Abstract
Background
This large population-based study was conducted to estimate the prevalence of Dupuytren’s disease in US adults and describe associated treatment patterns.
Methods
A total of 23,103 individuals from an Internet-based research panel representative of the US population completed a brief online survey designed to identify individuals with symptoms, diagnoses, and/or treatment experience indicative of Dupuytren’s disease (mean age = 50 years).
Results
The prevalence of Dupuytren’s disease defined as a self-reported physician diagnosis and/or surgical treatment was estimated as 1% (95% CI = 0.8–1.2), but the estimated prevalence is much higher (7.3%) when including self-reported symptoms of ropelike growth or hard bumps on the hand. The annual incidence proportion was estimated at about 3 cases per 10,000 adults. A total of 326 participants who reported relevant Dupuytren’s symptoms, treatment, and/or diagnosis completed a more in-depth survey focusing on timing of medical treatments after first symptom noticed, description of functional impairment, treatment patterns, and family history. From the second survey, most patients who reported seeking treatment for hand symptoms initially saw a primary care physician, and the mean time from noticing the first hand symptom to seeing a doctor was 23.1 months. At their first doctor visit for hand symptoms, only 9% of patients received a diagnosis of Dupuytren’s disease and 48% were advised to “wait and see” or received no treatment.
Conclusions
Results from the current study indicate a number of unmet medical needs, so strategies to raise physician awareness of disease symptoms and effective treatment options may be helpful.
Keywords: Dupuytren’s, Prevalence, Incidence, United States, Survey, Treatment, Epidemiology
Background
Dupuytren’s disease, also called Dupuytren’s contracture or palmar fibromatosis, is a condition in which the connective tissue under the skin of the palm contracts and toughens over time [2]. The fibroproliferative disorder affects the palmar fascia; ropelike collagen cords form, thicken, and shorten, causing permanent flexion contractures of joints and progressive flexion of one or more fingers. Typically, the metacarpophalangeal joint, the proximal interphalangeal joint, or both joints are involved, and contractures ultimately lead to hand deformity and impaired hand function [15]. The typical symptom is the presence of a nodule on the hand (a small knot or knob) followed by the formation of a ropelike growth on the palm (cord) and then permanent contracture of the fingers. Dupuytren’s disease usually presents in one hand first, and at some later time may appear in the other hand [2, 15]. Thickening of the lines in the palms of the hands or on knuckles and skin pitting and dimpling may also be present [21].
A recent review of epidemiologic studies of Dupuytren’s found that the majority have been conducted in Scandinavia or the UK [10]. Across the 42 studies included in the review, the prevalence estimates of Dupuytren’s disease varied from 0.2% [8] to 56% [4]; the large range was attributed to variation in age, population groups, and methods of data collection. Given that the prevalence of Dupuytren’s disease has been shown to have large geographic variability, likely due to genetic and environmental differences [10], the epidemiological data from other countries should not be applied to the US population. Using a Web-based survey design, the current study sought to estimate the prevalence and 1-year incidence proportion of Dupuytren’s disease in the general US population, describe hand symptom characteristics and functional impairment, and determine the time from first symptom noticed to medical treatment, treatment patterns, and family history.
