Abstract
We have characterized two new transgenic Xenopus lines enabling transgene expression using the Tet-On inducible system. An inducer line expresses the doxycycline- (Dox-) activated transcription factor rtTA under control of the ubiquitous promoter CMV. A responder line enables Dox-inducible expression of a dominant positive thyroid hormone receptor via a tetracycline responsive transgenic promoter (TRE). Dox-induced expression of transgenic GFP mRNA was detectable after 3 hours and increased up to 10- to 50-fold by 2 days depending on dose of Dox. Induced GFP mRNA expression returned to uninduced levels within 3 days upon Dox removal. Treatment of rtTA inducer and TRE responder double transgenic animals with Dox caused acceleration of metamorphic changes in thyroid hormone-response gene expression and morphology. These transgenic lines will be made available through the new Xenopus Stock Center and will serve as valuable tools for genetic analysis of development and metamorphosis.
Keywords: Xenopus, transgenesis, doxycycline, Tet-On, dominant positive thyroid hormone receptor
Introduction
The frog Xenopus has been a valuable model for the elucidation of gene function during early vertebrate development due to the combined use of the cell lineage fate map, cell microinjection, and embryo manipulation (Warkman and Krieg, 2007). Study of later embryonic stages is important for understanding organ development and metamorphic tissue remodeling (corresponding to events that occur during the fetal period in mammals), which may then help elucidate the developmental orgins of adult diseases (Blitz et al, 2006). However, inducible methods for altering gene expression in later development are limited in frogs compared to other model organisms. Thus, the utility of Xenopus as a developmental model would be greatly increased by further development of transgenic tools to manipulate post-embryonic gene expression.
Many post-embryological studies require simultaneous and precise temporal and spatial control of transgene expression. Binary transgene expression systems are capable of such controlled expression and are comprised of 1) a ligand-activated transgenic transcription factor that acts on 2) a cognate transgenic promoter controlling a gene of interest. These two components precisely regulate transgene expression by virtue of tissue-specific expression of the transgenic transcription factor activated by exogenous addition of the activating ligand. Two such binary methods have successfully expressed transgenes in a tissue-specific and inducible manner in frogs, the GAL4/UAS and Tet-On systems (Hartley et al, 2002; Das and Brown, 2004). The GAL4 transcripiton factor from yeast can bind and activate the transgenic UAS promoter. GAL4 can be controlled from ubiquitous (Hartley et al, 2002; Das and Brown, 2004) or tissue-specific promoters (Chae et al, 2002). Inducibility has been achieved by fusing Gal4 to the progesterone (Chae et al, 2002) or estrogen ((Das and Brown, 2004) receptor ligand binding domain. Commonly used in flies, the tissue-specific inducible versions of the Gal4/UAS system in frogs have not gone beyond proof of principle. A third binary strategy using Cre/lox has been developed, where Cre is inducibly expressed with the ubiquitous heat shock inducible promoter (Roose et al, 2009) or constitutively expressed from a muscle-specific promoter (Waldner et al, 2006) and acts on a separate transgene with appropriately placed lox sites to activate that transgene.
The Tet-On system, used extensively in mice, has been used to delimit the timing of thyroid hormone influence in hind limb innervation (Das and Brown, 2004), examine genes important for limb muscle development (Cai et al, 2007), and detect gene switching during liver metamorphosis (Mukhi et al, 2010). In the Tet-On system, the transgenic transcription factor rtTA (a re-engineered version of the bacterial Tet repressor fused to three copies of the minimal viral transactivation domain of VP16 to make a ligand-inducible transcriptional activator) regulates expression via a tetracycline-inducible promoter (seven copies of the bacterial tet operator upstream of a minimal CMV promoter) upon addition of the tetracycline mimic doxycycline (Dox).
Transgenic lines available from previous studies using the Tet-On system (Das and Brown, 2004; Cai et al, 2007) are of limited future use because the rtTA and TRE components are not separable, i.e., these components co-integrated into the same chromosomal position (the tet-inducible L-FABP:TRDN (Mukhi et al, 2010) were not reared to adulthood). Thus, no Tet-On lines are available that can be flexibly applied to a wide variety of research questions, highlighting the need to characterize new Dox-inducible transgenic Xenopus lines. Here, we characterize two transgenic Xenopus lines incorporating the Tet-On system, one inducer line with ubiquitous expression of rtTA and one responder line for Dox-dependent expression of a dominant positive thyroid hormone receptor (Buchholz et al, 2004). We examined expression induction characteristics to determine parameters important for overexpression studies. Development of these and future transgenic frog lines and depostion into the Xenopus stock center will significantly augment the utility of Xenopus as a model system by incorporating transgenic strategies into the major advantages of the Xenopus model system for studies of development and regeneration.
