Abstract
Indolic derivatives can affect fibril growth of amyloid forming proteins. The neurotransmitter serotonin (5-HT) is of particular interest, as it is an endogenous molecule with a possible link to neuropsychiatric symptoms of Parkinson disease. A key pathomolecular mechanism of Parkinson disease is the misfolding and aggregation of the intrinsically unstructured protein α-synuclein. We performed a biophysical study to investigate an influence between these two molecules. In an isolated in vitro system, 5-HT interfered with α-synuclein amyloid fiber maturation, resulting in the formation of partially structured, SDS-resistant intermediate aggregates. The C-terminal region of α-synuclein was essential for this interaction, which was driven mainly by electrostatic forces. 5-HT did not bind directly to monomeric α-synuclein molecules and we propose a model where 5-HT interacts with early intermediates of α-synuclein amyloidogenesis, which disfavors their further conversion into amyloid fibrils.
Abbreviations: AS, α-synuclein; 5-HT, serotonin; 5,7-HT, 5,7 dihydroxytryptamine; 5-HIAA, 5-hydroxyindoleacetic acid; ThioT, thioflavin T; TEM, transmission electron spectroscopy; DLS, dynamic light scattering; NAC-region, non-Aβ component region
Keywords: Protein misfolding, Amyloid, Aggregation, Parkinson disease, Neurodegeneration, Indoleamine
Research highlights
► The neurotransmitter serotonin (5-HT) suppresses amyloid fibril growth of alpha-synuclein (AS). ► 5-HT binds to intermediate aggregates of alpha-synuclein, not to monomeric AS. Consequently, 5-HT does not influence initial steps of amyloidogenesis. ► 5-HT promotes the accumulation of partially structured, SDS-resistant “on pathway” aggregates of AS. ► The C-terminal region of AS is essential for a charge dependent interaction. ► “On pathway” and “off-pathway” aggregations of AS might mechanistically overlap.
1. Introduction
The 14 kDa polypeptide α-synuclein (AS) is a signature protein of Parkinson disease (PD), a neuromotor disorder which is characterized by the death of dopaminergic neurons within the substantia nigra of the brain [1]. AS is the major constituent of proteinaceous amyloidogenic inclusions known as Lewy bodies, which are the histological hallmarks of PD [2–4].
AS is intrinsically unstructured [5]. It is composed of an N-terminal amphipathic region, a central aggregation prone NAC (non-Aβ-component) hydrophobic region, and an acidic C-terminal region. The high degree of conformational freedom within these regions influences folding and aggregation of AS, two processes that closely couple this molecule to neurotoxicity [6]. While AS adopts β-sheet conformation in amyloid fibrils [7], soluble folding intermediates assemble into aggregates of different size and shape [8–14]. A growing number of studies suggest that the neurotoxic agents of the disease are soluble, intermediately folded AS-oligomers rather than amyloid fibrils [8–13,15]. The generation of soluble aggregates depends on a number of factors such as natural mutations of the AS gene, molecular chaperones, and the interaction with several chemically and functionally unrelated small molecule ligands [6]. The non-homogeneous nature of these folding intermediates is reflected in their variable cytotoxic activity. Some conditions force the accumulation of non-toxic aggregates [16,17], while others promote pronouncedly neurotoxic species [9,12,13].
Several studies suggest that, in PD, the crucial interplay between AS and the neurotransmitter dopamine, dopamine oxidation intermediates, and other catechols promotes the generation of distinct aggregates and they may represent toxic species triggering dopaminergic cell death [18–25] and ultimately neuromotoric impairment. Intriguingly, it was suggested that an indolic oxidation intermediate of dopamine, namely 5,6-dihydroxylindole, interferes with the assembly of AS rather than dopamine itself [26,27]. Indole-scaffold derivatives affecting the assembly of different amyloid forming proteins have also been identified in various small molecule screening assays [28–32]. The indoleamine neurotransmitter serotonin (5-HT) [29] captured our particular attention, since in neurodegenerative diseases, altered levels of 5-HT are linked to neuropsychiatric impairments such as sleep abnormalities, depression, and anxiety [33–36]. In PD, altered 5-HT metabolism may be linked to the non-motor symptoms of the disease [35]. This led us to investigate whether 5-HT affects the folding and aggregation of AS, in analogous manner to what has been established for dopamine and its derivatives.
We found that, in an in vitro isolated system, 5-HT binds to and stabilizes AS aggregates of a heterogeneous composition, which consequently accumulate instead of growing into mature fibrils. This effect is particularly sensitive to charge perturbations, and we identify a prevalent role for electrostatic interaction with the C-terminal region of AS.
2. Materials and methods
Unless specified otherwise, all reagents were from Sigma (St. Louis, MO).
2.1. Protein expression and purification
The pRSETB vectors containing the human AS gene and the ASΔ96 gene were transformed into BL21 (DE3) Star cells (Invitrogen, Carlsbad, CA). AS was expressed in LB medium containing 100 μg/ml ampicillin. 15N-labeled AS (15N-AS) was expressed in minimal medium (6.8 g/l Na2HPO4, 3 g/l KH2PO4, 0.5 g/l NaCl, 1.5 g/l (15NH4)2SO4, 2 g/l glucose, 1 μg/l biotin, 1 μg/l thiamin, 100 μg/ml ampicillin, and 1 ml 1000× microsalts).
Cultures were grown at 37 °C to an OD600 of 0.7. The protein expression was induced by the addition of 1 mM IPTG. After 4 h, cells were harvested by centrifugation at 6000g for 15 min at 4 °C. The cells were resuspended in 20 mM Tris–HCl, 50 mM NaCl, pH 7.4 (buffer A), supplemented with a Complete® protease inhibitor mix (Roche, Basel, Switzerland) and disrupted on ice by sonification. After centrifugation, an acid precipitation step was performed by lowering the pH to 4 and stirring for 20 min at 4 °C. The precipitate was removed by centrifugation at 20,000g for 1 h and the supernatant was loaded onto a Source Q column which was previously equilibrated with buffer A. The elution was carried out using a gradient from 50 mM to 600 mM NaCl. The fractions containing protein were pooled, loaded on a Superdex 75 gel filtration column (GE Healthcare, Uppsala, Sweden), and eluted with H2O. The purified protein was aliquoted, lyophilized, and stored at − 80 °C.