Materials and Methods
Study Design
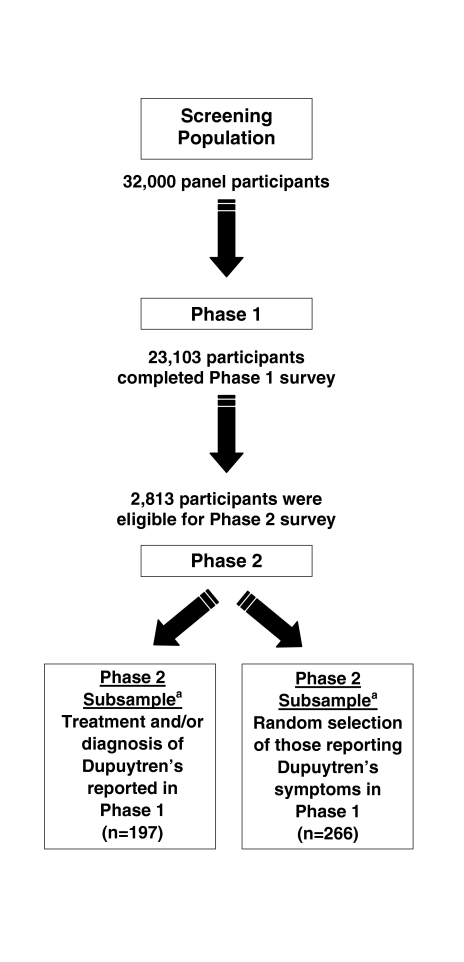
This cross-sectional, population-based survey was conducted in two phases (Fig. 1). In the initial phase (phase 1), panel participants from Knowledge Networks’ (KN) online panel were screened for the presence of current symptoms of Dupuytren’s disease, past surgical and/or needle aponeurotomy treatments, and a diagnosis of the disease. Data from phase 1 were used to estimate the prevalence of Dupuytren’s disease in the general US population and to determine eligibility to complete the full survey phase (phase 2). In phase 2, a subsample of participants meeting the eligibility criteria for Dupuytren’s disease were invited to complete the Dupuytren’s disease survey, which collected detailed information on severity of symptoms, timing and type of first symptoms noticed, treatment-seeking patterns, diagnoses, and brief medical and family histories. Phase 1 also collected information on Peyronie’s disease, and a parallel Peyronie’s disease survey was conducted in phase 2. Details and results regarding Peyronie’s disease are not reported in this paper, but will be reported in a future publication.
Fig. 1.
Study design. Data for this study were collected in two phases. In phase 1, panel participants were screened for the presence of symptoms, past treatment, and a diagnosis of Dupuytren’s disease. In phase 2, eligibility to complete the full survey was determined by participants’ responses to the screening items in phase 1. aParticipants for the Dupuytren’s phase 2 subsample populations were selected from the phase 2 eligible participants population to meet predetermined sample sizes
The KN panel, a proprietary Web-enabled panel of individuals who have agreed to participate in ongoing survey research, is the only known online panel based on a random-digit-dialing sample of the full US population, ensuring that the panel is representative of the US population [13]. KN provides panelist “points” that can be redeemed for cash at regular intervals for panel participation (for those with Internet access) or with Web-enabled technology to ensure that those who would not otherwise have access to the Internet are able to participate in KN surveys. The KN panel has been used to estimate prevalence previously in several different studies [19, 20, 24].
Following ethics committee approval, screening in phase 1 was fielded from 6 November 2007 through 9 December 2007, and the full survey in phase 2 was fielded from 20 March 2008 through 31 March 2008.
Participants
A total of 32,000 men and women aged 18 years and older residing in the USA were randomly selected from the KN online panel and invited to participate in the current study. Eligibility for the Dupuytren’s disease full survey (phase 2) was based on responses to the screening questions in phase 1. Specifically, phase 1 respondents eligible for the Dupuytren’s disease phase 2 survey were those who reported one or more of the following criteria at screening:
Ever received a diagnosis of Dupuytren’s disease or Dupuytren’s contracture by a doctor or other healthcare professional
Ever received either surgery or a needle aponeurotomy (or needle fasciotomy) to treat a hard, ropelike growth in the palm
- Currently have at least one of the following symptoms:
- A hard bump (knot) that developed on the palm or at the base of the fingers (excluding calluses, warts, or bumps caused by work done with hands)
- A hard, ropelike growth (cord) that developed in the palm of the hand
Note that participants who reported only dimpling in phase 1 had the potential for inaccurate identification of that symptom and thus were excluded from completing the survey in phase 2. Individuals who met the eligibility criteria for the phase 2 survey will be referred to as Dupuytren’s patients, unless otherwise specified.
Sampling Method for the Phase 2 Dupuytren’s Disease Survey
Patients invited to participate in phase 2 were selected using a stratified random sampling approach based on the following two strata within each disease: (1) participants reporting treatment (surgery or needle aponeurotomy) and/or diagnosis (stratum 1, meeting criteria 1 and 2) and (2) participants reporting symptoms (a ropelike growth or a hard bump) only (stratum 2, meeting criterion 3). To ensure an adequate sample size to evaluate treatment patterns, all eligible respondents in stratum 1 were invited to complete the phase 2 Dupuytren’s disease survey. In addition, a portion of respondents (n = 266) from stratum 2 were randomly selected to participate in the phase 2 survey. Because no respondents were allowed to complete both Dupuytren’s and Peyronie’s disease surveys, for the few respondents who were eligible to participate in both surveys, priority was given to the rarer Peyronie’s disease. As a result and a limitation, 4% of the eligible Dupuytren’s patients were not covered in the Dupuytren’s survey sample.
