
ACA T |
GCT A |
GCA A |
CAA Q |
AGA R |
ACA T |
ATG M |
GTG V |
GAT D |
|
|
Opt |
CAA |
GTG |
CGT |
GAA |
AGC |
CGT |
AAC |
CCG |
GGC |
AAC |
GCG |
GAA |
TTT |
GAA |
GAC |
765 |
| Ori |
CAA Q |
GTG V |
AGA R |
GAG E |
AGC S |
CGG R |
AAT N |
CCA P |
GGA G |
AAT N |
GCT A |
GAG E |
TTC F |
GAA E |
GAT D |
|
|
Opt |
CTG |
ATT |
TTT |
CTG |
GCG |
CGT |
AGC |
GCG |
CTG |
ATT |
CTG |
CGT |
GGC |
AGC |
GTG |
810 |
| Ori |
CTC L |
ATC I |
TTT F |
TTA L |
GCA A |
CGG R |
TCT S |
GCA A |
CTC L |
ATA I |
TTG L |
AGA R |
GGG G |
TCA S |
GTT V |
|
|
Opt |
GCG |
CAT |
AAA |
AGC |
TGC |
CTG |
CCG |
GCG |
TGC |
GTG |
TAT |
GGC |
AGC |
GCG |
GTG |
855 |
| Ori |
GCT A |
CAC H |
AAG K |
TCC S |
TGC C |
CTG L |
CCT P |
GCC A |
TGT C |
GTG V |
TAT Y |
GGA G |
TCT S |
GCC A |
GTA V |
|
|
Opt |
GCG |
AGC |
GGC |
TAT |
GAT |
TTT |
GAA |
CGT |
GAA |
GGC |
TAT |
AGC |
CTG |
GTG |
GGC |
900 |
| Ori |
GCC A |
AGT S |
GGA G |
TAC Y |
GAC D |
TTT F |
GAA E |
AGA R |
GAG E |
GGA G |
TAC Y |
TCT S |
CTA L |
GTC V |
GGA G |
|
|
Opt |
ATT |
GAT |
CCG |
TTT |
CGT |
CTG |
CTG |
CAG |
AAC |
AGC |
CAG |
GTG |
TAT |
AGC |
CTG |
945 |
| Ori |
ATA I |
GAC D |
CCT P |
TTC F |
AGA R |
CTG L |
CTT L |
CAA Q |
AAC N |
AGC S |
CAA Q |
GTA V |
TAC Y |
AGC S |
CTA L |
|
|
Opt |
ATT |
CGT |
CCG |
AAC |
GAA |
AAC |
CCG |
GCG |
CAT |
AAA |
AGC |
CAG |
CTG |
GTG |
TGG |
990 |
| Ori |
ATC I |
AGA R |
CCA P |
AAT N |
GAG E |
AAT N |
CCA P |
GCA A |
CAC H |
AAG K |
AGT S |
CAA Q |
CTG L |
GTG V |
TGG W |
|
|
Opt |
ATG |
GCG |
TGC |
CAT |
AGC |
GCG |
GCG |
TTT |
GAA |
GAC |
CTG |
CGT |
GTG |
AGC |
AGC |
1035 |
| Ori |
ATG M |
GCA A |
TGC C |
CAT H |
TCT S |
GCT A |
GCA A |
TTT F |
GAA E |
GAT D |
CTA L |
AGA R |
GTA V |
TCA S |
AGC S |
|
|
Opt |
TTT |
ATT |
CGT |
GGC |
ACC |
AAA |
GTG |
GTG |
CCG |
CGT |
GGC |
AAA |
CTG |
AGC |
ACC |
1080 |
| Ori |
TTC F |
ATC I |
AGA R |
GGG G |
ACG T |
AAA K |
GTG V |
GTC V |
CCA P |
AGA R |
GGG G |
AAG K |
CTT L |
TCC S |
ACT T |
|
|
Opt |
CGT |
GGC |
GTG |
CAG |
ATT |
GCG |
AGC |
AAC |
GAA |
AAC |
ATG |
GAA |
ACG |
ATG |
GAA |
1125 |
| Ori |
AGA R |
GGA G |
GTT V |
CAA Q |
ATT I |
GCT A |
TCC S |
AAT N |
GAA E |
AAC N |
ATG M |
GAG E |
ACT T |
ATG M |
GAA E |
|
|
Opt |
AGC |
AGC |
ACC |
CTG |
GAA |
CTG |
CGT |
AGC |
CGT |
TAT |
TGG |
GCG |
ATT |
CGT |
ACC |
1170 |
| Ori |
TCA S |
AGT S |
ACC T |
CTT L |
GAA E |
CTG L |
AGA R |
AGC S |
AGA R |
TAC Y |
TGG W |
GCC A |
ATA I |
AGG R |
ACC T |
|
|
Opt |
CGT |
AGC |
GGC |
GGC |
AAC |
ACC |
AAC |
CAG |
CAG |
CGT |
GCG |
AGC |
AGC |
GGC |
CAG |
1215 |
| Ori |
AGA R |
AGT S |
GGA G |
GGG G |
AAC N |
ACC T |
AAT N |
CAA Q |
CAG Q |
AGG R |
GCT A |
TCC S |
TCG S |
GGC G |
CAA Q |
|
|
Opt |
ATT |
AGC |
ATT |
CAG |
CCG |
ACC |
TTT |
AGC |
GTG |
CAG |
CGT |
AAC |
CTG |
CCG |
TTT |
1260 |
| Ori |
ATC I |
AGC S |
ATA I |
CAA Q |
CCT P |
ACG T |
TTC F |
TCA S |
GTA V |
CAG Q |
AGA R |
AAT N |
CTC L |
CCT P |
TTT F |
|
|
Opt |
GAT |
CGT |
CCG |
ACC |
ATT |
ATG |
GCG |
GCG |
TTT |
ACC |
GGC |
AAC |
ACC |
GAA |
GGC |
1305 |
| Ori |
GAC D |
AGA R |
CCA P |
ACC T |
ATT I |
ATG M |
GCA A |
GCA A |
TTC F |
ACT T |
GGG G |
AAT N |
ACA T |
GAG E |
GGG G |
|
|
Opt |
CGT |
ACC |
AGC |
GAT |
ATG |
CGT |
ACC |
GAA |
ATT |
ATT |
CGT |
CTG |
ATG |
GAA |
AGC |
1350 |
| Ori |
AGA R |
ACA T |
TCT S |
GAC D |
ATG M |
AGA R |
ACC T |
GAA E |
ATC I |
ATA I |
AGG R |
CTG L |
ATG M |
GAA E |
AGT S |
|
|
Opt |
GCG |
CGT |
CCG |
GAA |
GAT |
GTG |
AGC |
TTT |
CAG |
GGC |
CGT |
GGC |
GTG |
TTT |
GAA |
1395 |
| Ori |
GCA A |
AGA R |
CCA P |
GAA E |
GAT D |
GTG V |
TCT S |
TTC F |
CAG Q |
GGG G |
CGG R |
GGA G |
GTC V |
TTC F |
GAG E |
|
|
Opt |
CTG |
AGC |
GAT |
GAA |
AAA |
GCG |
ACC |
AGC |
CCG |
ATT |
GTG |
CCG |
AGC |
TTT |
GAT |
1440 |
| Ori |
CTC L |
TCG S |
GAC D |
GAA E |
AAG K |
GCA A |
ACG T |
AGC S |
CCG P |
ATC I |
GTG V |
CCC P |
TCC S |
TTT F |
GAC D |
|
|
Opt |
ATG |
AGC |
AAC |
GAA |
GGC |
AGC |
TAC |
TTT |
TTC |
GGC |
GAT |
AAC |
GCG |
GAA |
GAA |
1485 |
| Ori |
ATG M |
AGT S |
AAT N |
GAA E |
GGA G |
TCT S |
TAT Y |
TTC F |
TTC F |
GGA G |
GAC D |
AAT N |
GCA A |
GAG E |
GAG E |
|
|
Opt |
TAT |
GAT |
AAC |
TAA |
1497 | |||||||||||
| Ori |
TAC Y |
GAC D |
AAT N |
TAA * |
Opt= codon optimized sequence in bold letters; Ori= Non-codon optimized original sequence
2.6. Vectors for antigen delivery via regulated delayed lysis system
The NP gene was amplified from plasmid pCGGAS-NP (kindly provided by Dr. Andrew Pekosz) by PCR using the primer pair RDLF-3 and RDLP-2. The PCR product was digested using NcoI and XmaI sites and cloned into plasmid pYA3681 yielding pYA4702. The codon-optimized NP gene from pUC-57-WSN-NP was amplified using the primer pair RDLF-5 and RDLRP-7. The PCR product was digested with NcoI and cloned in pYA3681 yielding pYA4858. The correct orientation of the NP gene was confirmed by restriction digestion with PstI and by sequencing. All derivatives of pYA3681 were sequenced by the primer set Ptrc-F and Ptrc-R, respectively. Negative control vector pYA4651 encoding the ply gene from S. pneumonia cloned in pYA3681 was constructed by Wei Xin. All vectors were transferred to appropriate S. Typhimurium strains by electroporation. All DNA constructs were confirmed by sequencing at the core facility at Arizona State University, using ABI Prism fluorescent BigDye terminators.