Results and Discussion
The effect of HS4 insulators
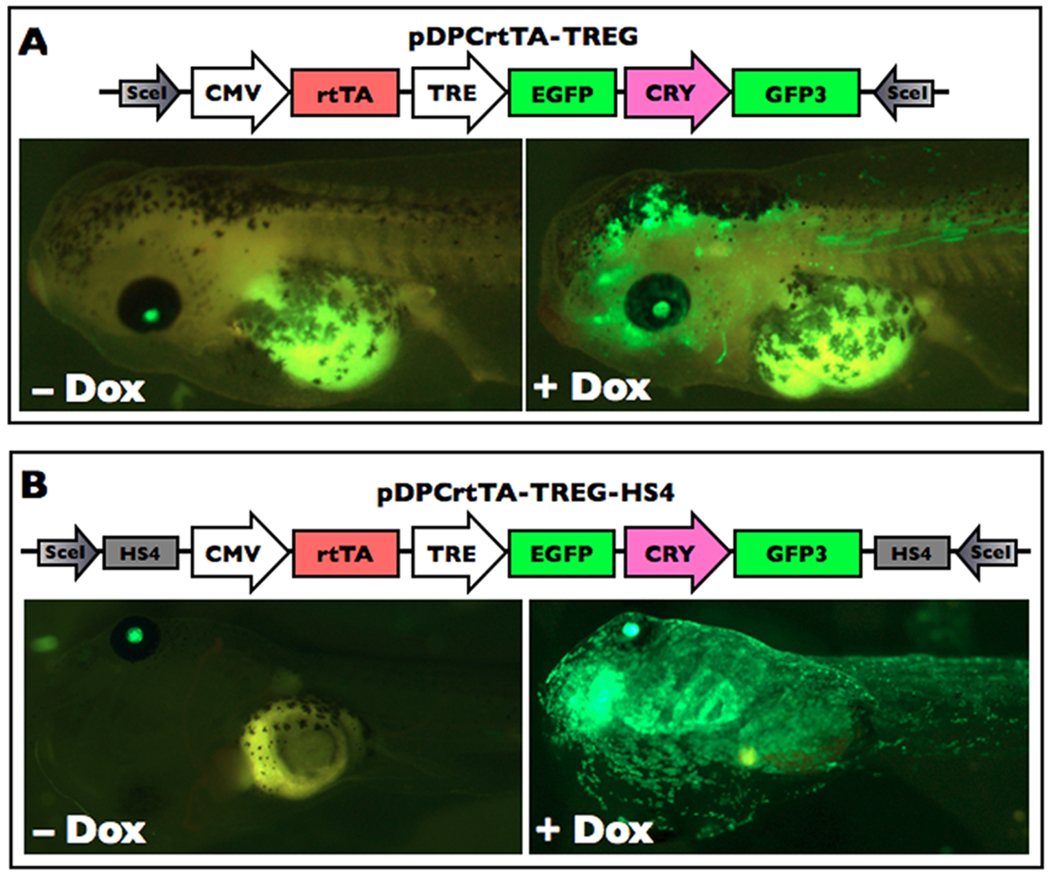
Variable expression levels from the same transgenesis construct in different transgenic animals and across tissues in the same transgenic animal even with ubiquitous promoters are often observerd and attributed to random insertion into different sites in the genome (Bell et al, 2001). Insulator sequences are cis-regulatory elements that can reduce the influence of enhancers or heterochromatin silencing mechanisms operating at the site of transgene insertion (Gaszner and Felsenfeld, 2006). Two frog transgenesis studies incorporated the chicken β-globin 5'HS4 insulator (Allen and Weeks, 2005; Sekkali et al, 2008) and substantially reduced transgene expression variegation within transgenic animals. We examined the effectiveness of these HS4 insulators to improve uniformity of transgene expression from a Dox-inducible system after meganuclease transgenesis. To do this, we made two transgenesis constructs having the Dox-activated transcription factor rtTA under the control of the ubiquitous promoter CMV and green fluorescent protein GFP regulated by the modified tetracycline response element TREtight (pDPCrtTA-TREG and pDPCrtTA-TREG-HS4), where the only difference between them was the presence of a pair of directly repeated HS4 insulator elements (Fig. 1). Both constructs also had a crystallin:GFP expression cassette for constitutive expression of GFP in the lens as a marker of transgenesis (Fu et al, 2002).
Figure 1.
Chick 5'HS4 insulator sequences improve uniformity of ubiquitous Dox-induced GFP expression. A. Exemplar tadpoles are transgenic for the construct pDPCrtTA-TREG. This construct lacks the HS4 insulators but has the CRY:GFP cassette, as indicated by lens-specific GFP fluorescence. The tadpoles were treated with and without 50 ug/mL Dox for four days and visualized using fluorescence microscropy with GFP2 filter sets. The expression of GFP in the body is present but variegated in the presence of Dox and lacking in the absence of Dox. B. Exemplar tadpoles are transgenic for the construct pDPCrtTA-TREG-HS4. This construct has a pair of HS4 direct repeats and also has the CRY:GFP cassette, as indicated by lens-specific GFP fluorescence. The tadpoles were treated with and without 5 ug/mL Dox for five days and visualized using fluorescence microscropy with GFP2 filter sets. Again, the expression of GFP in the body is lacking in the absence of Dox, but in this case GFP fluorescence is uniform in the presence of Dox.
Using the meganuclease method of transgenesis, we obtained 19 and 31 normal transgenic embryos at NF stage 38–39 (Nieuwkoop and Faber, 1994) with and without insulator elements, respectively, as indicated by GFP expression in one or both eyes. No GFP fluorescence was observed in the bodies of transgenic animals with and without insulators in the absence of Dox (Fig. 1). Transgenic tadpoles were then treated with 5 ug/mL Dox and scored for GFP expression uniformity after five days (Fig. 1, Table 1). Uniform ubiquitous GFP expression was seen in 53% of the insulated transgenic animals having lens GFP expression compared to 42% in the non-insulated transgenic animals. The percent mosaic animals was the same between insulated and non-insulated constructs and likely reflects the fact that insulators do not affect the incidence of episomal expression or the time at which integration occurs (i.e., one-cell or two-cell stage vs. later stages which account for mosaicism). The lack of visible ubiquitous GFP expression in 10% of the non-insulated transgenic animals with GFP fluorescence in one or both eyes accounts for the difference in uniformity seen in transgenic animals with and without insulated transgenes. These results are consistent with results observed previously in frogs (Allen and Weeks, 2005; Sekkali et al, 2008) in that HS4 insulators gave reduced incidence of position-dependent silencing of the transgene. Therefore, we included insulators in subsequent transgenesis constructs.
Table 1.