ASΔ96 was expressed in LB-medium containing 100 μg/ml ampicillin. The purification was similar to that of AS, except that a heat precipitation step was performed instead of acid precipitation (80 °C, 10 min under stirring). Also, an SP-sepharose column (GE Healthcare, Uppsala, Sweden) was used instead of a Source Q column.
2.2. Thioflavin T (ThioT) and turbidity measurements
Unless otherwise described, a 100 μM AS or ASΔ96 solution in 20 mM Tris–HCl, 150 mM NaCl, 3 mM NaN3, pH 7.4, was agitated in a thermomixer (Eppendorf, Hamburg, Germany) at 37 °C and 1200 rpm. Ten microliter aliquots were taken at the indicated time points and diluted into a five micromolar ThioT solution prepared in the same buffer. Spectra of the samples were recorded on a Cary Eclipse fluorescence spectrometer (Varian, Palo Alto, CA) at 25 °C and 482 nm upon excitation at 442 nm. The slit widths were set to 10 nm and 20 nm for excitation and emission, respectively.
The turbidity of the same samples was measured on a Cary Eclipse fluorescence spectrometer (Varian, Palo Alto, CA) at 25 °C with excitation and emission wavelengths set at 360 nm using a slit width of 5 nm for both excitation and emission.
The values are averages including S.E. of three independent determinations.
2.3. Circular dichroism
Far-UV-circular dichroism measurements were performed at 25 °C on a J-715 spectropolarimeter (Jasco, Tokyo, Japan) with a response time of 2 s and with a data point resolution of 0.1 nm using a 0.1 cm quartz cuvette. The spectra were recorded by diluting the samples in 20 mM NaPi, pH 7.4. Three scans were averaged to obtain smooth spectra.
To eliminate the signal contribution of residual monomeric AS from the aggregate spectrum, the ratio of monomer to oligomer AS was determined by 1H-NMR under the assumption that no other species is present in solution. This was then subtracted from the original spectrum.
Mean residue ellipticity calculations and secondary structure deconvolution analysis were performed on the DICHROWEB platform [37] by applying the SELCON3 algorithm [38].
2.4. Dynamic light scattering (DLS)
Stokes radii were determined using a DynaPro instrument (Protein Solutions, Lakewood, NJ) in a 1.5 mm pathlength 12 μl quartz cuvette at 25 °C. Samples were centrifuged before measuring. The values are averages including S.E. of three independent determinations.
2.5. NMR spectroscopy
All NMR spectra were acquired at 300 K on a Bruker Avance DRX 500 MHz NMR spectrometer equipped with a 5 mm TXI triple-resonance probe and z-axis gradients. One-dimensional 1H-NMR spectra were recorded on a solution containing 50 μM 5-HT and 100 μM AS in 20 mM Tris–d11, 150 mM NaCl, 3 mM NaN3, pH 7.4, and containing 10% (vol./vol.) of D2O. The solution was shaken for 7 days at 1200 rpm at 37 °C. Every 24 h, a one-dimensional 1H NMR spectrum with Watergate solvent suppression was acquired with 1024 scans. Two-dimensional 1H-15N-HSQC spectra were recorded on a 100 μM AS solution in 50 mM NaPi, 50 mM NaCl, 3 mM NaN3, pH 6.5, in the absence and in the presence of 1 mM 5-HT. Ten percent D2O were added for field-frequency locking. Data were processed using NMRPipe [39].
2.6. Transmission electron spectroscopy
A protein suspension was placed onto carbon-coated grids, blot dried, and rinsed in distilled water for 1 min. Negative staining was performed by applying 1% uranyl acetate for 1 min and blotting the liquid with filter paper. Afterwards, the samples were air-dried and viewed with a Zeiss EM 901 transmission electron microscope.
2.7. Ultrafiltration assays
A 100 μM AS solution in 20 mM Tris–HCl, 150 mM NaCl, pH 7.4, was agitated in a thermomixer (Eppendorf, Hamburg, Germany) at 37 °C and 1200 rpm in the presence of equimolar amounts of 5-HT. Fifty microliters of the solution were then removed at given times and spun at 13,000g for 5 min through an Amicon Ultra-0.5 centrifugal filter device (Millipore, Billerica, MA) with a membrane cutoff of 3 kDa. The amount of 5-HT recovered in the flow-through was detected by fluorescence emission spectroscopy after excitation of 295 nm. Emission spectra were recorded between 300 and 400 nm on a Cary Eclipse fluorescence spectrometer.
2.8. Denaturing SDS–PAGE
A 100 μM AS solution in 20 mM Tris–HCl, 150 mM NaCl, 3 mM NaN3, pH 7.4, was agitated at 37 °C and 1200 rpm in the absence and presence of 1 mM 5-HT. Two microliter aliquots were collected at given times and resolved on a 4%–12% SDS–PAGE gel. Proteins were visualized by silver staining.
3. Results
3.1. 5-HT promotes the accumulation of partially structured aggregates
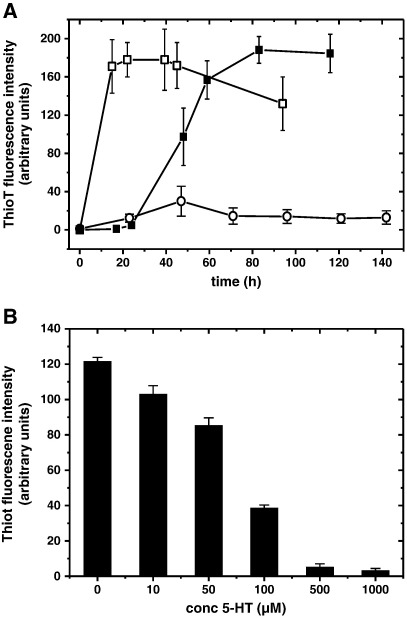
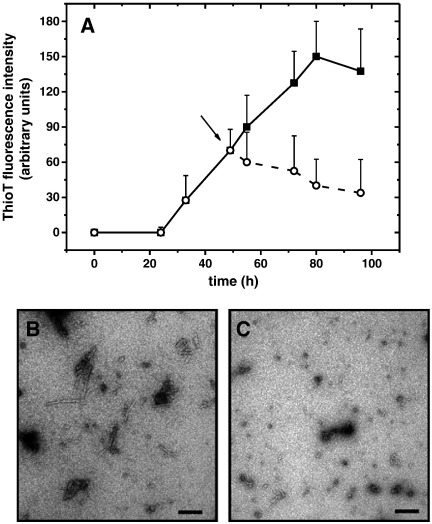
We first tested whether 5-HT was able to influence the growth of AS amyloid fibrils by agitating an AS sample at 37 °C over several days. Fibril growth was monitored by the dye Thioflavin T (ThioT), which exhibits a fluorescence emission maximum at 482 nm when bound to mature fibrils but not to monomeric or pre-fibrillar species [40]. ThioT fluorescence increased in the absence of 5-HT indicated that fibril growth proceeded following an initial lag phase, reaching a plateau around 80 h (Fig. 1A). ThioT fluorescence was suppressed when a 10-fold excess of 5-HT was present (Fig. 1A). The addition of different amounts of 5-HT to an agitating AS solution (Fig. 1B) showed that 5-HT was able to abolish the assembly of AS into mature amyloid fibrils in a concentration dependent way, even at sub-stoichiometric levels. Unless explicitly stated, the following experiments were all performed at a 10-fold 5-HT excess to ensure complete AS saturation.