Questionnaire Content
The phase 1 questionnaire included questions assessing the presence of various hand-related symptoms, diagnosis of Dupuytren’s disease or Dupuytren’s contracture, and past surgical procedures or needle aponeurotomy treatments for hand-related symptoms. Real-life pictures corresponding with each symptom [ropelike growth (cord), hard bump, and dimpling] were included in the screener to help patients accurately assess the presence of these symptoms.
The Dupuytren’s survey developed specifically for the phase 2 portion of this study focused on the presence and severity of current symptoms, family history, and treatment history for hand symptoms (e.g., specific symptom first noticed, time from noticing first symptom until seeking medical treatment, other symptoms noticed, types of treatment received and success of each treatment type, length of treatment/duration of care, number of surgeries and/or needle aponeurotomies, diagnoses received, types of physicians treating and/or diagnosing the disease).
Data Analysis
Prevalence Estimates
The prevalence estimates of Dupuytren’s disease were based on self-reported data from phase 1 and adjusted to represent the US population by using statistical weights provided by KN. These weights are designed to (1) account for the known sources of deviation from an equal-probability selection design during formation of the KN panel, (2) reduce bias due to noncoverage of households without telephones, and (3) reduce the nonresponse bias potentially introduced during data collection for outcomes highly correlated with demographic and geographic characteristics.
Five different prevalence estimates for Dupuytren’s disease were calculated. The numerators for the prevalence estimates of Dupuytren’s disease were derived using the following five definitions, ordered from the most to the least stringent, and the denominator was the number of individuals who completed phase 1:
Definition 1: Patient has received a diagnosis of Dupuytren’s disease or Dupuytren’s contracture from a doctor or other healthcare professional
Definition 2: Patient meets the criterion in definition 1 or has ever had surgery and/or a needle aponeurotomy to treat a hard, ropelike growth in the palm of the hand
Definition 3: Patient meets the criteria in definition 2 or has a hard, ropelike growth (like a cord) in the palm of the hand
Definition 4: Patient meets the criteria in definition 3 or has a hard bump (like a knot) on the palm or at the base of finger
Definition 5: Patient meets the criteria in definition 4 or has noticeable dimpling (like a pit) on the skin of the palm
Note that the definition 5 is the least stringent definition and includes patients who reported only dimpling in phase 1. These patients were excluded from completing the survey in phase 2, as discussed previously. Although the incidence proportion of Dupuytren’s disease based on definition 5 was not available, the corresponding prevalence estimate is provided from screening data from phase 1.
Incidence Proportion
The 1-year incidence proportion for Dupuytren’s disease in 2007 was based on (1) time of first diagnosis by a doctor or other healthcare professional (corresponding to definition 1) and (2) the earliest time when a hard bump or ropelike growth was noticed (corresponding to definition 4). The incidence proportion was defined as the number of new cases, i.e., participants who reported 2007 as their year of onset (diagnosis or symptom), divided by the number of people who were free of the condition (i.e., at risk of developing the condition) on the first day of 2007. It is a function of the prevalence and the proportion of new cases among all cases. The prevalence was estimated using data from phase 1 as described previously. The proportion of new cases among all cases was estimated using data from the survey completed in phase 2.
Analysis of Phase 2 Dupuytren’s Disease Survey
Because patients reporting a diagnosis or treatment (stratum 1) were oversampled in the phase 2 survey, to get an unbiased estimate for the Dupuytren’s patients (definition 4), summary statistics (e.g., mean, standard error, or percentage) of disease symptoms, family history, and treatment patterns collected in the phase 2 survey were calculated using weighted averages of the stratum-specific estimates [9]. The weight for each stratum was the proportion of the respondents in the stratum among Dupuytren’s patients, which was estimated using phase 1 data. No imputations were made for missing values. When calculating percentages, patients who did not answer a particular question were excluded from the denominator for that question.