2.7. SDS-PAGE and immunoblots
To evaluate NP protein synthesis from plasmids in E. coli and S. Typhimurium strains, bacterial cells were grown overnight at 37°C in LB containing 0.2% arabinose. Aliquots (1 ml) were taken, centrifuged at low speed, and resuspended in 2× SDS-PAGE loading buffer and boiled for 10 min. The samples were centrifuged for 10 min, diluted 1:10 in 2× sample loading buffer and 10 µl was loaded onto 12.5% SDS-PAGE gels for separation by electrophoresis as previously described [44]. Proteins were transferred onto nitrocellulose membranes and blocked with 5% skim milk for 1 h at room temperature. Membranes were rinsed with PBS-0.05% Tween 20 (T20) three times. For analyzing NP synthesis blots were incubated with rabbit polyclonal anti-influenza A NP antibody (Abcam) for 1 h with constant shaking. After washing with PBS-T20, the membranes were incubated with goat anti-rabbit IgG alkaline phosphatase conjugate (Sigma) for 1 h and developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyphosphate (BCIP) (Sigma). Membranes were washed with water and air dried.
2.8. Virus strain propagation, purification, and titration
rWSN virus was provided by Dr. Andrew Pekosz (Johns Hopkins University, Baltimore, MD). It is a mouse-adapted strain created by reverse genetics and is lethal to mice in doses above 103 TCID50 [45, 46]. The virus was propagated and titrated in Madin-Darby canine kidney cells cultured in RPMI-1640 (Gibco) containing 2 µg/ml acetyl-trypsin (Sigma). The virus was passed through a 30% (w/v) sucrose cushion at 11,620 × g for 3 h in a Surespin Sorvall 630 rotor using a WK ultra 90 centrifuge (Thermo Electron Corp.). The resulting pellet was resuspended in phosphate buffered saline (PBS) pH 7.2 and centrifuged at 11,620 × g for 1 h. The viral pellet was finally dissolved in 500 µl of PBS and kept frozen at −80°C until used.
2.9. Immunization of mice
All animal experiments were done in BSL-2 level containment in our animal facilities at The Biodesign Institute, Arizona State University, according to approved ASU IACUC protocols. Five-week old female BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) and were allowed to acclimate for 1 week before immunization. Each group of mice was deprived of food and water for 4 h prior to oral immunization. Recombinant S. Typhimurium strains χ11017(pYA4858) (SifA+), χ11246(pYA4858) (SifA−), χ11017(pYA3681), χ11246(pYA3681) and χ11246(pYA4651) were individually grown in LB broth with 0.2% arabinose and 0.2% mannose to an OD600 of 0.85. The cultures were centrifuged at 4,000 × g for 15 min at room temperature and suspended in buffered saline containing 0.01% gelatin (BSG) to a final concentration of 5 × 109 CFU/ml. Bacteria were titrated on LB agar supplemented with arabinose. Mice were immunized by the peroral (PO) route with 20 µl (1 × 109 CFU), intranasally (IN) 10 µl (1 × 107 CFU) or by the intraperitoneal (IP) route with 100 µl (1 × 105 CFU). Food and water were returned to orally immunized mice 30 min after vaccine administration. Vectors without any expressed antigen gene (pYA3681) or that expressed the ply gene from S. pneumonia (pYA4651) and BSG immunized mice were used as negative controls in the following experiments.
2.10. Experimental design
2.10.1.Trial 1
To evaluate the effect of the sifA deletion (χ11246) on the immunogenicity and protective immunity conferred by RASV strains encoding the codon-optimized NP (pYA4858) of influenza virus, BALB/c mice (n=8) were orally immunized with parent χ11017(pYA4858) (SifA+), mutant χ11246(pYA4858) (SifA−), vector controls χ11017(pYA3681) (SifA+) and χ11246(pYA3681) (SifA−) or with BSG at week zero and boosted three times at weeks 1, 4 and 7 post primary immunization (PPI). Blood collected at weeks 3 and 6 PPI by cheek pouch bleeding was monitored for the presence of antibodies against NP or S. Typhimurium LPS by ELISA. For assaying antigen specific IFN-γ secreting T cells, spleens were aseptically collected at week 8 PPI from 2–3 mice, pooled and processed for ELISPOT [47]. The remaining mice (n=5) in each group were challenged with rWSN (100 LD50) at week 8 PPI (14 weeks of age) and observed for morbidity and mortality for an additional 3 weeks.
2.10.2. Trial 2
It is generally accepted that multiple boosters are required for effective vaccination [48], hence we used three boosters in the previous trial. It was of interest to determine if decreasing the number of booster immunizations from three doses in the previous trial to two would be as effective in protecting against the rWSN virus (100 LD50) challenge. Therefore, the groups of mice (n=8) were immunized orally with strains encoding codon-optimized NP χ11246(pYA4858) (SifA−), an irrelevant antigen (Ply) from χ11246(pYA4651) or BSG at week zero and boosted twice at week 1 and 4 PPI. Spleens and blood were harvested from 3 mice from each group, 4 days after the final boost and ELISPOTs and ELISA were performed to detect antigen-specific T cells and NP and LPS specific antibodies. The remaining mice in each group were challenged with rWSN (100 LD50) at week 5 PPI (at 10 weeks of age) and observed for morbidity and mortality for 3 additional weeks.
2.10.3. Trial 3
To determine the immunogenicity and protective efficacy of using the SifA− strain when administered via different routes, mice were immunized via PO, IN or IP routes with RASV χ11246(pYA4858) (NP+ SifA−) and χ11246(pYA4651) (Ply+ SifA−) as a negative control, at week 0 and boosted thrice at weeks 1, 4 and 7 PPI. Spleens were harvested from 3 mice four days after the final immunization and analyzed for production of antigen-specific IFN-γ secreting cells by ELISPOT and for NP147–155 specific proliferation. The remaining mice in each group were challenged with rWSN (100 LD50) at week 8 PPI (14 weeks of age) and observed for morbidity and mortality for an additional 3 weeks.
2.11. Virus Challenge
For virus challenge, mice were anaesthetized with 0.05 ml/20 g body weight of a ketamine cocktail (21.0 mg ketamine, 2.4 mg xylazine, and 0.3 mg acepromazine) administered intraperitoneally. Sedated mice were intranasally (IN) infected with a 100 LD50 (1 × 105 TCID50) of rWSN in a total volume of 30 µl, 15 µl per nostril for all experiments. Groups of mice were IN infected with 30 ul of the serially diluted purified rWSN virus from 1× 107 – 1 × 102 TCID50 at 8 weeks of age and the LD50 determined by the method of Reed and Muench [49]. To rule out any age dependent variation in LD50 doses, similar experiments were performed with mice at 10 and 14 weeks of age. No difference was observed in terms of virus-associated morbidity and mortality in mice at 8, 10 or 14 weeks of age. An aliquot of the virus used for challenge was back-titrated on MDCK to ascertain the exact dose given to mice.