Comparison of Dox-induced GFP expression in transgenic tadpoles with (pDPCrtTA-TREG-HS4) and without (pDPCrtTA-TREG) HS4 insulators. NF stage 38–39 tadpoles were treated with 5 ug/mL Dox for 5 days. Tadpoles lacking eye-specific GFP expression are not included.
| GFP in body (number and percent) | ||||
|---|---|---|---|---|
| pDPCrtTA-TREG | GFP in eyes* | none | non-uniform** | uniform*** |
| without HS4 | n = 31 | 3 (10%) | 15 (48%) | 13 (42%) |
| with HS4 | n = 19 | 0 (0%) | 9 (47%) | 10 (53%) |
Indicates tadpoles with one or two eyes with GFP expression.
Non-uniform GFP expression on the same side as the green eye.
For tadpoles with one green eye, uniform GFP expression was on the same side as the green eye.
Production and characterization of the rtTA inducer line
To reduce the time and expence of rearing and testing large numbers of potential founders, we adopted a strategy that would enable preliminary testing of the F0 tadpoles to increase the chances of useful adult founders being produced. To this end, both CMV:rtTA and TRE:GFP cassettes were included on the same transgenesis construct so we could evaluate expression levels and uniformity of Dox-induced GFP in the F0 tadpoles. We performed transgenesis using the construct pDPCrtTA-TREG-HS4 (diagram in Fig. 1B) and after two weeks obtained 93 tadpoles with no green eyes, 81 with one green eye, and 66 with two green eyes. No animals exhibited GFP fluorescence in the body in the absence of Dox. All 240 survivors with 0, 1, or 2 green eyes were treated with Dox for 2 days and scored for GFP expression (Table 2). The majority of animals lacking green eyes had non-uniform Dox-induced GFP expression, indicating that they had episomal (non-integrated) plasmids, mosaic expression (plasmids integrated into a subset of cells and not in the cell lineage leading to the lens), or that the CRY:GFP was silenced despite the HS4 insulators. Some green-eyed animals lacked ubiquitous GFP expression, even with the insulators, suggesting that an expanded core HS4 insulator sequence is worth trying to improve the results (Aker et al, 2007). Because we wanted transgenic animals with the highest levels of rtTA activity in the presence of Dox, we reared to adulthood 11 of the 46 uniform GFP expressing tadpoles which had particularly bright Dox-induced GFP fluorescence.
Table 2.
GFP expression anaysis to select inducer transgenic founders to rear to adulthood. Meganuclease transgenesis was carried out using pDPCrtTA-TREG-HS4 and the surviving two-week old F0 tadpoles were treated with 5 ug/mL Dox for 2d, changed daily.
| GFP in body (number and percent) | |||||
|---|---|---|---|---|---|
| none | non-uniform* | uniform | |||
| GFP in eyes | one side | both sides | one side | both sides | |
| 0 (n = 93) | 20 (22%) | 52 (56%) | 21 (23%) | none | none |
| 1 (n = 81) | 7 (9%) | 51 (63%)** | 6 (7%) | 17 (21%) | none |
| 2 (n = 66) | none | 8 (12%) | 29 (44%) | none | 29 (44%) |
Non-uniformity due to mosaicism, episomal expression, or both.
In one case, the green eye and green in the body were on opposite sides.
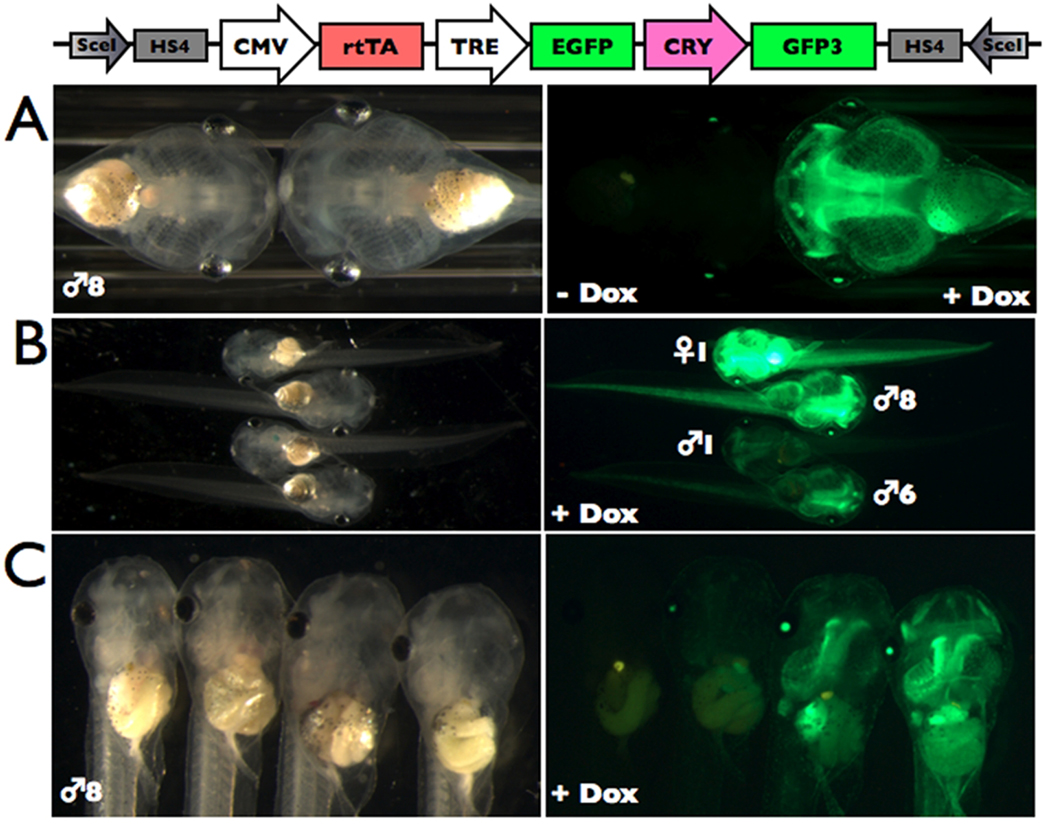
We bred each transgenic founder to wild-type adults and found that 7 of 11 were germ-line. The frequency of offspring with GFP expression in the eyes was 5–26% across founders indicating that the germ line was mosaic for transgene insertion. None of the offspring had visible transgene expression in the absence of Dox (Fig. 2A, and data not shown), and the offspring from each founder had a different Dox-induced transgene expression level (Fig. 2B, and data not shown). Also, in founders ♂3 and ♂8, Dox-induction resulted in a range of GFP fluorescence levels among transgenic siblings (Fig. 2C, and data not shown). A possible interpretation for these different levels is that transgene integration may have occurred in two chromosomal locations resulting in three brightness classes (and after the two-cell stage given the mosaic germ line), but the results could also represent independent insertions in different groups of germ cells. Data from F2 offspring from each brightness class would be needed for futher analysis. Founder ♀1 had the highest expression levels but the animal died, so we used founder ♂8 for subsequent experiments.