Results
Of the 32,000 men and women (16,000 of each gender) aged 18 years and older who were randomly selected from the pool of KN panel members and invited to participate in phase 1, a total of 23,103 completed the screening survey for a response rate of 72%. Over 400 (n = 463) Dupuytren’s patients (197 of them reported a Dupuytren’s diagnosis and/or surgical/needle aponeurotomy treatment) were invited to complete a full survey in phase 2; of those, 376 (81%) responded to the survey invitation. Of the responders, 326 (87%) consented and completed the Dupuytren’s disease survey in phase 2.
Demographic Characteristics
Table 1 presents unweighted demographic characteristics for all respondents in phase 1 as well as respondents to the Dupuytren’s disease survey in phase 2. The mean age of all respondents in phase 1 was nearly 50 years (range, 18–101 years), similar to the phase 2 respondents reporting only symptoms (mean age, 49 years). Those reporting a diagnosis or treatment were older on average (mean age, 59 years) and had a higher proportion of males (63%) compared with the general participants or those with only symptoms. The majority of respondents across both phases were white and had completed more than a high school level of education.
Table 1.
Summary of demographic characteristics
| Characteristic | Phase 1 participants (N = 23,103) | Phase 2 participants (N = 326) | |
|---|---|---|---|
| With hard bump or ropelike growth and no diagnosis/treatment (n = 161) | With diagnosis/treatment (n = 165) | ||
| Age (years), n (%) | |||
| 18–29 | 2201 (10) | 27 (17) | 6 (4) |
| 30–44 | 7165 (31) | 37 (23) | 19 (12) |
| 45–59 | 7112 (31) | 47 (29) | 56 (34) |
| 60+ | 6625 (29) | 50 (31) | 84 (51) |
| Gender, n (%) | |||
| Male | 11,420 (49) | 73 (45) | 104 (63) |
| Female | 11,683 (51) | 88 (55) | 61 (37) |
| Race, n (%) | |||
| White, non-Hispanic | 18,735 (81) | 127 (79) | 132 (80) |
| Black, non-Hispanic | 1,788 (8) | 12 (7) | 10 (6) |
| Other, non-Hispanic | 615 (3) | 7 (4) | 9 (5) |
| Hispanic | 1,342 (6) | 14 (9) | 6 (4) |
| 2+ races, non-Hispanic | 623 (3) | 1 (1) | 8 (5) |
| Education, n (%) | |||
| Less than high school | 1,421 (6) | 23 (14) | 11 (7) |
| High school | 5,592 (24) | 46 (29) | 36 (22) |
| Some college | 7,634 (33) | 44 (27) | 50 (30) |
| Bachelor’s degree or higher | 8,456 (37) | 48 (30) | 68 (41) |
Prevalence and Incidence Proportion
The estimated prevalence of Dupuytren’s based on the five definitions ranged from 0.5% to 11% (Table 2). The estimated prevalence is <1% based on self-reported diagnosis of Dupuytren’s disease or Dupuytren’s contracture, and only 1% based on self-reported diagnosis and/or surgery/needle aponeurotomy (or fasciotomy). The estimated 1-year incidence proportion of Dupuytren’s (number of new cases in 2007/size of the population at risk at the beginning of 2007) ranged from 0.03% for the first diagnosis by a doctor or other healthcare professional to 1.4% for the first symptom noticed (hard bump or ropelike growth; Table 3) [23]. The estimated annual number of new cases of physician-diagnosed Dupuytren’s, based on the year 2007 data, was approximately 3 cases per 10,000 adults (or 70,505 adults based on current census data) [23]. If first symptoms noticed was used, the estimated annual number would be 140 cases per 10,000 adults (or 3,290,224 adults).
Table 2.
Estimated prevalence of Dupuytren’s disease in the United States in 2007
| Disease definition | Phase 1 participants (N = 23,103) | |
|---|---|---|
| Prevalence (%) | 95% CI | |
| Definition 1: Diagnosis | 0.5 | 0.4–0.7 |
| Definition 2: Diagnosis or treatment | 1.0 | 0.8–1.2 |
| Definition 3: Diagnosis, treatment, or ropelike growth | 3.0 | 2.7–3.3 |
| Definition 4: Diagnosis, treatment, ropelike growth, or hard bump | 7.3 | 6.8–7.8 |
| Definition 5: Diagnosis, treatment, or any symptom | 10.9 | 10.3–11.5 |
Prevalence estimates are weighted estimates from 23,103 respondents
CI confidence interval
Table 3.