The challenged mice were inspected daily for signs of infection such as ruffled fur, hunched posture, and weighed on alternate days till 21 days to monitor the progression of infection. Percent weight loss was calculated for individual mice in each group by comparing their daily weight to their pre-challenge weight. Mice that succumbed to infection or had to be euthanized were promptly removed.
2.12. ELISA
IgG responses against NP or LPS in sera were determined by ELISA [50]. Briefly, 96-well flat-bottom polystyrene microtiter plates (Dynatech Laboratories Inc., Chantilly, VA) were coated with 2 µg/ml of purified NP protein (kindly provided by Dr. Troy Randall, (Trudeau Institute, Saranac Lake, NY) or LPS (Sigma) suspended in carbonate coating buffer (pH=9.5) and incubated at 4°C overnight. Free binding sites were blocked with phosphate buffered saline (PBS)-0.05% T20 containing 3% bovine serum albumin (BSA) for 2 h at room temperature. Sera were serially two-fold diluted in PBS/3% BSA and 100 µl was incubated in duplicate wells for 1 h at room temperature. Plates were washed thrice with PBS-T20 and incubated for 1 h with a 1:1000 dilution of either biotinylated goat anti-mouse IgG or IgG1 or IgG2a (Southern Biotechnology Inc., Birmingham, AL). After washing as above, the plates were incubated for 1 h with streptavidin-alkaline phosphatase conjugate (Southern Biotechnology Inc., Birmingham, AL) and developed by incubating with p-nitrophenyl phosphate (Sigma) for 30 min and read by an automated ELISA plate reader (SpectraMax, Molecular Devices, Sunnydale, CA) at 405 nm. Endpoint titers were expressed as the reciprocal log2 value of the last positive sample dilution. Absorbance two times higher than pre-immune serum, used as baseline values, were considered positive.
2.13. IFN-γ ELISPOT
Influenza NP-specific CD8+ or CD4+ T cells secreting IFN-γ were enumerated using an IFN-γ ELISPOT assay. The spleens from 2–3 immunized mice from each group were aseptically collected at week 5 or 8 PPI. Single cell suspensions were prepared from each spleen and the cells were pooled from mice within a group for the production of IFN-γ by stimulation with NP protein (provided by Dr. Troy Randall) or NP147–155 peptide. ELISPOT assays were performed as described elsewhere [47].
2.14. Cell proliferation
Lymphocyte proliferation assays were performed to assess influenza peptide specific (NP147–155) cell-mediated responses. Single-cell suspensions prepared from spleens were plated at a concentration of 5 × 105 cells/well and stimulated with the NP147–155 peptide TYQRTRALV (20 µg/ml) for 7 days. Vision blue dye™ from the fluorescence cell viability assay kit (Biovision, Mountain View, CA) was added according to the manufacturer’s instructions and plates were read at excitation 530 nm and emission 590 nm.
2.15. Statistical analyses
Differences in antibody titers between groups, cell proliferation and quantitative difference in numbers of IFN-γ secreting cells between the groups were determined using analysis of variance (ANOVA) and statistically different means were further analyzed using Bonferroni’s test or by Tukey’s method. Survival analysis was analyzed using the log rank test (GraphPad Prism; GraphPad Software).
3. Results
3.1 Antigen synthesis in strains with vectors encoding non-codon-optimized and codon-optimized NP
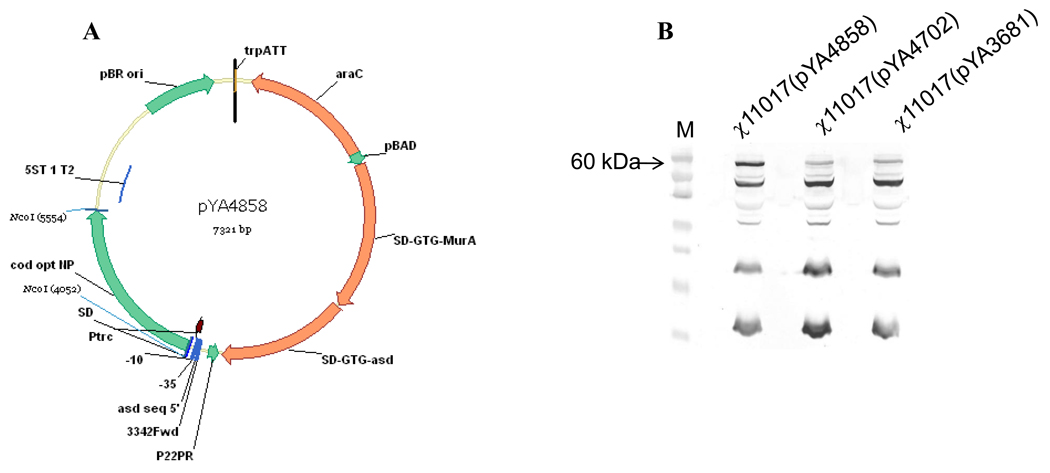
To evaluate the effect of a sifA deletion on protective immunity induced by an RASV strain with regulated delayed lysis attributes, we introduced a sifA deletion into strain χ11017 yielding χ11246. The deletion of sifA was confirmed by PCR and the lysis phenotype was confirmed by growing strains on LB plates with or without arabinose. The complete coding sequence of the NP gene was cloned into the regulated lysis vector pYA3681 yielding pYA4702 as was the entire coding sequence of the codon-optimized NP gene cloned into pYA3681 yielding pYA4858 (Fig.1A). Synthesis of NP was analyzed in χ11017(pYA4702) and χ11017(pYA4858) by western blots using rabbit polyclonal anti-NP antibody (Fig. 1B). NP was not synthesized in χ11017(pYA4702) at significant levels whereas a prominent 60 kDa band was observed for the χ11017(pYA4858) culture (Fig. 1B) as detected by western blots using the rabbit polyclonal anti-NP antibody. Low molecular weight bands of non-specific nature were also detected in the western blot. Similar levels of NP proteins were synthesized by strains χ11017 (SifA+) and χ11246 (SifA−) as analyzed by western blots (data not shown).
Fig. 1.
A. Map of plasmid pYA4858 c arrying the codon-optimized NP gene from influenza virus in the regulated delayed lysis plasmid pYA3681.
B. Detection of NP in cell free lysates of strain χ11017 (SifA+) encoding codon-optimized NP (pYA4858); non-codon-optimized NP (pYA4702) and vector control (pYA3681) using rabbit polyclonal anti-NP sera by western blot analysis. Arrow indicates 60 kDa NP. M= Molecular size marker.