Figure 2.
Dox-induced GFP expression varied across and within founder offspring. Five-day old tadpoles transgenic for pDPCrtTA-TREG-HS4 were treated with 50 ug/mL Dox for three days and shown under brightfield and green fluorescence microscopy. A. All transgenic offspring from each founder lacked visible GFP expression in the absence of Dox, and showed induction of GFP in the presence of Dox. An exemplar tadpole from ♂8 is shown. B. The offspring from four founders (♀1, ♂8, ♂6, ♂1) vary in GFP brightness, likely due to chromosomal insertion site effects. C. A range of Dox-induced GFP expression levels was observed among transgenic sibling offspring from some founders. F1 offspring from ♂8 are shown. A non-transgenic tadpole (left) is shown for comparison.
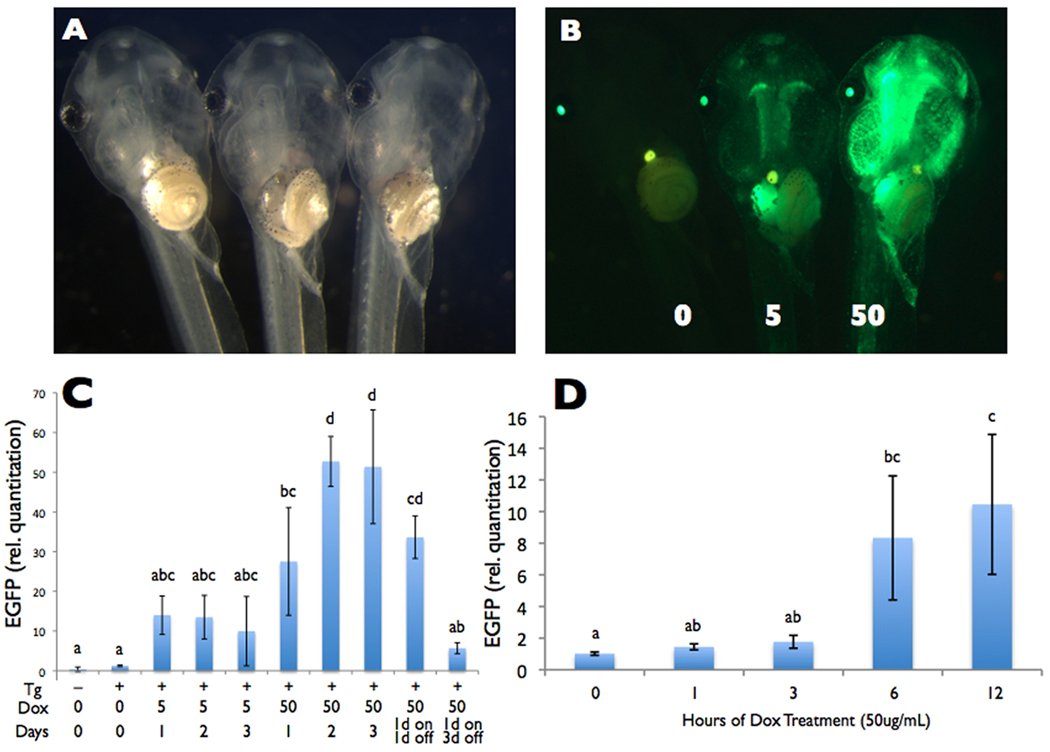
We observed a dose-dependent induction of GFP fluorescence levels after 1 day with 5 and 50 ug/mL Dox (Fig. 3A,B). These doses had been used previously in frogs (Das and Brown, 2004; Cai et al, 2007; Mukhi et al, 2010), and higher doses of Dox are known to inhibit metamorphic events at least in part due to inhibition of matrix metalloproteinases (Schrieber, pers. comm.). To quantify these transgene induction levels and examine the time course of induction, we treated animals with 5 or 50 ug/mL Dox, harvested their tails after 1, 2, and 3 days of treatment, and quantified the relative GFP expression levels by real time reverse transcriptase PCR (Fig. 3C). In the absence of Dox, transgenic animals had GFP mRNA expression levels comparable to non-transgenic animals. The level of transgene induction was 10 and 50 fold in 5 and 50 ug/mL Dox, achieved by 1 and 2 days, respectively. We then determined the time at which induction could first be detected using 50 ug/mL by measuring the relative GFP mRNA levels in tails collected at 0, 1, 3, 6, and 12 hours after Dox treament (Fig. 3D). We detected Dox-induced mRNA expression by 6 hours after Dox-treatment.
Figure 3.