Fig. 1.
(A) ThioT amyloid growth kinetics of 100 μM AS in the absence (■) and in the presence of 1 mM 5-HT (○); 100 μM AS seeded with approximately 100 nM of 5-HT induced aggregates (□). (B) ThioT fluorescence of 100 μM AS after 5 days in dependence of increasing concentrations of 5-HT.
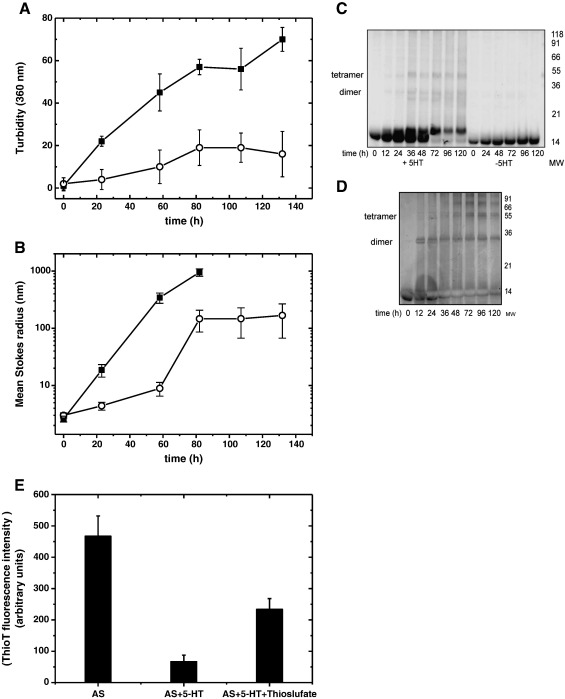
In parallel to ThioT fluorescence, the turbidity of the sample solutions was measured, which defines the formation of light scattering particles. The turbidity of the AS solution in the absence of 5-HT (Fig. 2A) increased already during the ThioT lag-phase (Fig. 1A). This is in accordance to previous studies [41,42], which describe the build-up of pre-amyloid, ThioT-negative aggregates preceding fibril assembly. A less prominent increase in turbidity was observed in the presence of 5-HT, suggesting the accumulation of non-amyloidogenic aggregates.
Fig. 2.
(A) Turbidity of 100 μM AS in the absence (■) and presence of 1 mM 5-HT (○). (B) DLS of 100 μM AS in the absence (■) and presence of 1 mM 5-HT (○). The mean Stokes radii of the predominating AS species of each sample were plotted against the time. (C) Denaturing SDS–PAGE aggregation profile of 100 μM AS agitating at 37 °C shows that SDS-resistant species appear over time in the presence of 1 mM 5-HT. (D) Denaturing SDS–PAGE aggregation profile of 100 μM AS agitating at 37 °C in the presence of 10 mM sodium thiosulfate. (E) The amyloid inhibiting effect of 5-HT measured at t = 100 h in the absence and in the presence of 10 mM of the reducing agent sodium thiosulfate.
We employed dynamic light scattering (DLS) for a more detailed analysis of the size composition of these aggregates. Fig. 2B shows the Stokes radii of the most prominent AS aggregates appearing in solution over time in the absence or in the presence of 5-HT. Monomeric AS had a Stokes radius of 2.6 nm, consistent with previous reports [43]. We also observed for AS alone that higher order species with mean radii up to 1 μm were generated over time, corresponding to the assembly of amyloid fibrils. The addition of 5-HT prevented the maturation of these amyloid fibrils in favor of aggregates with an initial mean size of up to 10 nm, which increased to approximatively 150 nm within 3–4 days (Fig. 2B). As shown by SDS–PAGE analysis, these aggregates were composed of differently sized SDS-resistant oligomers (Fig. 2C). Ultracentrifugation analysis indicated that the aggregates partitioned in the soluble fraction (Fig. S1). Therefore, 5-HT does not suppress the assembly of AS completely but rather promotes the accumulation of soluble intermediate aggregates instead of mature amyloid fibrils, as suggested for other ligands [17,19,23,44].
Fig. 2C also shows a time-dependent shift of monomeric AS to a slightly higher sized band. We speculate that this might derive from a covalent reaction between AS and an oxidized derivative of 5-HT. In support to this, we noticed that this band shift was not observed under reducing conditions (Fig. 2D), suggesting that a covalent 5-HT:AS adduct might indeed form during the reaction. However, this adduct would not account for fibril suppression as 5-HT was still able to suppress amyloid growth in the presence of the reducing agent thiosulfate (Fig. 2E) [45]. Also, SDS-resistant dimer and tetramer bands appeared even under reducing conditions (Fig. 2D). These results both indicate that 5-HT was able to efficiently suppress fibril formation independently of an oxidative adduct accumulating during the reaction.