3.2. Immune responses and evaluation of protection against viral challenge
3.2.1. Trial 1
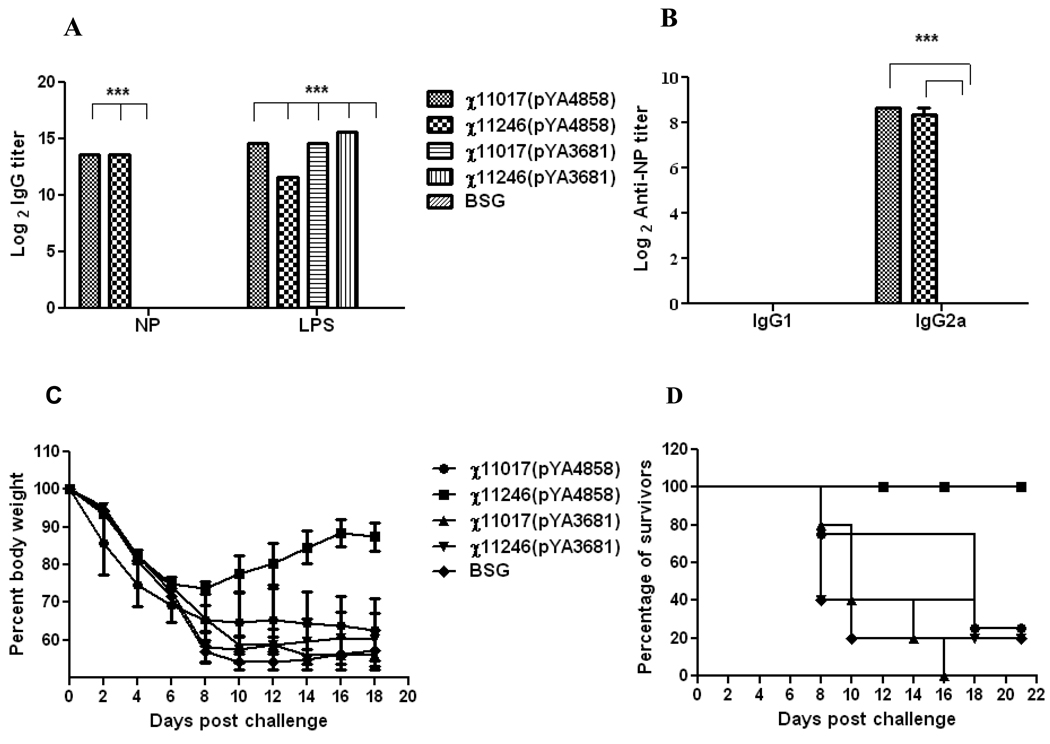
Mice orally immunized with RASV χ11017(pYA4858) (SifA+) and χ11246(pYA4858) (SifA−) induced significantly (P<0.001) higher antibody high titers against influenza NP and against Salmonella LPS as compared to BSG (Fig. 2A). The antibody titers elicited against NP by immunization with either χ11017(pYA4858) (SifA+) or χ11246(pYA4858) (SifA−) were similar indicating that both RASV strains were equally immunogenic. The antibody titers elicited against LPS by χ11017(pYA4858) (SifA+), χ11246(pYA4858) (SifA−) and the vector controls were similar indicating that all vectors invaded the host cells and colonized lymphoid tissues equally well. The antibody responses against influenza NP were skewed towards IgG2a, a typical Th1-type response elicited by RASV (Fig. 2B) [34].
Fig. 2.
Trial 1 (A&B). Antibody titers detected by ELISA in orally immunized mice, 6 weeks after three booster doses with the recombinant attenuated Salmonella strains χ11017(pYA4858) (SifA+), χ11246(pYA4858) (SifA−) encoding influenza NP or with the vector controls χ11017(pYA3681) (SifA+), χ11246(pYA3681) (SifA−) or BSG.
A. Induction of IgG titers against Influenza NP protein and purified S. Typhimurium LPS.
B. Induction of IgG1 and IgG2a responses against influenza NP.
Pooled serum samples (n=8) from mice within a group were assayed and analyzed by two-way ANOVA followed by Bonferroni test. ***P<0.001.
C. Weight loss and D. percent survival of mice (n=5) given three booster oral doses after an intranasal challenge with 100 LD50 of rWSN influenza virus at 8 weeks PPI.
Mice infected with the rWSN influenza strain showed ruffled fur, hunched posture, trembling and a continuous weight loss as signs of infection from the second day after challenge that progressed with time. Mice immunized with χ11246(pYA4858) (SifA−) recovered from influenza infection earlier as indicated by the alleviation of symptoms by 6 days after challenge, than mice immunized with χ11017(pYA4858) (SifA+) and with vector control groups that continued to loose weight and became sicker. This is also evident by weight recovery data of mice immunized with χ11246(pYA4858) (SifA−) as compared to mice immunized with χ11017(pYA4858) (SifA+) or with vector controls χ11017(pYA3681) (SifA+), χ11246(pYA3681) (SifA−) or BSG (Fig. 2C). Mice immunized with strain χ11246(pYA4858) (SifA−) survived whereas mice immunized with χ11017(pYA4858) (SifA+) and vector controls χ11017(pYA3681) and χ11246(pYA3681) or with BSG commenced dying 8 days after challenge.
All mice immunized orally with χ11246(pYA4858) were protected (100%) against the 100 LD50 rWSN virus challenge as compared to 25% survivors in the group immunized with χ11017(pYA4858) and 0% to 20% survivors in the groups immunized with χ11017(pYA3681) and χ11246(pYA3681) (vector controls) or BSG (Fig. 2D).
3.2.2. Trial 2
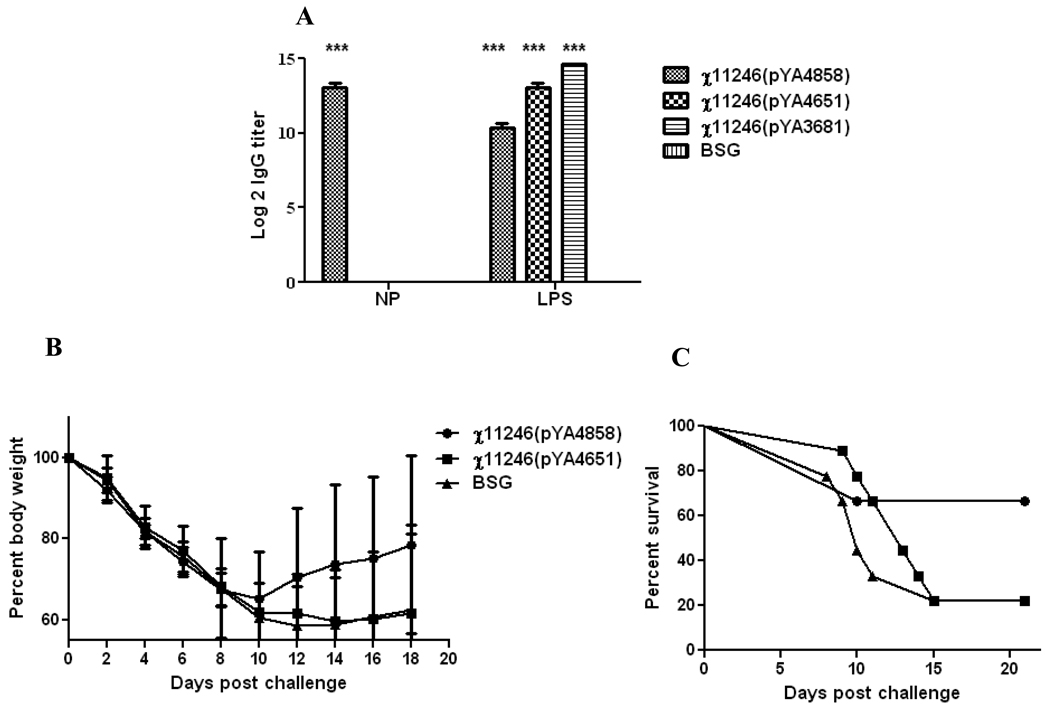
To determine the optimal number of booster immunizations required to protect mice from lethal virus challenge, we reduced the number of booster immunizations from 3 in the previous trial to 2 immunizations in this trial given at 1 and 4 weeks PPI. The mice were challenged with the rWSN influenza virus (100 LD50) at week 5 PPI. Mice immunized with RASV χ11246(pYA4858) (SifA−) elicited significantly higher (P<0.001) IgG antibodies against Influenza NP as compared to the mice immunized with χ11246(pYA4651) (SifA−) encoding irrelevant Ply antigen or with BSG (Fig. 3A). The titers against LPS were lower in the mice immunized with χ11246(pYA4858) as compared to the vector control group probably due to attenuation of the strain resulting from over synthesis of NP. The antibody levels obtained at 5 weeks PPI were similar to the ones obtained after two immunizations at 6 weeks PPI in the previous trial (Fig. 2A). Measurement of antigen specific IFN-γ secreting T cells in Trial 2 was done by stimulating the splenocytes harvested from immunized mice in each group at 4 week PPI, 4 days after the last immunization with either purified NP protein or with NP147–155 peptide or ConA as a positive control in an ELISPOT assay. There were no influenza-specific IFN-γ secreting T cells after stimulation with either the NP protein or the NP147–155 peptide (data not shown).