Dose-dependent Dox-induced GFP expression. A and B) Five-day old offspring from founder ♂8 transgenic for pDPCrtTA-TREG-HS4 were treated with 0, 5, or 50 ug/mL Dox for one day and shown under A) brightfield and B) green fluorescence microscopy. In the absence of Dox, GFP is not visible in the body. In the presence of Dox, GFP is induced to a higher level with 50 compared to 5 ug/mL. A representative tadpole from the highest brightness class within each Dox treatment is shown. C and D) For a time course of dose-dependent GFP mRNA expression, GFP mRNA levels in the tail relative to the housekeeping gene rpL8 were measured (C) at 0, 1, 2, and 3 days of 5 and 50 ug/mL Dox treatment and (D) at 0, 1, 3, 6, and 12 hrs. of 50 ug/mL Dox treatment in 5- to 8-day old offspring from founder ♂8 transgenic for pDPCrtTA-TREG-HS4 using real-time reverse transcriptase PCR. Non-transgenic tadpoles were included as controls. C. Sample size was n=3, and no tadpoles were pooled. To avoid variation in the results due to variation in GFP expression amongs siblings in founder ♂8 (seen in Fig. 2C), tadpoles from the highest brightness class were used for all time points (except Day 0), done by selecting similarly bright individuals from a surplus of Dox-treated tadpoles after 24 hours. D. Because tadoles could not be preselected based on GFP brightness prior to tissue harvesting, we used a sample size of n=3 and pooled four tadpoles per sample to decrease the chances of obtaining unrepresentative results, given the expected levels of variability in GFP expression among siblings in the same treatment. Letters above the bars represent significance groups based on ANOVA and Bonferroni post-hoc pairwise comparisons, α = 0.05.
After Dox treatment (3 days, 50 ug/mL, NF stage 45), visible GFP expression very gradually declined in the absence of Dox in all founders until it was nearly gone after four weeks, a period when the animals increased greater than 10-fold in mass (data not shown). To determine whether the visible GFP may be due to continued mRNA expresssion or high GFP protein stability, we treated tadpoles with 50 ug/mL Dox for 1 day then measured GFP mRNA levels after 1 and 3 days after Dox removal (Fig. 3C). At 1 day after removal of Dox, the GFP mRNA levels were not significanlty reduced, but by 3 days in the absence of Dox, the GFP mRNA levels were nearly back to the uninduced levels, despite persistent GFP fluorescence.
Production and characterization of the TRE responder line
We chose to use a dominant positive thyroid hormone receptor (dpTR) for the responder transgene as a test case because it had been previously shown to function as expected under control of the heat shock promoter (Buchholz et al, 2004). Specifically, the presence of dpTR was sufficient to initiate changes in gene expression and morphology associated with thyroid hormone present during natural metamorphosis. The responder transgenesis construct, pDRTREdpTR-HS4 (diagram in Fig. 4), was injected using the meganuclease procedure, and we obtained 271 with no red eyes, 31 with one red eye, and 5 with two red eyes. We reared 18 half- and full-transgenic tadpoles with the brightest red eyes to adulthood (16 females and 2 males) and crossed them with wild-type animals. Of the 18 founders, 2 had germ-line expression, 2 were sterile (no eggs after three attempts at 3 mo. intervals), and 14 were not germline. Comapred to the high germ-line transgenesis efficiency observed for the rtTA-expressing line, the frequency of germ-line transmission for the dpTR line was lower probably because CRY:DsRed alone was available for selecting tadoles to rear, while rtTA line founders were screened through ubiquitous Dox-induced GFP expression. These results emphasize the mosaic nature of transgenic animals produced by the meganuclease procedure. Based on this experience, we recommend generating larger numbers of founders to have a sufficient number of germ line founders to screen in the F1 generation and/or engineering a means of screening founders as tadpoles to avoid time and labor rearing non-germ line animals.
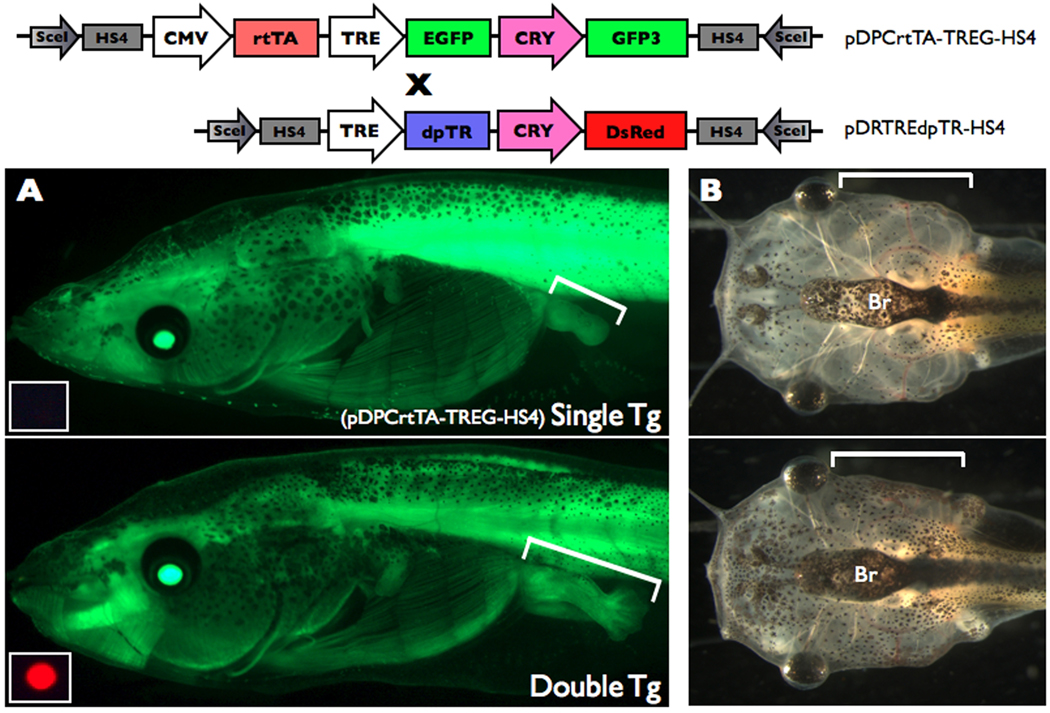
Figure 4.