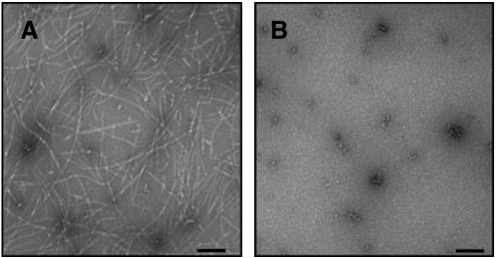
To visualize the morphology of the observed aggregates, we recorded TEM images of samples collected at the end of the amyloid growth curve. In the absence of 5-HT, the sample was composed predominantly of entangled fibrils of varying length and approximate 5 nm width, along with a minor amount of smaller globular aggregates (Fig. 3A). In the presence of 5-HT, heterogeneously sized, small spherical (< 50 nm) and large granular (100–200 nm) aggregates were the predominant species (Fig. 3B).
Fig. 3.
TEM images of 100 μM AS in the absence (A) and in the presence (B) of 1 mM 5-HT. Samples were collected after 7 days agitation at 37 °C, 1200 rpm. Scale bars correspond to 200 nm.
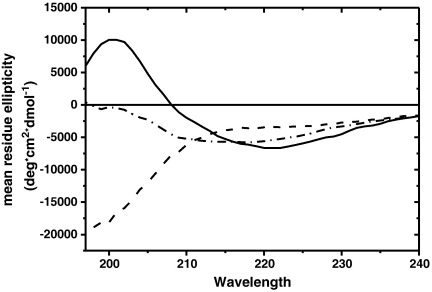
In order to structurally characterize these aggregates, we used far-UV circular dichroism spectroscopy which proved very useful to distinguish the secondary structure conformation of differently folded AS species [46]. The spectrum of monomeric AS featured intrinsically unstructured patterns, i.e., a minimal signal readout, followed by a strong negative signal minimum below 210 nm (Fig. 4, dashed line). In contrast, the CD spectrum of mature AS amyloid fibrils was typical of β-sheets (Fig. 4, solid line), which corresponds to the conformation that AS adopts in amyloid fibers [7]. The signal showed a negative minimum around 220 nm and a positive ellipticity below 210 nm. For 5-HT generated AS aggregates, we observed a prominent CD-signal increase below 210 nm and a slight decrease of the CD-signal around 220 nm, compared to monomeric AS (Fig. 4, dash-dotted line). This corresponded to a decrease of the unstructured signal component in favor of the structured component, accounting for a reduced conformational flexibility of AS within these aggregates. As shown by secondary structure deconvolution of the respective CD spectra (Table 1), structural disorder decreased from 91.9% for monomeric AS to 56.8% for intermediate aggregates. This was paralleled by a gradual increase of the beta-sheet component from 2.1% for monomeric AS to 34.9% for the intermediate aggregates and finally to 59.8% for the amyloid fiber sample. This indicates that the aggregates observed in the presence of 5-HT are partially structured and display a significant beta-sheet structural content.
Fig. 4.
CD spectra of 10 μM unagitated AS (dashed line); AS fibrils (solid line) and AS:5-HT = 1:10 (dash-dotted line; corrected with respect to the residual monomeric component) after 100 h agitation at 37 °C, 1200 rpm.
Table 1.
Secondary structure deconvolution analysis of the CD spectra as shown in Fig. 4.
| α-Helix | β-Sheet | Unstructured | |
|---|---|---|---|
| Monomeric AS | 6.8% | 2.1% | 91.9% |
| 5-HT-induced aggregates | 10.3% | 32.2% | 56.8% |
| Amyloid fibers | 5.8% | 59.8% | 34.2% |
We noticed that, in aging aggregate preparations stored at RT containing AS and 5-HT, short amyloid fibrils were slowly formed after 6–8 weeks (Fig. S2(A)). We thus assumed that the observed aggregates might represent kinetically stabilized “on-pathway” components of amyloid assembly. To discriminate between “on-pathway” and “dead-end,” we examined if they could act as nucleation seeds to promote AS amyloidogenesis. Nucleation consists of the early formation of transient, pre-fibrillar intermediates, which then convert into amyloids [6]. For this purpose, we induced fibril growth in the absence and in the presence of 5-HT-generated aggregates. Fig. 1A and Fig. S2(B) show that amyloidogenesis was accelerated when these aggregates were used for seeding, identifying them as “on-pathway.”
3.2. 5-HT binds to intermediate aggregates
We observed that sub-stoichiometric amounts of 5-HT were sufficient to affect AS amyloidogenesis (Fig. 1B and Fig. S2(C)). Also, fibril growth was interrupted by the addition of 5-HT during the amyloid mid-growth phase (Fig. 5). This led us to suppose that, rather than binding to monomeric AS, this neurotransmitter binds to and stabilizes intermediate AS aggregates once they have formed.
Fig. 5.
(A) ThioT amyloid growth kinetics of 100 μM AS (■), AS:5-HT = 1:10, with 5-HT added at mid-amyloid growth (arrow) (○). (B) TEM images of 100 μM AS at mid-amyloid growth in the absence of 5-HT and (C) after 100 h in the presence of 1 mM 5-HT added at mid-amyloid growth. Scale bars correspond to 200 nm.
To prove our hypothesis, we used two-dimensional heteronuclear correlation NMR spectroscopy, which is suitable for measuring binding of a given ligand to a protein. The 1H-15N HSQC spectrum of monomeric AS recorded at 25 °C showed limited signal dispersion typical for intrinsically unstructured proteins (Fig. S3). For 5-HT binding to monomeric 15N-AS, we expected significant shifts or intensity changes for some of the signals, as previously observed for other AS ligands [16,17,47,48]. However, this did not occur upon the addition of 5-HT, as the spectra are virtually unchanged. This argues against binding of 5-HT to AS monomers.