Fig. 3.
Trial 2. A. Induction of IgG titers against influenza NP protein and purified S. Typhimurium LPS as detected by ELISA in orally immunized mice given two booster immunizations, 4 weeks PPI with the recombinant attenuated Salmonella strain χ11246(pYA4858) (SifA−) encoding influenza NP or with the negative controls χ11246(pYA4651) (SifA−) encoding an irrelevant Ply antigen or the empty vector control χ11246(pYA3681) or BSG. Pooled serum samples (n=3) from mice within a group were assayed and analyzed by two-way ANOVA followed by Bonferroni test. ***P<0.001.
B. Weight loss and C. percent survival of mice (n=5) orally immunized with two boosters after an intranasal challenge with 100 LD50 of rWSN influenza virus at 5 weeks PPI.
Following challenge with 100 LD50 of rWSN, mice immunized with RASV χ11246(pYA4858) (NP+) (SifA−) recovered from influenza infection and commenced to regain weight whereas mice receiving either an irrelevant antigen (Ply) or BSG continued to loose weight and did not recover (Fig. 3B). Mice boosted twice with χ11246(pYA4858) (NP+) (SifA−) were significantly protected against 100 LD50 of rWSN of influenza virus (66% survival) as compared to 22% survival of mice in groups immunized with χ11246(pYA4651) delivering S. pneumoniae Ply as a negative control and BSG (P>0.05) (Fig. 3C).
3.2.3. Trial 3
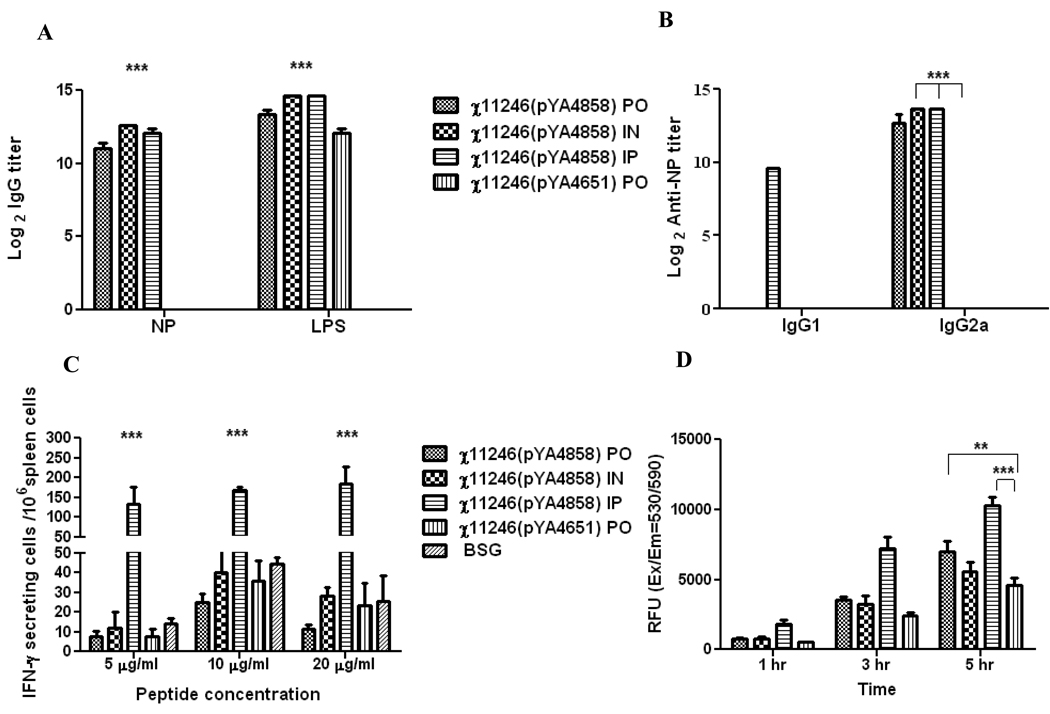
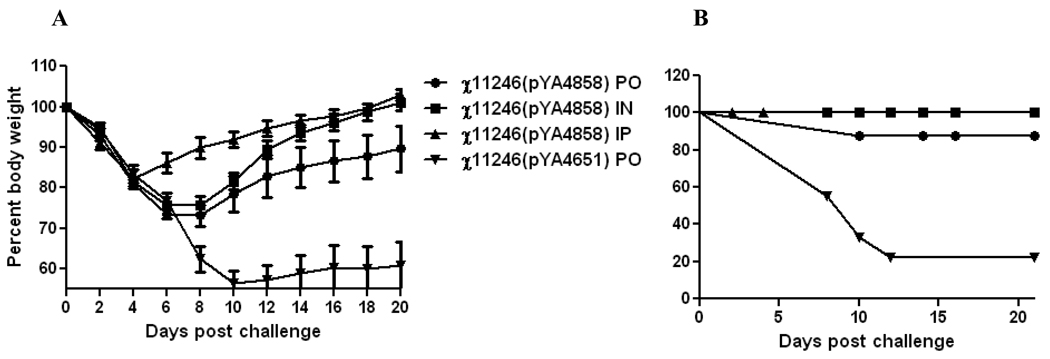
To investigate the efficacy of the SifA− vaccine strain when administered via different routes, mice were boosted thrice (as in Trial 1) with RASV strains χ11246(pYA4858) (NP+) (SifA−) and χ11246(pYA4651) (SifA−) (Ply+) via PO, IN and IP routes. Mice immunized with RASV χ11246(pYA4858) (NP+) (SifA−) via all three routes (PO, IN and IP) elicited significantly higher (P<0.001) IgG antibodies against influenza NP and Salmonella LPS as compared to the mice orally immunized with χ11246(pYA4651) (SifA−) encoding irrelevant Ply antigen or with BSG (Fig. 4A). The resulting antibody responses against NP from these immunizations were of the Th1-type (IgG2a) in all cases, except that χ11246(pYA4858) (NP+) (SifA−) administered via the IP route induced a mixed IgG2a (Th1 type) and IgG1 (Th-2 type) response (Fig. 4B).
Fig. 4.
Trial 3. (A & B). Antibody titers detected by ELISA in mice immunized via oral (PO) intranasal (IN) or intraperitoneal (IP) routes, 6 weeks after three booster immunizations with the recombinant attenuated Salmonella strains χ11246(pYA4858) (SifA−) expressing influenza NP or BSG. (A). Induction of IgG titers against Influenza NP protein and purified S. Typhimurium LPS by ELISA. (B). Induction of IgG1 and IgG2a responses against influenza NP protein by ELISA. Pooled serum samples (n=12) from mice within a group were assayed and analyzed by two-way ANOVA followed by Bonferroni test. ***P<0.001.
C. ELISPOT analysis of IFN-γ production by NP147–155 specific CD8+ T cells. Mice were boosted thrice with χ11246(pYA4858) (NP+) (SifA−) via PO, IN and IP routes. Splenocytes (n=3) from immunized mice were harvested at 8 weeks PPI and stimulated with NP147–155 peptide for 48 h. Statistical analysis was performed by ANOVA followed by Tukey's method with 95% confidence interval. *** P<0.0001
D. Cell proliferation assay. Splenocytes (n=3) harvested from these mice were stimulated with NP147–158 peptide (20 µg/ml) for 6 days and incubated with the Vision blue dye (Biovision). Plates were read at Ex 530 and Em 590nm.Relative fluorescence units (RFU) were calculated by subtracting background reading from unstimulated cells from the stimulated cells. Data were analyzed by two-way ANOVA followed by Bonferroni test. **, P < 0.05; ***, P < 0.01. PO =oral; IN= intranasal and IP= intraperitoneal.