Dox-induced dpTR causes metamorphic phenotypes. Double transgenic tadpoles for the two constructs shown were treated with 50 ug/mL Dox beginning at NF stage 49 for 12 days (4 tads/200mL with changing and feeding every 2nd day). Tadpoles singly transgenic for pDPCrtTA-TREG-HS4 was similarly treated and used as a control. A. GFP expression present all over the body in single (top) and double (bottom) transgenic animals from Dox-activated rtTA induced GFP from the TRE:GFP cassette in pDPCrtTA-TREG-HS4 was visualized using fluorescence microscopy. Hindlimbs are indicated by brackets. The small inset at the bottom left of each green fluorescence image is the red fluorescence image cropped around the eye to reveal the absence or presence of the pDRTREdpTR-HS4 transgene. The same camera settings were used in both panels. Representative individuals from n=13 single transgenics and n=7 double transgenics for Dox-treated tadpoles starting at NF49–52. B. The gills in the double transgenic animals (bottom) and the single transgenic tadpole (top) are indicated by brackets. The same camera settings were used in both panels. Representative individuals from n=3 single transgenics and n=2 double transgenics for Dox-treated tadpoles starting at NF49.
While F1s are maturing, we tested the validity of the binary system by breeding our one mature pDRTREdpTR-HS4 founder to the CMV:rtTA transgenic founder ♂8 characterized above, and 20% of the offspring were transgenic for pDRTREdpTR-HS4. We selected a number of tadpoles from NF stages 49–52 for each of the four predicted genotypes of the progeny: 1) non-transgenic (n=11), 2) single transgenic pDRTREdpTR-HS4 (n=10), 3) single transgenic for pDPCrtTA-TREG-HS4 (n=13), and double transgenic (n=7), as detected by GFP and/or DsRed fluorescence in the eye. Even though large clutch sizes contribute to Xenopus as a model system, we have modest sample sizes here because young females produce relatively small clutches and the frequency of single transgenic and especially double transgenic animals is low in a cross where each founder has ~20% transgenic offspring due to a mosaic germline. In the absence of Dox, these tadpoles had no GFP fluorescence in the body (from the TRE:GFP cassette in pDPCrtTA-TREG-HS4) (data not shown). Througout the 12 days of Dox treatment, all tadpoles with green eyes (i.e. transgenic for pDPCrtTA-TREG-HS4) had similar levels of GFP fluorescence in the body (Fig. 4A, and data not shown). Phenotypic effects of Dox-induced dpTR were first detected by 3 days and most dramatic at Day 12. Because we used tadpoles of different starting stages (NF49–52), limb stage comparisons were made among tadpoles of the same starting stage. All seven double transgenic tadpoles had limbs at least one limb stage beyond the other groups which were all similar to each other (Fig. 4A, and data not shown). In addition, Dox treatment initiated gill resorption only in the double transgenic animals, and only when Dox treatment was started at stage NF 49 (Fig. 4B). These Dox-induced metamorphic phenotypes in the double transgenic animals correspond to phenotypes obtained by heat shock-induced dpTR expression and treatment of non-transgenic tadpoles with thyroid hormone (Buchholz et al, 2004), which can be induced as early as NF 45. It is not clear why the later stages (i.e., NF 50–52) lacked gill resorption. Most likely, there was variation among animals in the ability to induce the dpTR transgene as seen for GFP transgene induction.
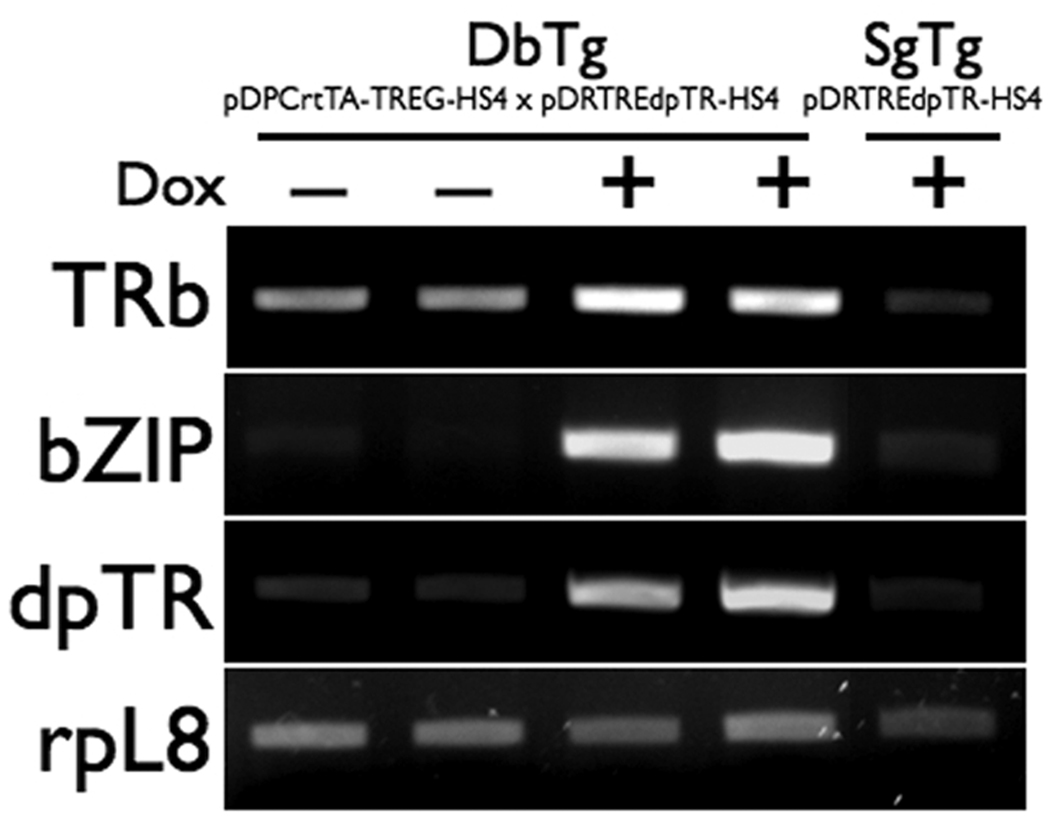
The tail is completely resorbed during natural metamorphosis, but it is not dramatically shortened by dpTR (Buchholz et al, 2004). However, because gene induction occurs in response to dpTR, we examined the expression of two well-known thyroid hormone-response genes (thyroid hormone receptor beta and TH-responsive basic leucine zipper) after Dox induction of dpTR at NF52–54 (Fig. 5). The dpTR transgene had minimal levels of expression in the absence of Dox and was strongly induced by Dox only in the double transgenic animals. The presence of dpTR in turn induced expression of TH-regulated genes, as seen previously (Fig. 5) (Buchholz et al, 2004). The tadpole singly transgenic for pDPTREdpTR-HS4 was used as another control and had a similar gene expression profile as the non-Dox treated double transgenic animals (Fig. 5).