Estimated incidence proportion of Dupuytren’s disease in the United States in 2007
| Dupuytren’s disease | Incidence proportion (%) | 95% CI | Current US population estimatea |
|---|---|---|---|
| First diagnosed | 0.028 | 0.000–0.078 | 70,505 adults |
| First symptomb noticed | 1.442 | 0.907–1.977 | 3,290,224 adults |
Incidence estimates are weighted estimates from 23,103 respondents in phase 1 and 326 respondents in phase 2
CI confidence interval
aBased on current extrapolated US population data (18 years and over): 235,016,000 adults [23]
bHard bump or ropelike growth
Dupuytren’s Disease Symptoms and Family History
Table 4 presents the weighted estimates of the disease symptoms among the Dupuytren’s patients (definition 4). The most commonly reported symptom at the time of the survey was a hard bump on one or both hands (69%), followed by dimpling in one hand (30%) and a ropelike growth (cord) in one hand (23%, Table 4). More than one third of Dupuytren’s patients indicated that their fingers were bent to the extent that it interfered with everyday activities, such as picking up items, putting on gloves, buttoning a shirt, or washing hands or face (data not shown in table).
Table 4.
Summary of current Dupuytren’s disease symptoms among Dupuytren’s patients
| Survey question | Statistic or category | % of Participants (n = 326) |
|---|---|---|
| Hard bump (knot) on the palm or at the base of fingers | In one hand | 46 |
| In both hands | 23 | |
| Dimpling (pit) on the skin of palm | In one hand | 30 |
| In both hands | 21 | |
| Ropelike growth (cord) in the palm of hand | In one hand | 23 |
| In both hands | 14 | |
| One or more finger(s) bent toward palm | In one hand | 20 |
| In both hands | 19 | |
| Able to flatten palm on an even surface | Can flatten both palms | 74 |
| Can flatten palm of one hand only | 20 | |
| Cannot flatten either palm | 7 | |
| Hand symptom noticed first | A hard bump (knot) | 61 |
| A hard, ropelike growth (cord) | 12 | |
| One or more finger(s) bent toward palm | 11 | |
| Dimpling (pit) | 9 | |
| Other | 6 |
Percents are weighted averages of the stratum-specific estimates
The first hand symptom noticed was most commonly a bump or a knot that had developed on the palm or at the base of the fingers (61%). A substantially lower percentage of Dupuytren’s patients reported that a ropelike growth was the first symptom noticed, followed by fingers bent toward the palm or dimpling (Table 4). Nearly all Dupuytren’s patients (82%) indicated that the first symptom noticed appeared only in one hand.
Eighteen percent of Dupuytren’s patients reported that an immediate family member had one or more symptoms consistent with Dupuytren’s: hard bump (10%), bent fingers (9%), dimpling (5%), and a ropelike growth (5%). Only 3% of patients indicated that an immediate family member had a diagnosis of Dupuytren’s disease, Dupuytren’s contracture, or palmar fibromatosis.
Dupuytren’s Disease Treatment Patterns
One hundred ninety-two Dupuytren’s patients reported seeing a doctor for the treatment of hand symptoms. Of these, 63% talked to a doctor when a hand symptom was first noticed, and 37% spoke to a doctor at a later date. Of these treatment-seeking patients, 70% initially sought treatment from a primary care physician (PCP). Only 12% sought initial treatment from a hand specialist/hand surgeon. The remaining 18% sought initial treatment from an orthopedic specialist/orthopedic surgeon, plastic surgeon, rheumatologist, or other type of healthcare professional.
The mean time from noticing the first hand symptom to seeing a doctor was 23.1 months (SE = 5.6 months). The most common first symptom that led patients to seek treatment was a hard bump (48%), followed by ropelike growth (12%), pit or dimpling (11%), and bent fingers (10%). Only 9% of patients seeking treatment for a hand symptom received a diagnosis of Dupuytren’s from the first doctor. Other diagnoses received from the first doctor seen included arthritis/rheumatoid arthritis (25%), carpal tunnel (22%), tendonitis (15%), ganglia (10%), and trigger finger (10%), and 12% reported some “other” diagnosis. The first physician seen for a hand symptom provided an average duration of care of approximately 8.6 months.