A significantly higher (P<0.0001) number of influenza NP147–155 peptide-specific IFN-γ secreting cells were detected in splenocytes harvested from mice at 8 weeks PPI receiving χ11246(pYA4858) (NP+) (SifA−) via the IP route than in mice immunized orally (PO) or by the intranasal (IN) route (Fig. 4C). To assess the influenza NP147–155 peptide-specific cell mediated responses, splenocytes harvested from immunized mice at 8 week PPI were stimulated with the NP147–158 peptide. The degree of proliferation was measured by increase in the fluorescence of vision blue dye. Background readings from the negative control mice was subtracted from the readings from NP147–158 stimulated splenocytes. The splenocytes harvested from mice immunized via the PO (P<0.05) or IP (P<0.01) route proliferated in response to NP147–158 peptide as compared to splenocytes harvested from mice immunized with the negative controls (Fig. 4D). The non-specific proliferation in mice orally immunized with χ11246(pYA4651) might be due to the high peptide (20 µg/ml) concentration used in the experiment.
Mice infected with influenza virus showed ruffled fur, hunched posture, and trembling and weight loss as signs of infection and started dying commencing at 8 days after challenge. Mice immunized with χ11246(pYA4858) (NP+) (SifA−) via the PO and IN route recovered from infection by day 6 after challenge while those immunized by the IP route recovered earlier by 4 days after challenge as indicated by recovery from symptoms of influenza infection and weight gain (Fig. 5A). Mice immunized with χ11246(pYA4858) (NP+) (SifA−) via the PO, IN and IP route of immunization were protected 80%, 100% and 100%, respectively, from the influenza virus challenge as compared to 22% in the χ11246(pYA4651) (SifA−) (Ply+) immunized group (P<0.0002) (Fig. 5B).
Fig. 5.
Trial 3. Weight l oss and survival data of mice after three boosters with 11246(pYA4858) (NP+) (SifA−) via PO, IN and IP routes and χ11246(pYA4651)(Ply+)(SifA−) as a negative control at 8 weeks PPI.
A. Weight loss and B. Percent survival of mice after three booster immunizations and an intranasal challenge with 100 LD50 of rWSN influenza virus (n=8) at 8 weeks PPI.
4. Discussion
Currently available vaccines against the influenza virus rely on the humoral immune responses for protection against the virus although some can induce CD4+ T cell responses [51, 52]. NP147–155 is an immunodominant MHC class I epitope (H-2Kd restricted) [53] and induces CD8+ T-cell responses and accelerated viral clearance in BALB/c mice [10].
Salmonella vaccines generate a strong DTH and CD4+ T-cell responses [18, 26] but little or no MHC-1-restricted CD8+ cytotoxic T cells (CTL) to foreign antigens since Salmonella faces difficulty in reaching the cytosol of the host cell [20]. Our attempts to deliver NP to the cytosol through T3SS failed since influenza NP could not be secreted through the T3SS machinery even after deletion of the RNA binding domain spanning the amino acids (91–108) near the N-terminus of the gene (unpublished data).
In this report, a novel bacterial delivery system for an influenza virus NP utilizing an orally administered RASV displaying a regulated delayed lysis in vivo phenotype is described. An irreversible chromosomal deletion of the sifA gene was introduced into the regulated delayed lysis strain χ11017 yielding χ11246 carrying mutations to enhance complete lysis and antigen delivery [34 & Table 1]. The resultant strain invaded cells, reached the SCVs and escaped into the cytosol by virtue of the sifA deletion [54], and eventually lysed due to unavailability of DAP and arabinose in vivo, releasing its payload of antigenic peptides/proteins [34].
We included the coding sequence of the complete NP protein in the plasmid vectors (pYA4702 and pYA4858) to include a complete repertoire of the epitopes restricted by several different MHC-I molecules so as to make a vaccine effective for a larger population. Expression of a non-codon optimized NP gene (pYA4702) in Salmonella resulted in low levels of protein synthesis and immunization with the χ11017(pYA4702) strain did not result in protection against a lethal virus challenge (data not shown). Therefore, the coding sequence of NP (pYA4858) was codon-optimized for maximal expression in Salmonella strains χ11017 (SifA+) or χ11246 (SifA−) (Fig.1B).
To evaluate the effect of sifA deletion on protective immunity conferred by an RASV strain with regulated delayed lysis attributes, we compared immunogenicity and protection induced by strains χ11246(pYA4858) (SifA−) and χ11017(pYA4858) (SifA+) in BALB/c mice. Persistence and amount of antigen are important factors for conferring the protective T-cell responses [48, 55]. To determine the optimal number of doses required for conferring immunity, mice were orally primed and given 3 booster doses 2 weeks apart with χ11246(pYA4858) (SifA−) and χ11017(pYA4858) (SifA+) strains and challenged at 8 weeks PPI with a lethal influenza virus strain (rWSN).
Both RASV strains χ11246(pYA4858) (SifA−) and χ11017(pYA4858) (SifA+) effectively induced antibody responses to the NP protein (P<0.001) and LPS indicating that all strains effectively invaded host cells. The induced antibody response in Trial 1 is completely skewed toward a Th1-type response as judged by the abundance of IgG2a subtype antibody and the lack of detectable IgG1 (Fig. 2B). Salmonella vaccines generally elicit the Th1 type but Th2 type antibodies are also detected [34]. Infection with viruses elicit IgG2a antibodies [56] which are more effective in combating viral infection than IgG1 subtypes [56]. The Th1 helper cells direct a cell-mediated immune response and promote class switching to IgG2a while Th2 cells provide help for B cells and class switching to IgG1 antibodies [57]. NP elicits non-neutralizing antibodies [58] detectable in serum of naturally infected or vaccinated humans and animals [59] and enhance protection in mice upon a sub-lethal challenge [60]. However, no NP antibodies specific protection was observed in our experiments since similar levels of anti-NP antibodies were elicited by both χ11017(pYA4858) SifA+ and χ11246(pYA4858) SifA− strains but the protection offered by χ11246(pYA4858) SifA− was substantially higher than induced by χ11017(pYA4858) SifA+.
IFN-γ is the signature cytokine for Th1-type responses and mediates protection for intracellular bacteria like Mycobacterium, and some viruses [48]. Unexpectedly, we could not detect any influenza NP (antigen or peptide NP147–155 specific) IFN-γ production in the splenocytes of the immunized mice 1 week after the last booster dose by ELISPOT assay. We might need to evaluate more mice at additional time points post immunization to examine this point. However, IFN-γ is not required for recovery from primary infection with influenza virus [61, 62] and for the induction of secondary influenza virus–specific CTL to lethal influenza A infection in mediastinal lymph nodes, a site in which production of antigen-specific CTL correlates with protection. Therefore, we plan to test MLN in addition to spleens for the presence of CTLs and the role of cytokines like TNF-α, IL-2 and IL-12 in future experiments.
Our results showed that vaccination with NP does not provide sterilizing immunity against the virus [10]. Mice immunized with the χ11246(pYA4858) SifA− strain initially lost weight until 8 days after rWSN influenza virus challenge but recovered thereafter and were completely protected in contrast to the groups immunized with χ11017(pYA4858)SifA+ or the vector controls χ11246(pYA3681) or χ11017(pYA3681) (Fig. 2C). Hence we concluded that the difference in protection was due to the ability of the SifA− strain to deliver the NP antigen to the cytosol better than the SifA+ strain in the absence of detectable IFN-γ and protective antibody and was probably due to induction of a robust CTL response, an important parameter that remains to be determined.
Vaccine boosters enhance the protection by affecting the quantity and quality of memory T cells and produce higher-affinity T-cell clones [55] but too much antigen can deter the immune responses by inducing apoptosis or clonal anergy [48]. Therefore, the number of vaccine boosters was reduced from 3 to 2 and mice were challenged at 4 weeks after priming with the rWSN influenza virus in trial 2. The antibody levels obtained at 4 weeks after priming in trial 2 were similar (P<0.001) (Fig 3A) to those obtained during the trial 1 at 6 weeks (Fig. 2A) with no detectable IFN-γ secreting T cells in splenocytes of immunized mice. However, only 66% of the mice immunized with χ11246(pYA4858) SifA− strain were protected against the rWSN strain of influenza virus challenge as compared to 22% mice immunized with an irrelevant Ply antigen χ11246(pYA4651) from S. pneumonia (Fig.3C). These data confirmed that antibody responses did not play a role in the protection afforded by the χ11246(pYA4858) SifA− vaccine. The decrease in protective efficacy might have resulted from either one less dose of vaccine or an earlier virus challenge at 4 weeks after priming. Multiple boosters enhance the affinity of the memory T cells [48]. A certain threshold level of effector T cells is required for protection against the disease [48] which apparently was not reached at 4 weeks post immunization. Also, efficient boosting of T cells requires long intervals between the booster shots [63]. Hence, it would be interesting to determine in future experiments the effect of two booster doses given at 6 and 12 weeks after priming.