Figure 5.
Dox-induced dpTR caused changes in gene expression. Tadpoles doubly transgenic for the two constructs shown were treated with and without 50 ug/mL Dox beginning at NF stage 52–54 for three days. A tadpole singly transgenic for pDRTREdpTR-HS4 was similarly treated with Dox and used as a control. Tails were harvested for the analysis. Each lane of the gel represents an individual tadpole. The presence of Dox upregulated the transgene dpTR, which in turn induced expression of the two thyroid hormone response genes, thyroid hormone receptor beta (TRβ) and thyroid hormone-induced basic leucine zipper (bZIP). Consistent expression of the control gene ribosomal protein L8 (rpL8) in each lane indicates comparable sample quality.
In conclusion, we have produced and characterized new transgenic Xenopus lines enabling transgene expression using the Tet-On inducible system. Future production of inducer lines with rtTA controlled by tissue-specific promoters and TRE responder lines regulating different genes of interest will create a great variety of possible crosses that can inducibly target genes of interest to specific tissues. The transgenic lines characterized here will be deposited at the newly established Xenopus stock center at Woods Hole and will serve as a foundation for future studies using Tet-On transgene induction in frogs.
Experimental Procedures
Plasmid construction
To make pDPCrtTA-TREG, a PCR product containing rtTA 2S-M2 from pCS2-rtTA2S-M2 (gift from B. Das and D. Brown) using primers DRB141 5' GGGGACCGGTATGTCTAGACTGGACAAGAGC and DRB142 5' GGGGGAATTCCTATAGTTCTAGAGGCTCGAG was cloned into pDPCG-Sce (Rankin et al, 2009) to make pDPCrtTA-Sce. Compared to the original transactivator rtTA, rtTA 2S-M2 is more stable in eukaryotic cells, has reduced background inducibility in the absence of Dox, and is more sensititve to Dox (Urlinger et al, 2000). The EcoRI, BglII, and AgeI sites of pDPCrtTA-Sce were removed by cutting and blunting with Klenow, and then a TREtight fragment from pTREtight (Clontech) was cloned into the NotI site. The TREtight (modified tetracycline response element) promoter has 7 tet operator sequences adjacent to a CMV minimal promoter. The rtTA-TREtight fragment was cloned in between CMV and GFP in pDPCG-Sce to get pDPCrtTA-TREG. To make pDPCrtTA-TREG-HS4, pDPCG-Sce was cut with SceI and an NheI/AfeI phosphorylated linker was put in: DRB 196 5’ GCTAGCCATCATAGCGCTTTAT DRB 197 5’ AGCGCTATGATGGCTAGCTTAT, which also reconstituted the two SceI sites. A synthetic gene (www.DNA20.com) containing two direct repeats of chick 5' HS4 insulator elements (Allen and Weeks, 2005) separated by a mutliple cloning site was ligated into the NheI and AfeI sites. Then, the CMV to GFP fragment of pDPCrtTA-TREG was cloned in to make pDPCrtTA-TREG-HS4. To make the responder construct, the flag-tagged dominant positive thyroid hormone receptor from F-dpTR (Buchholz et al, 2004), the CRY:DsRed cassette from pDRCG-Sce (Rankin et al, 2009) and the inducer plasmid pDPCrtTA-TREG-HS4 were used to make the vector pDRTREdpTR-HS4 via standard cloning methods. All portions of plasmids involving PCR or Klenow were sequence verified, and the two plasmid sequences (pDPCrtTA-TREG-HS4 and pDRTREdpTR-HS4) are available though GenBank (accession numbers JF330265 and JF330266).
Transgenesis and production of founders
Transgenesis was carried out using the SceI meganuclease procedure (Ogino et al, 2006; Pan et al, 2006; Rankin et al, 2009). Briefly, the SceI reaction (400ng of DNA, 2.0 uL SceI buffer, 2.0 uL of bovine serum albumin, 2uL of SceI in 20 uL total volume of the reaction mixture) was incubated at 37°C for 40 min. then injected into dejellied zygotes (10nL per zygote) at room temperature using a Picospritzer III (Parker Hannifin Corp.). Embryos were cultured at 18°C and sorted daily to remove dead and dying embryos. Founder individuals were reared to sexual maturity for breeding within 12–18 months. Founders were bred to wild type.