Among those patients who sought treatment for a hand symptom, 48% were either told by the first doctor seen to “wait and see” or simply received no treatment; others received physical therapy, splint, steroid injection, prescription medication, over-the-counter medication, surgery, needle aponeurotomy, other treatment, or referral to a specialist. Of the 101 patients who reported receiving at least one treatment, the most commonly reported first-time treatments were prescription medication (40%), physical therapy (21%), splinting (19%), steroid injection (19%), surgery (18%), and over-the-counter medication (10%, data not shown in table). These were also most commonly reported as the second treatment. Very few patients received more than two treatments (n < 15). Table 5 presents the most commonly reported treatments received anytime after seeing a doctor. The mean time from noticing the first symptom until receiving the first treatment ranged from 7 months (SE = 4.9) for referral to a specialist to more than 7 years for over-the-counter medication (data not shown in table).
Table 5.
Treatment among patients reporting a doctor visit for the treatment of a hand symptom (n = 192)
| Most commonly reported treatment | Patients reporting treatment (%) | Time to first treatment since first symptom (months) | ||
|---|---|---|---|---|
| N | Mean (SE) | Range | ||
| Prescription medication | 30 | 32 | 28.2 (11.0) | 0–150 |
| Surgery | 20 | 54 | 28.2 (15.2) | 0–151 |
| Steroid injection | 19 | 23 | 19.1 (6.4) | 0–120 |
| Physical therapy | 18 | 22 | 31.4 (17.0) | 0–147 |
| Splint | 16 | 27 | 28.0 (15.7) | 0–148 |
Time to first treatment is summarized among those receiving treatment. Percent, mean, and SE are weighted averages of the stratum-specific estimates
SE standard error
Among patients with a Dupuytren’s diagnosis (n = 93), over half (52%) received the diagnosis from their PCP. Other types of physicians providing a Dupuytren’s diagnosis included hand specialist/surgeons (20%) and orthopedic specialist/orthopedic surgeons (16%).
Summary of Surgical Procedures
Of the Dupuytren’s patients reporting seeing a doctor, 20% reported they had received surgery to treat a hand symptom; of these, 62% reported one surgery, 29% reported two surgeries, and 9% reported three or more surgeries (Table 6). Hand specialists or hand surgeons performed nearly two thirds (62%) of first-time surgeries and orthopedic specialists or surgeons performed 27% of first-time surgeries.
Table 6.
Summary of hand surgeries and needle aponeurotomies used to treat hand symptoms
| Patients who had surgery (n = 89) | Patients who had a needle aponeurotomy (n = 44) | |
|---|---|---|
| No. of procedures | ||
| 1 | 55 (62%) | 25 (57%) |
| 2 | 26 (29%) | 14 (32%) |
| 3 | 4 (4%) | 3 (7%) |
| 4 | 2 (2%) | 0 (0%) |
| 5 or more | 2 (2%) | 2 (5%) |
| Did the first procedure correct the problem? | ||
| Yes | 71 (80%) | 17 (39%) |
| Hand symptoms after first procedure | ||
| Symptoms returned in the same place | 14 (20%) | 3 (18%) |
| Symptoms appeared in a new spot on same hand | 9 (13%) | 2 (12%) |
| Symptoms appeared in other hand | 18 (25%) | 3 (18%) |
| Symptoms did not come back and no new symptoms have appeared | 34 (48%) | 10 (59%) |
| How soon symptoms returned after surgery | ||
| No. of patients whose symptoms returned in the same place | 14 | 3 |
| Months till return, mean (SE) | 44.1 (10.6) | 26.3 (17.2) |
| Months till return, median | 30.0 | 16.0 |
Percentages are calculated from the single stratum (patients having had surgery or a needle aponeurotomy to treat a hand symptom)
SE standard error
The type of surgery performed was unknown to 40% of Dupuytren’s patients, 16% of patients reported complete fasciectomy, 7% reported partial fasciectomy, and 12% reported percutaneous fasciotomy. Of those Dupuytren’s patients who reported the time of surgery and the first-noticed hand symptom or diagnosis (n = 68), the mean time from first noticing a hand symptom until first surgery was 18.1 months (SE = 3.5 months), and the mean time from first diagnosis to first surgery (n = 25) was 8.8 months (SE = 2.9 months).