To compare the vaccine efficacy when administered by different routes i.e., oral, IN and IP, we immunized mice with χ11246(pYA4858) SifA− delivering influenza NP or with the irrelevant antigen control χ11246(pYA4651) expressing Ply from S. pneumonia. Mice immunized via the IP route elicited IgG1 and IgG2a responses as compared to completely biased IgG2a response elicited in the groups of mice immunized via the PO or IN route (Fig. 4B). We could not detect a significant number of IFN-γ secreting cells in splenocytes harvested from mice immunized via mucosal surfaces (PO and IN) while a lot of influenza NP147–155 specific IFN-γ secreting cells were detected in mice immunized IP with the χ11246(pYA4858) SifA− strain (Fig. 4C). This indicated that IFN-γ is not a correlate of protection for the influenza infection since all mice were consistently protected against the viral challenge irrespective of the route of administration of the RASV. Th1 and CD8+ T cells are the effector mechanisms required for protective immunity [48]. In vitro stimulation of the splenocytes with the peptide NP147–155 indicated the presence of peptide-specific CD8+ T cells (Fig. 4D). Immunization of mice with χ11246(pYA4858) SifA− via IN and IP routes resulted in complete protection. Orally immunized mice might have received less amount of antigen probably due to the stresses of passage through the gastro-intestinal track and showed less protection than IN or IP inoculated mice after a lethal challenge with the rWSN strain of influenza virus. To conclude, a SifA− regulated delayed lysis strain of Salmonella encoded and delivered NP antigen presumably to the cytosol and functioned well in vivo via multiple routes. It remains to be seen if the SifA− mutant could present encoded NP antigen in the context of class I molecules. However, the best measure for the efficacy of a vaccine is the protection afforded against the target pathogen determined after a lethal challenge. Next, we plan to challenge mice immunized with χ11246(pYA4858) SifA− RASV with a heterologous strain of influenza virus. To further expand the spectrum of conserved elements in this vaccine, we plan to design new plasmids carrying conserved T-cell epitopes from HA sequences and the immunodominant M2e epitope from the influenza virus [64]. This RASV vaccine would provide broad based cellular immunity and could be used for delivering foreign antigens of any viral or intracellular bacterial pathogen that requires access of the antigen to the host cell cytosol without being hindered by its nature like presence of high stability regions, glycosylation and three dimensional structure.
Acknowledgments
We would like to thank Dr. Andrew Pekosz (Washington University, St. Louis, MO. Current address: Johns Hopkins University, Baltimore, MD) for providing the influenza virus strains, Dr. Troy Randall (Trudeau Institute, Saranac lake, NY) for providing purified NP protein used in this study, Jacquelyn Kilbourne for expert assistance with animal experiments, Dr. Wei Xin for providing plasmid pYA4651, and Drs. Praveen Alamuri and Kenneth Roland for critically reviewing the manuscript. This work was supported by NIH grant AI065779 to R.C.III.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li YC, Norton EC, Dow WH. Influenza and pneumococcal vaccination demand responses to changes in infectious disease mortality. Health Services Research. 2004;39(4 Pt 1):905–926. doi: 10.1111/j.1475-6773.2004.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein SL, Tumpey TM, Misplon JA, Lo CY, Cooper LA, Subbarao K, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis. 2002;8(8):796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bui HH, Peters B, Assarsson E, Mbawuike I, Sette A. Antibody and T cell epitopes of influenza A virus, knowledge and opportunities. Proc Natl Acad Sci. 2007;104(1):246–251. doi: 10.1073/pnas.0609330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C, et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72(7):5648–5653. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tite JPH-JC, O'Callaghan D, Dougan G, Russell SM, Gao XM, et al. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. II. Protection from influenza infection and mechanism of protection. Immunology. 1990;71(2):202–207. [PMC free article] [PubMed] [Google Scholar]
- 6.Wraith DC, Vessey AE, Askonas BA. Purified influenza virus nucleoprotein protects mice from lethal infection. J GenVirol. 1987;68(2):433–440. doi: 10.1099/0022-1317-68-2-433. [DOI] [PubMed] [Google Scholar]
- 7.Wesley RD, Tang M, Lager KM. Protection of weaned pigs by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of H3N2 swine influenza virus. Vaccine. 2004;22(25–26):3427–3434. doi: 10.1016/j.vaccine.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Altstein AD, Gitelman AK, Smirnov YA, Piskareva LM, Zakharova LG, Pashvykina GV, et al. Immunization with influenza A NP-expressing vaccinia virus recombinant protects mice against experimental infection with human and avian influenza viruses. Arch Virol. 2006;151(5):921–931. doi: 10.1007/s00705-005-0676-9. [DOI] [PubMed] [Google Scholar]
- 9.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 10.Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23(46–47):5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Epstein SL, Stack A, Misplon JA, Lo CY, Mostowski H, Bennink J, et al. Vaccination with DNA encoding internal proteins of influenza virus does not require CD8+ cytotoxic T lymphocytes: either CD4+ or CD8 + T cells can promote survival and recovery after challenge. Inter Immun. 2000;12(1):91–101. doi: 10.1093/intimm/12.1.91. [DOI] [PubMed] [Google Scholar]
- 12.Cardenas L, Clements JD. Oral immunization using live attenuated Salmonella spp. as carriers of foreign antigens. Clin Micro Rev. 1992;5(3):328–342. doi: 10.1128/cmr.5.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galan JE, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci. 1989;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bollen WS, Gunn BM, Mo H, Lay MK, Curtiss R., III Presence of wild-type and attenuated Salmonella enterica strains in brain tissues following inoculation of mice by different routes. Infect Immun. 2008;76(7):3268–3272. doi: 10.1128/IAI.00244-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava IK, Liu MA. Gene vaccines. Ann Inter Med. 2003;138(7):550–559. doi: 10.7326/0003-4819-138-7-200304010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Hess J, Schaible U, Raupach B, Kaufmann SHE. Exploiting the immune system: toward new vaccines against intracellular bacteria. Adv Immun. 2000;75:1–88. doi: 10.1016/s0065-2776(00)75001-2. [DOI] [PubMed] [Google Scholar]
- 17.Sizemore DR, Branstrom AA, Sadoff JC. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science. 1995;270(5234):299–303. doi: 10.1126/science.270.5234.299. [DOI] [PubMed] [Google Scholar]
- 18.Tite JP, Gao XM, Hughes-Jenkins CM, Lipscombe M, O'Callaghan D, Dougan G, et al. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. III. Delivery of recombinant nucleoprotein to the immune system using attenuated Salmonella typhimurium as a live carrier. Immunology. 1990;70(4):540–546. [PMC free article] [PubMed] [Google Scholar]
- 19.McSorley SJAS, Costalonga M, Reinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16(3):365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 20.Sadoff JC, Ballou WR, Baron LS, Majarian WR, Brey RN, Hockmeyer WT, et al. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988;240(4850):336–338. doi: 10.1126/science.3281260. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal A, Kumar S, Jaffe R, Hone D, Gross M, Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990;172(4):1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess J, Gentschevt I, Miko D, Welzel M, Ladel C, Goebel W, et al. Superior efficacy of secreted over somatic antigen display in recombinant Salmonella vaccine induced protection against listeriosis. Proc Natl Acad Sci. 1996;93:1458–1463. doi: 10.1073/pnas.93.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentschev I, Dietricha G, Spreng S, Kolb-Ma¨urer A, Brinkmann V, Grode L, et al. Recombinant attenuated bacteria for the delivery of subunit vaccines. Vaccine. 2001;19:2621–2628. doi: 10.1016/s0264-410x(00)00502-8. [DOI] [PubMed] [Google Scholar]