Doxycycline treatments and transgene expression analysis
Tadpoles at one week old at a density up to 50 tadpoles/30mL in a petri dish and at stage NF stage 49–52 (Nieuwkoop and Faber, 1994) at a density of 2–7 tadpoles/200mL were treated with 0, 5, or 50 ug/mL Dox for 1–12 days in the dark to avoid photo-breakdown of Dox. Water and Dox were changed every 1–4 days without feeding. Fluorescent protein expression in live animals was observed using a Leica fluorescence dissection microscope with CFP, GFP2, and RFP filter sets and photographed with a Leica DFC420 digital camera.
To measure transgenic GFP mRNA expression, total RNA was extracted from whole bodies of single two-week old tadpoles using TriReagent (Fisher), contaminating genomic DNA was removed using DNA-free (Ambion), and 2ug total RNA per sample was used to make cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative PCR using primers and FAM-labelled TaqMan probes (Applied Biosystems) for GFP (F-5'TGTCCACACAATCTGCCCTTTC, R-5'GCAGCTGTTACAAACTCAAGAAGGA, P-5'TTTCGTTGGGATCTTTC) and the housekeeping gene rpL8 (F-5'AGAAGGTCATCTCATCTGCAAACAG, R-5'CTTCAGGATGGGTTTGTCAATACGA, P-5'CAACCCCAACAATAGCT) was carried out and analyzed using the delta delta Ct method (Livak and Schmittgen, 2001) on a 7300 Real Time PCR System (Applied Biosystems). Three biological replicates per treatment group were used. Analysis of variance followed by Bonferroni post-hoc pairwise comparisons (JMP Statistical Software) was used to test for significant differences between treatment groups with α = 0.05.
Acknowledgements
Helpful comments and suggestions came from R. Kerney. Support for this research was from NIH R03 5F32DK010069-03 and NSF IOS 0950538 to DRB and NIH R01DK070858 to AMZ.
Grant Sponsors: NIH R03 HD059995 and NSF IOS 0950538 to DRB and NIH R01 DK070858 to AMZ.
References Cited
- Aker M, Tubb J, Groth AC, Bukovsky AA, Bell AC, Felsenfeld G, Kiem HP, Stamatoyannopoulos G, Emery DW. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum Gene Ther. 2007;18:333–343. doi: 10.1089/hum.2007.021. [DOI] [PubMed] [Google Scholar]
- Allen BG, Weeks DL. Transgenic xenopus laevis embryos can be generated using phiC31 integrase. Nat Methods. 2005;2:975–979. doi: 10.1038/nmeth814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AC, West AG, Felsenfeld G. Insulators and boundaries: Versatile regulatory elements in the eukaryotic genome. Science. 2001;291:447–450. doi: 10.1126/science.291.5503.447. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Andelfinger G, Horb ME. Germ layers to organs: Using xenopus to study "later" development. Semin Cell Dev Biol. 2006;17:133–145. doi: 10.1016/j.semcdb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Tomita A, Fu L, Paul BD, Shi YB. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol. 2004;24:9026–9037. doi: 10.1128/MCB.24.20.9026-9037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Das B, Brown DD. Changing a limb muscle growth program into a resorption program. Dev Biol. 2007;304:260–271. doi: 10.1016/j.ydbio.2006.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J, Zimmerman LB, Grainger RM. Inducible control of tissue-specific transgene expression in xenopus tropicalis transgenic lines. Mech Dev. 2002;117:235–241. doi: 10.1016/s0925-4773(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Das B, Brown DD. Controlling transgene expression to study xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2004;101:4839–4842. doi: 10.1073/pnas.0401011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Buchholz D, Shi YB. Novel double promoter approach for identification of transgenic animals: A tool for in vivo analysis of gene function and development of gene-based therapies. Mol Reprod Dev. 2002;62:470–476. doi: 10.1002/mrd.10137. [DOI] [PubMed] [Google Scholar]
- Gaszner M, Felsenfeld G. Insulators: Exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- Hartley KO, Nutt SL, Amaya E. Targeted gene expression in transgenic xenopus using the binary Gal4-UAS system. Proc Natl Acad Sci U S A. 2002;99:1377–1382. doi: 10.1073/pnas.022646899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Cai L, Brown DD. Gene switching at xenopus laevis metamorphosis. Dev Biol. 2010;338:117–126. doi: 10.1016/j.ydbio.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of xenopus laevis (daudin) New York: Garland Publishing; 1994. p. 252. [Google Scholar]
- Ogino H, McConnell WB, Grainger RM. Highly efficient transgenesis in xenopus tropicalis using I-SceI meganuclease. Mech Dev. 2006;123:103–113. doi: 10.1016/j.mod.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Pan FC, Chen Y, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in xenopus. Dev Dyn. 2006;235:247–252. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- Rankin SA, Hasebe T, Zorn AM, Buchholz DR. Improved cre reporter transgenic xenopus. Dev Dynamics. 2009 doi: 10.1002/dvdy.22043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose M, Sauert K, Turan G, Solomentsew N, Werdien D, Pramanik K, Senkel S, Ryffel GU, Waldner C. Heat-shock inducible cre strains to study organogenesis in transgenic xenopus laevis. Transgenic Res. 2009;18:595–605. doi: 10.1007/s11248-009-9253-4. [DOI] [PubMed] [Google Scholar]
- Sekkali B, Tran HT, Crabbe E, De Beule C, Van Roy F, Vleminckx K. Chicken beta-globin insulator overcomes variegation of transgenes in xenopus embryos. FASEB J. 2008;22:2534–2540. doi: 10.1096/fj.07-098111. [DOI] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: Novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldner C, Sakamaki K, Ueno N, Turan G, Ryffel GU. Transgenic xenopus laevis strain expressing cre recombinase in muscle cells. Dev Dyn. 2006;235:2220–2228. doi: 10.1002/dvdy.20880. [DOI] [PubMed] [Google Scholar]
- Warkman AS, Krieg PA. Xenopus as a model system for vertebrate heart development. Semin Cell Dev Biol. 2007;18:46–53. doi: 10.1016/j.semcdb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]