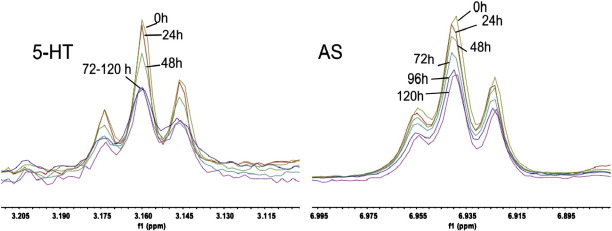
We expected that the 1H-resonance signals of 5-HT should be sensitive to an interaction with AS aggregates. To show this, we recorded 1H-NMR spectra of an agitating 5-HT:AS mixture and monitored signal variations over time. The signals of both 5-HT (Fig. 6, left) and AS (Fig. 6, right) displayed decreasing intensity within the first 4 days of agitation. The decrease in AS signal intensities was indicative of aggregate formation. As these are well beyond the NMR size limit, they yield extremely broad and undetectable signals. Their formation can thus be seen indirectly in the NMR spectra by the decrease of signal intensities. For 5-HT, a significant reduction of resonance intensities was observable only after 48 h, which can be explained by binding of 5-HT to accumulating aggregates rather than to monomers. A signal decrease was not observed for 5-HT in the absence of AS (Fig. S4). Since there were no detectable chemical shift changes of 5-HT and we did not find a saturation transfer difference (STD) effect on 5-HT (see supplementary data, Fig. S5), the binding of 5-HT to AS aggregates has to be relatively tight (Kd < mM). By finding the same results under reducing conditions, we could exclude that a covalent interaction between AS and an oxidized derivative of 5-HT might prevent STD (see supplementary data, Fig. S5).
Fig. 6.
Overlay of one-dimensional 1H spectra of 10 μM 5-HT agitated with 100 μM AS at 37 °C. The well separated signal of the 1-CH2 group of the 2-aminoethyl chain of 5-HT (left) and an aromatic side chain signal of AS (right) were taken as representative signals.
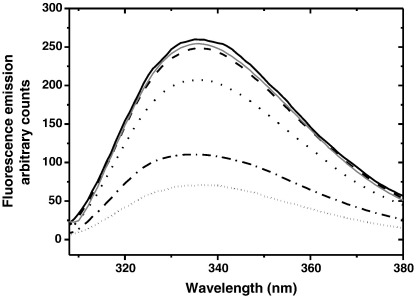
An analogous result was observed by ultrafiltration analysis (Fig. 7). AS was agitated in the presence of 5-HT, and aliquots collected at different times of amyloidogenesis were centrifuged through an ultrafiltration device with a membrane cutoff of 3 kDa, which is permeable for unbound 5-HT but not for 5-HT bound to AS. As a consequence, binding of 5-HT to AS was expected to reduce the recovery of free 5-HT in the flow-through. The assay shows that monomeric AS (t = 30 min) was not able to retain 5-HT, which was recovered in the filtrate. In contrast, the recovery of 5-HT was significantly lower (74% less) for a sample collected at t = 100 h, indicating that a considerable amount of 5-HT remained bound to forming aggregates. These results show that 5-HT binds to AS aggregates rather than to monomeric AS.
Fig. 7.
Fluorescence emission spectra of 5-HT recovered in the flow through after ultrafiltration through a 3 kDa cutoff membrane: 5-HT in the absence of AS after 0 h (black solid line) and after 100 h agitation at 37 °C, 1200 rpm (dark gray solid line). 5-HT in the presence of AS after agitation at 37 °C, 1200 rpm for 30 min (dashed line), 24 h (dotted line), 48 h (dash-dotted line), and 100 h (short dotted line).
3.3. The interaction between 5-HT and AS is charge sensitive
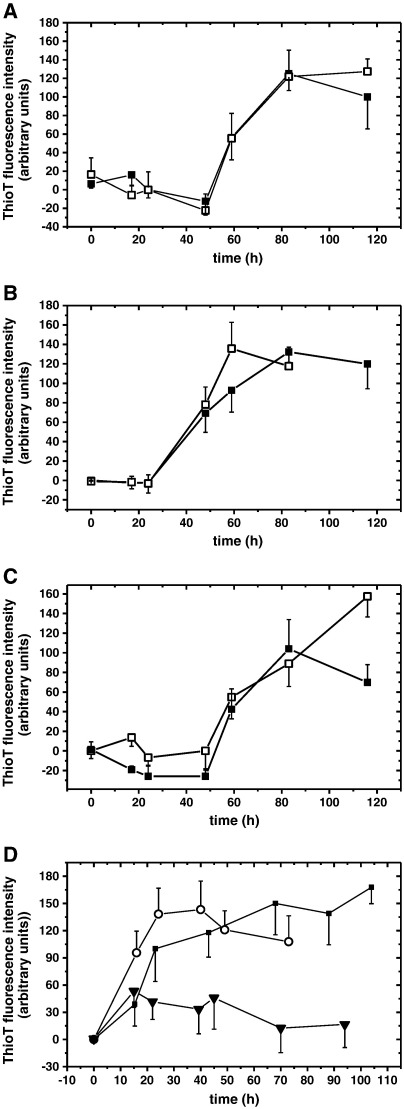
The above results indicate that the 5-HT binding regions might become exposed during amyloidogenesis. The acidic C-terminal region of AS becomes more accessible after the release of long-range contacts with the NAC core domain [49,50] and ligands such as catechols, polyamines, and some cations target this region [48,51–53]. To test if 5-HT binds to the C-terminal region, we tested an AS truncation mutant (ASΔ96) lacking the whole C-terminal region (aa 97–140). Fig. 8A shows the amyloid growth curve of ASΔ96, in the absence and in the presence of 5-HT. In direct contrast to what we observed for full-length AS (Fig. 1), 5-HT had a minimal effect on ASΔ96 amyloid growth (see also Fig. S2(D) and Fig. S2(E)). This suggests that the C-terminal region of AS is required for the efficient suppression of 5-HT mediated amyloid growth.
Fig. 8.
ThioT amyloid growth kinetics of (A) 150 μM ASΔ96 in the absence (■) and in the presence of 1 mM 5-HT (□); (B) 100 μM AS in the absence (■) and in the presence of 1 mM 5-HT (□) with 300 mM NaCl; (C) 100 μM AS in the absence (■) and in the presence of 1 mM 5-HT (□) at pH 11.6; (D) 100 μM AS in the absence (■) and in the presence of 1 mM 5,7-HT (▼) or 1 mM 5-HIAA (○).