- 24.Galán JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284(5418):1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 25.Galán JE. Salmonella interactions with host cells: Type III Secretion at Work. Annu revcelldevbiol. 2001;17(1):53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Konjufca V, Wanda SY, Jenkins MC, Curtiss R., III A recombinant attenuated Salmonella enterica serovar Typhimurium vaccine encoding Eimeria acervulina antigen offers protection against E. acervulina challenge. Infect Immun. 2006;74(12):6785–6796. doi: 10.1128/IAI.00851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rüssmann HSH, Poblete F, Fu Y, Galán JE, Donis RO. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281(5376):565–568. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 28.Evans DTCL, Gillis J, Lin KC, Harty B, Mazzara GP, et al. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J Virol. 2003;77(4):2400–2409. doi: 10.1128/JVI.77.4.2400-2409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LM, Briones G, Donis RO, Galan JE. Optimization of the delivery of heterologous proteins by the Salmonella enterica serovar Typhimurium type III secretion system for vaccine development. Infect Immun. 2006;74(10):5826–5833. doi: 10.1128/IAI.00375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Branger CG, Torres-Escobar A, Sun W, Perry R, Fetherston J, Roland KL, et al. Oral vaccination with LcrV from Yersinia pestis KIM delivered by live attenuated Salmonella enterica serovar Typhimurium elicits a protective immune response against challenge with Yersinia pseudotuberculosis and Yersinia enterocolitica. Vaccine. 2009;27(39):5363–5370. doi: 10.1016/j.vaccine.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Wang S, Scarpellini G, Gunn B, Xin W, Wanda SY, et al. Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc Natl Acad Sci. 2009;106(2):593–598. doi: 10.1073/pnas.0811697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xin W, Li Y, Mo H, Roland KL, Curtiss R., III PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae. Infect Immun. 2009;77(10):4518–4528. doi: 10.1128/IAI.00486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakayama K, Kelly SM, Curtiss R., III Construction of an Asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Nat Biotechnol. 1988;6:693–697. [Google Scholar]
- 34.Kong W, Wanda SY, Zhang X, Bollen W, Tinge SA, Roland KL, et al. Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc Natl Acad Sci. 2008;105(27):9361–9366. doi: 10.1073/pnas.0803801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brumell JH, Tang P, Zaharik ML, Finlay BB. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar Typhimurium in the cytosol of epithelial cells. Infect Immun. 2002;70(6):3264–3270. doi: 10.1128/IAI.70.6.3264-3270.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beuzón CR, Méresse S, Unsworth KE, Ruíz-Albert J, Garvis S, Waterman SR, et al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. The EMBO Journal. 2000;19(13):3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karasova D, Sebkova A, Vrbas V, Havlickova H, Sisak F, Rychlik I. Comparative analysis of Salmonella enterica serovar Enteritidis mutants with a vaccine potential. Vaccine. 2009;27:5265–5270. doi: 10.1016/j.vaccine.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 38.Micheal A, Stratford R, Khan S, Dalgleish A, Pandha H. Novel strains of Salmonella typhimurium as potential vectors for gene delivery. FEMS Mircrobiology Letters. 2004;238:345–351. doi: 10.1016/j.femsle.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 39.Curtiss R, III, Porter SB, Munson M, Tinge SA, Hassan JO, Gentry-Weeks C, Kelly SM. Nonrecombinant and recombinant avirulent Salmonella live vaccines for poultry. In: Blanken-ship LC, Bailey JS, Cox NA, Stern NJ, Meinersmann RJ, editors. Colonization control of human bacterial enteropathogens in poultry. New York, NY: Academic Press; 1991. pp. 169–198. [Google Scholar]
- 40.Kang HY, Dozois CM, Tinge SA, Lee TH, Curtiss R., III Transduction-mediated transfer of unmarked deletion and point mutations through use of counterselectable suicide vectors. J bacteriol. 2002;184(1):307–312. doi: 10.1128/JB.184.1.307-312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertani G. Studies on lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. J bacteriol. 1951;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hitchcock PJ, Brown TM. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning; A Laboratory Manual. Second ed. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 45.McCown MF, Pekosz A. The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J Virol. 2005;79(6):3595–3605. doi: 10.1128/JVI.79.6.3595-3605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCown MF, Pekosz A. Distinct domains of the influenza a virus M2 protein cytoplasmic tail mediate binding to the M1 protein and facilitate infectious virus production. J Virol. 2006;80(16):8178–8189. doi: 10.1128/JVI.00627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sedgwick JD, Holt PG. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immun Met. 1983;57(1–3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- 48.Seder RA, Hill AVS. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406(6797):793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 49.Reed JDM. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–496. [Google Scholar]
- 50.Harlow E, Lane D. Antibodies: a laboratory manual. CSHL Press; 1988. [Google Scholar]
- 51.Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J Immunol. 2011;186(2):987–993. doi: 10.4049/jimmunol.1002664. [DOI] [PubMed] [Google Scholar]
- 52.Doherty PC, Kelso A. Towards a broadly protective influenza vaccine. J Clin Invest. 2008;118(10):3273–3275. doi: 10.1172/JCI37232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deliyannis G, Jackson DC, Ede NJ, Zeng W, Hourdakis I, Sakabetis E, et al. Induction of long-term memory CD8+ T cells for recall of viral clearing responses against influenza virus. J Virol. 2002;76(9):4212–4221. doi: 10.1128/JVI.76.9.4212-4221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrovska LAR, Barber L, Clare S, Simmons CP, Stratford R, et al. Salmonella enterica serovar Typhimurium interaction with dendritic cells: impact of the sifA gene. Cell Micro. 2004;6(11):1071–1084. doi: 10.1111/j.1462-5822.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 55.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 56.Markine-Goriaynoff D, van der Logt J, Truyens C, Nguyen TD, Heessen FWA, Bigaignon G, et al. IFN -γ independent IgG2a production in mice infected with viruses and parasites. Inter Immun. 2000;12(2):223–230. doi: 10.1093/intimm/12.2.223. [DOI] [PubMed] [Google Scholar]
- 57.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infec Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 58.Andrew ME, Coupar BE, Boyle DB, Ada GL. The roles of influenza virus haemagglutinin and nucleoprotein in protection: analysis using vaccinia virus recombinants. Scand J Immunol. 1987;25(1):21–28. doi: 10.1111/j.1365-3083.1987.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 59.De Boer GF, Back W, Osterhaus A. An ELISA for detection of antibodies against influenza A nucleoprotein in humans and various animal species. Arch Virol. 1990;115(1):47–61. doi: 10.1007/BF01310622. [DOI] [PubMed] [Google Scholar]
- 60.Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol. 2008;181(6):4168–4176. doi: 10.4049/jimmunol.181.6.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178(5):1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nguyen HHVF, Vu HL, Novak MJ, McGhee JR, Mestecky J. Gamma interferon is not required for mucosal cytotoxic T-lymphocyte responses or heterosubtypic immunity to influenza A virus infection in mice. J Virol. 2000;74(12):5595–5601. doi: 10.1128/jvi.74.12.5495-5501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wherry E, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunology. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 64.Ameiss K, Ashraf S, Kong W, Pekosz A, Wu WH, Milich D, et al. Delivery of woodchuck hepatitis virus-like particle presented influenza M2e by recombinant attenuated Salmonella displaying a delayed lysis phenotype. Vaccine. 2010;28:6704–6713. doi: 10.1016/j.vaccine.2010.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roland K, Curtiss R, III, Sizemore D. Construction and evaluation of a delta cya delta crp Salmonella typhimurium strain expressing avian pathogenic Escherichia coli O78 LPS as a vaccine to prevent airsacculitis in chickens. Avian Dis. 1999;43(3):429–441. [PubMed] [Google Scholar]