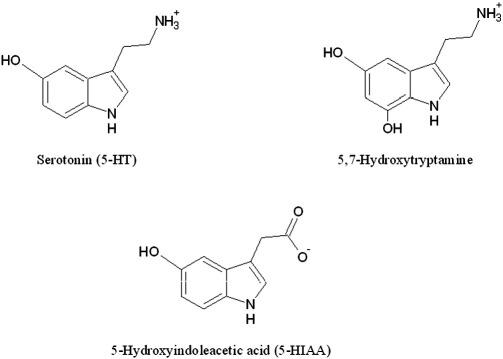
As the C-terminal region of AS has a negative net charge and 5-HT is cationic at physiological pH [54], electrostatic forces might drive the interaction. This was indeed the case since 5-HT was no longer able to inhibit amyloidogenesis when the ionic strength of the solution was increased (300 mM NaCl) (Fig. 8B, Fig. S2(F), Fig. S2(G)). A similar effect was observed after neutralizing the positive charge of the aliphatic 5-HT amino group by increasing the pH of the buffer above its pKa-value of 9.97 (Fig. 8C, Fig. S2(H), Fig. S2(I)) [54]. 5-HT scaffold modifications provided further indications for charge effects. 5,7-hydroxytryptamine (5,7-HT), which differs from 5-HT by an additional hydroxy group at the aromatic ring (Fig. 9), was also able to suppress amyloid assembly (Fig. 8D and Fig. S2(J)). In contrast, 5-hydroxyindoleacetic acid (5-HIAA), a 5-HT metabolite where the positively charged aliphatic amino group of 5-HT is replaced by a negatively charged carboxy group (Fig. 9), did not suppress amyloid growth (Fig. 8D and Fig. S2(K)). In contrast, dopamine mediated inhibition of AS fibrillization is not significantly modulated by electrostatic forces since high salt conditions (500 mM NaCl) had no effect [26]. Collectively, these results demonstrate that charge perturbations critically affect the ability of 5-HT to efficiently interfere with AS amyloidogenesis.
Fig. 9.
Molecular formulae of the three compounds used in this study.
4. Discussion
The intrinsic structural disorder of the PD key protein AS accounts for ligand binding promiscuity and conformational variance. Both issues are coupled to the generation of pathogenic AS aggregates. Yet, the exact circumstances dictating the formation of these species are unclear. We demonstrated that the neurotransmitter 5-HT profoundly influenced amyloidogenesis of AS, which was altered in favor of “on-pathway” intermediates.
We show that 5-HT targets the C-terminal region of AS, as reported for dopamine and other functionally unrelated ligands [48,51–53]. In contrast to them, 5-HT does not bind directly to monomeric AS molecules. Consequently, 5-HT does not influence initial steps of amyloidogenesis. Rather, 5-HT intervenes in the AS fibril assembly at a given intermediate growth stage, when partially folded soluble aggregates have already accumulated. This interrupts their further maturation. Reinke et al. [32] proposed a similar mechanism for a set of indolic derivatives which bind preferentially to pre-amyloid intermediates of the Alzheimer disease peptide Aβ. This suggests common interaction properties between indoles and different amyloid-forming proteins. The link between 5-HT and the non-motor symptoms of PD [33,35] might account for a pathophysiological relationship between this particular neurotransmitter and AS in PD.
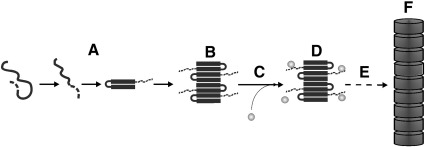
Based on our results, we outline a model for the 5-HT-mediated AS aggregation (Fig. 10). The initial phase involves the 5-HT-independent interaction of AS monomers to form the early small intermediates (Fig. 10A). This might be initiated by the loosening of intramolecular constraints between the C-terminal region and the hydrophobic core region, as proposed by Dedmon et al. [49,50]. The C-terminal region is not assembled within the β-sheet scaffold which is generated during fiber elongation but remains rather unstructured and solvent exposed (Fig. 10B) [55,56]. We suggest that this facilitates the consequent interaction with 5-HT (gray spheres) (Fig. 10C), which takes place during the pre-fibrillar phase. Since the C-terminal region of AS is critical for the further maturation of pre-fibrils into amyloid fibers [55], it is likely that binding of 5-HT to this region interferes with the assembly of mature fibrils (Fig. 10F). As a result, fibril generation is inhibited (Fig. 10E), resulting in the accumulation of the partially structured and kinetically stabilized intermediate aggregates (Fig. 10D).
Fig. 10.
An in vitro model for the 5-HT-modulated suppression of AS amyloidogenesis. In a first step, amyloid-promoting conformational rearrangements of AS occur (A). During the consequent build-up of intermediate aggregates (B), the C-terminal region of AS (dashed line) remains solvent exposed [55,56]. 5-HT molecules (gray spheres) bind to the C-terminal region (C) and arrest the build-up of mature amyloid fibrils (F). Instead, intermediate, partially structured, toxic aggregates become stabilized (D). Their conversion into amyloid fibrils is kinetically disfavored (E).
It remains to be established whether the 5-HT/AS interaction is either pathological or physiologically relevant in vivo. Despite the promotion of “on pathway” aggregation by 5-HT, the occurrence of SDS-resistant oligomeric intermediates (Fig. 2C) similar to those observed for dopamine induced “off-pathway” aggregation [17,26] suggests some mechanistic overlap between these two processes. We speculate that an interaction between 5-HT and AS in serotonergic neurons might trigger neurotoxic effects paralleling those suggested for dopamine and AS in dopaminergic neurons. This is supported by the occurrence of AS-positive Lewy inclusions in serotonergic neurons [57], which might imply molecular events similar to those eventually leading to dopaminergic cell death.
The following are the supplementary materials related to this article.
Acknowledgments
This work was supported by the Austrian Science Fund (project no. P22400 to SFF and P20020 to KZ). RC is an NHMRC Senior Research Fellow. DICHROWEB is supported by grants to the BBSRC Centre for Protein and Membrane Structure and Dynamics (CPMSD).
References
- 1.Lees A.J., Hardy J., Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M., Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc. Natl Acad. Sci. USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windisch M., Wolf H., Hutter-Paier B., Wronski R. The role of alpha-synuclein in neurodegenerative diseases: a potential target for new treatment strategies? Neurodegener. Dis. 2008;5:218–221. doi: 10.1159/000113707. [DOI] [PubMed] [Google Scholar]
- 5.Weinreb P.H., Zhen W., Poon A.W., Conway K.A., Lansbury P.T. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 6.Uversky V.N. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J. Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 7.Vilar M., Chou H.T., Lührs T., Maji S.K., Riek-Loher D., Verel R., Manning G., Stahlberg H., Riek R. The fold of alpha-synuclein fibrils. Proc. Natl Acad. Sci. USA. 2008;105:8637–8642. doi: 10.1073/pnas.0712179105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayed R., Head E., Thompson J.L., McIntire T.M., Milton S.C., Cotman C.W., Glabe C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 9.Lashuel H.A., Hartley D., Petre B.M., Walz T., Lansbury P.T., Jr. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 10.Outeiro T.F., Putcha P., Tetzlaff J.E., Spoelgen R., Koker M., Carvalho F., Hyman B.T., McLean P.J. Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS ONE. 2008;4:e1867. doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsika E., Moysidou M., Guo J., Cushman M., Gannon P., Sandaltzopoulos R., Giasson B.I., Krainc D., Ischiropoulos H., Mazzulli J.R. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J. Neurosci. 2010;30:3409-3344. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danzer K.M., Haasen D., Karow A.R., Moussaud S., Habeck M., Giese A., Kretzschmar H., Hengerer B., Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright J.A., Wang X., Brown D.R. Unique copper-induced oligomers mediate alpha-synuclein toxicity. FASEB J. 2009;23:2384–2393. doi: 10.1096/fj.09-130039. [DOI] [PubMed] [Google Scholar]
- 14.Kim H.Y., Cho M.K., Kumar A., Maier E., Siebenhaar C., Becker S., Fernandez C.O., Lashuel H.A., Benz R., Lange A., Zweckstetter M. Structural properties of pore-forming oligomers of alpha-synuclein. J. Am. Chem. Soc. 2009;131:17482–17489. doi: 10.1021/ja9077599. [DOI] [PubMed] [Google Scholar]
- 15.Karpinar D.P., Balija M.B., Kügler S., Opazo F., Rezaei-Ghaleh N., Wender N., Kim H.Y., Taschenberger G., Falkenburger B.H., Heise H., Kumar A., Riedel D., Fichtner L., Voigt A., Braus G.H., Giller K., Becker S., Herzig A., Baldus M., Jäckle H., Eimer S., Schulz J.B., Griesinger C., Zweckstetter M. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson's disease models. EMBO J. 2009;28:3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamberto G.R., Binolfi A., Orcellet M.L., Bertoncini C.W., Zweckstetter M., Griesinger C., Fernández C.O. Structural and mechanistic basis behind the inhibitory interaction of PcTS on alpha-synuclein amyloid fibril formation. Proc Natl Acad Sci USA. 2009;106:21057–21062. doi: 10.1073/pnas.0902603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrnhoefer D.E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 18.Leong S.L., Cappai R., Barnham K.J., Pham C.L. Modulation of alpha-synuclein aggregation by dopamine: a review. Neurochem. Res. 2009;34:1838–1846. doi: 10.1007/s11064-009-9986-8. [DOI] [PubMed] [Google Scholar]
- 19.Cappai R., Leck S.L., Tew D.J., Williamson N.A., Smith D.P., Galatis D., Sharples R.A., Curtain C.C., Ali F.E., Cherny R.A., Culvenor J.G., Bottomley S.P., Masters C.L., Barnham K.J., Hill A.F. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19:1377–1379. doi: 10.1096/fj.04-3437fje. [DOI] [PubMed] [Google Scholar]
- 20.Conway K.A., Rochet J.C., Bieganski R.M., Lansbury P.T. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 21.Periquet M., Fulga T., Myllykangas L., Schlossmacher M.G., Feany M.B. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J. Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Outeiro T.F., Klucken J., Bercury K., Tetzlaff J., Putcha P., Oliveira L.M., Quintas A., McLean P.J., Hyman B.T. Dopamine-induced conformational changes in alpha-synuclein. PLoS ONE. 2009;4:e6906. doi: 10.1371/journal.pone.0006906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norris E.H., Giasson B.I., Hodara R., Xu S., Trojanowski J.Q., Ischiropoulos H., Lee V.M. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J. Biol. Chem. 2005;280:21212–21219. doi: 10.1074/jbc.M412621200. [DOI] [PubMed] [Google Scholar]
- 24.Mazzulli J.R., Mishizen A.J., Giasson B.I., Lynch D.R., Thomas S.A., Nakashima A., Nagatsu T., Ota A., Ischiropoulos H. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J. Neurosci. 2006;26:10068–10078. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alves da Costa C., Dunys J., Brau F., Wilk S., Cappai R., Checler F. 6-Hydroxydopamine but not 1-methyl-4-phenylpyridinium abolishes alpha-synuclein antiapoptotic phenotype by inhibiting its proteasomal degradation and by promoting its aggregation. J. Biol. Chem. 2006;281:9824–9831. doi: 10.1074/jbc.M513903200. [DOI] [PubMed] [Google Scholar]
- 26.Pham C.L., Leong S.L., Ali F.E., Kenche V.B., Hill A.F., Gras S.L., Barnham K.J., Cappai R. Dopamine and the dopamine oxidation product 5,6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of alpha-synuclein in a pH-dependent manner. J. Mol. Biol. 2009;387:771–785. doi: 10.1016/j.jmb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Bisaglia M., Mammi S., Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J. Biol. Chem. 2007;282:15597–15605. doi: 10.1074/jbc.M610893200. [DOI] [PubMed] [Google Scholar]
- 28.Latawiec D., Herrera F., Bek A., Losasso V., Candotti M., Benetti F., Carlino E., Kranjc A., Lazzarino M., Gustincich S., Carloni P., Legname G. Modulation of alpha-synuclein aggregation by dopamine analogs. PLoS ONE. 2010;5:e9234. doi: 10.1371/journal.pone.0009234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inbar P., Bautista M.R., Takayama S.A., Yang J. Assay to screen for molecules that associate with Alzheimer's related beta-amyloid fibrils. Anal. Chem. 2008;80:3502–3506. doi: 10.1021/ac702592f. [DOI] [PubMed] [Google Scholar]
- 30.Morshedi D., Rezaei-Ghaleh N., Ebrahim-Habibi A., Ahmadian S., Nemat-Gorgani M. Inhibition of amyloid fibrillation of lysozyme by indole derivatives—possible mechanism of action. FEBS J. 2007;274:6415–6425. doi: 10.1111/j.1742-4658.2007.06158.x. [DOI] [PubMed] [Google Scholar]
- 31.Cohen T., Frydman-Marom A., Rechter M., Gazit E. Inhibition of amyloid fibril formation and cytotoxicity by hydroxyindole derivatives. Biochemistry. 2006;45:4727–4735. doi: 10.1021/bi051525c. [DOI] [PubMed] [Google Scholar]
- 32.Reinke A.A., She H.Y., Gestwicki J.E. A chemical screening approach reveals that indole fluorescence is quenched by pre-fibrillar but not fibrillar amyloid-beta. Bioorg. Med. Chem. Lett. 2009;19:4952–4957. doi: 10.1016/j.bmcl.2009.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox S.H., Chuang R., Brotchie J.M. Serotonin and Parkinson's disease: on movement, mood, and madness. Mov. Disord. 2009;24:1255–1266. doi: 10.1002/mds.22473. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri K.R., Schapira A.H. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 35.Taylor T.N., Caudle W.M., Shepherd K.R., Noorian A., Jackson C.R., Iuvone P.M., Weinshenker D., Greene J.G., Miller G.W. Nonmotor symptoms of Parkinson's disease revealed in an animal model with reduced monoamine storage capacity. J. Neurosci. 2009;29:8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasten M., Kertelge L., Brüggemann N., van der Vegt J., Schmidt A., Tadic V., Buhmann C., Steinlechner S., Behrens M.I., Ramirez A., Binkofski F., Siebner H., Raspe H., Hagenah J., Lencer R., Klein C. Nonmotor symptoms in genetic Parkinson disease. Arch. Neurol. 2010;67:670–676. doi: 10.1001/archneurol.67.6.670. [DOI] [PubMed] [Google Scholar]
- 37.Whitmore L., Wallace B.A. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers. 2008;89:392–400. doi: 10.1002/bip.20853. [DOI] [PubMed] [Google Scholar]
- 38.Sreerama N., Venyaminov S.Y., Woody R.W. Estimation of the number of helical and strand segments in proteins using CD spectroscopy. Protein Sci. 1999;8:370–380. doi: 10.1110/ps.8.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delaglio F., Grzesiek S., Vuister G.W., Zhu G., Pfeifer J., Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 40.LeVine H., III Quantification of beta-sheet amyloid fibril structures with thioflavin T. Meth. Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 41.Yamin G., Uversky V.N., Fink A.L. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 2003;542:147–152. doi: 10.1016/s0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Uversky V.N., Fink A.L. Conformational behavior of human alpha-synuclein is modulated by familial Parkinson's disease point mutations A30P and A53T. Neurotoxicology. 2002;23:553–567. doi: 10.1016/s0161-813x(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 43.Falsone S.F., Kungl A.J., Rek A., Cappai R., Zangger K. The molecular chaperone Hsp90 modulates intermediate steps of amyloid assembly of the Parkinson-related protein alpha-synuclein. J. Biol. Chem. 2009;284:31190–31199. doi: 10.1074/jbc.M109.057240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng X., Munishkina L.A., Fink A.L., Uversky V.N. Molecular mechanisms underlying the flavonoid-induced inhibition of alpha-synuclein fibrillation. Biochemistry. 2009;48:8206–8212. doi: 10.1021/bi900506b. [DOI] [PubMed] [Google Scholar]
- 45.Zhou W., Gallagher A., Hong D.P., Long C., Fink A.L., Uversky V.N. At low concentrations, 3,4-dihydroxyphenylacetic acid (DOPAC) binds non-covalently to alpha-synuclein and prevents its fibrillation. J. Mol. Biol. 2009;388:597–610. doi: 10.1016/j.jmb.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uversky V.N., Fink A.L. Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim. Biophys. Acta. 2004;1698:131–153. doi: 10.1016/j.bbapap.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Bodner C.R., Maltsev A.S., Dobson C.M., Bax A. Differential phospholipid binding of alpha-synuclein variants implicated in Parkinson's disease revealed by solution NMR spectroscopy. Biochemistry. 2010;49:862–871. doi: 10.1021/bi901723p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernández C.O., Hoyer W., Zweckstetter M., Jares-Erijman E.A., Subramaniam V., Griesinger C., Jovin T.M. NMR of alpha-synuclein–polyamine complexes elucidates the mechanism and kinetics of induced aggregation. EMBO J. 2004;23:2039–2046. doi: 10.1038/sj.emboj.7600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dedmon M.M., Lindorff-Larsen K., Christodoulou J., Vendruscolo M., Dobson C.M. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J. Am. Chem. Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 50.Bertoncini C.W., Jung Y.S., Fernandez C.O., Hoyer W., Griesinger C., Jovin T.M., Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc. Natl Acad. Sci. USA. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee E.N., Cho H.J., Lee C.H., Lee D., Chung K.C., Paik S.R. Dequalinium-induced protofibril formation of alpha-synuclein. J. Biol. Chem. 2006;281:3463–3472. doi: 10.1074/jbc.M505307200. [DOI] [PubMed] [Google Scholar]
- 52.Lowe R., Pountney D.L., Jensen P.H., Gai W.P., Voelcker N.H. Calcium(II) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 2004;13:3245–3252. doi: 10.1110/ps.04879704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazzulli J.R., Armakola M., Dumoulin M., Parastatidis I., Ischiropoulos H. Cellular oligomerization of alpha-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence. J. Biol. Chem. 2007;282:31621–31630. doi: 10.1074/jbc.M704737200. [DOI] [PubMed] [Google Scholar]
- 54.Pratuangdejkul J., Nosoongnoen W., Guerin G.A., Loric S., Conti M., Launay J.M., Manivet P. Conformational dependence of serotonin theoretical pK(a) prediction. Chem. Phys. Lett. 2006;420:538–544. [Google Scholar]
- 55.Qin Z., Hu D., Han S., Hong D.P., Fink A.L. Role of different regions of alpha-synuclein in the assembly of fibrils. Biochemistry. 2007;46:13322–13330. doi: 10.1021/bi7014053. [DOI] [PubMed] [Google Scholar]
- 56.Chen M., Margittai M., Chen J., Langen R. Investigation of alpha-synuclein fibril structure by site-directed spin labeling. J. Biol. Chem. 2007;282:24970–24979. doi: 10.1074/jbc.M700368200. [DOI] [PubMed] [Google Scholar]
- 57.Braak H., Ghebremedhin E., Rüb U., Bratzke H., Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.