Summary of Needle Aponeurotomy
Of the Dupuytren’s patients reporting a doctor visit, 2% reported a needle aponeurotomy to treat a hand symptom; of these, 57% reported one needle aponeurotomy, 32% reported two needle aponeurotomies, and 11% reported three or more needle aponeurotomies (Table 6). The first needle aponeurotomy was performed by a hand specialist/hand surgeon in 45% of the patients reporting a needle aponeurotomy and by an orthopedic specialist or orthopedic surgeon in 32%.
Of those Dupuytren’s patients who reported the time of needle aponeurotomy and the first noticed hand symptom or diagnosis (n = 34), the mean time from first noticing a hand symptom until first needle aponeurotomy was 19.4 months (SE = 6.5 months), and the mean time from first diagnosis to first needle aponeurotomy is not reported here given the small number of patients providing complete data (n = 4).
Summary of Treatment Effectiveness
For surgery and needle aponeurotomies, additional information from patients was collected on their effectiveness (Table 6). Of patients having had surgery performed by any doctor, 80% reported when asked that the first surgery corrected the hand symptoms. The corresponding percentage for a needle aponeurotomy was 39%. However, this information should be considered carefully due to the fact that improvement was self-reported by each patient and not measured objectively.
Discussion
The primary goals of the research study presented in this paper were to estimate the prevalence and incidence of Dupuytren’s in the general US adult population as well as to describe symptom progression and treatment patterns associated with this condition. As expected, the prevalence and incidence of diagnosed patients was very low (0.5%), but higher prevalence estimates were found when patients who self-reported a diagnosis, treatment, or any hand symptom were considered (11%). This study is the first large-scale study of a representative sample of the US population, and the estimates derived using the more stringent definitions are generally consistent with the 4–6% prevalence rates found in other studies of general populations [3, 6, 25].
The incidence proportion of Dupuytren’s in the general US population estimated that the annual number of new cases of physician-diagnosed disease, based on 2007 data, was approximately 3 cases per 10,000 adults. The estimated incidence of Dupuytren’s in the USA appears to be comparable with the estimated incidence (34.4 per 100,000) for the British population in 2004 among men aged 40–84 years [12].
In the phase 2 survey, more than two thirds of Dupuytren’s patients reported having a hard bump on one or both hands. In addition, one third reported that their hand symptoms interfered with everyday activities. Yet nearly 40% of those reporting a diagnosis, treatment, or a hand symptom indicated they had never sought medical treatment.
Of those patients seeking treatment for a hand symptom, over one third did not seek treatment immediately, with the average time from first noticing a symptom until seeing the first doctor being approximately 2 years. In general, patients who did seek treatment initially went to their PCP. Only 9% of those seeking treatment for hand symptoms reported receiving a diagnosis of Dupuytren’s disease. Other initial diagnoses reported by patients for their hand symptoms include arthritis or rheumatoid arthritis, carpal tunnel syndrome, tendonitis, and trigger finger. It is not clear from the data in the current study if other diagnoses given by physicians were accurate diagnoses of the patient’s symptoms at the time of assessment, if physicians or patients confused Dupuytren’s with other similar conditions, and/or if no diagnosis was given because no conclusion was made about the hand symptoms. The difference in prevalence between hand symptoms and an actual diagnosis of Dupuytren’s in the current study suggests that Dupuytren’s may be underdiagnosed. Recognition of symptoms and accurate diagnosis by physicians is critical as early detection and treatment may help slow the disease’s progression. These data indicate the need for increased physician awareness, particularly by PCPs, of Dupuytren’s hand symptoms and available treatments.
Both surgical and nonsurgical treatment options (including needle aponeurotomies/injections) are currently used to treat Dupuytren’s. Of the Dupuytren’s patients reporting a doctor visit, 20% of patients reported one or more hand surgeries. Most surgeries were performed by a hand specialist or hand surgeon, and fasciectomy was the most frequent type of surgery performed. In the literature, surgery is the most common treatment for Dupuytren’s [15–17, 21], and fasciectomy is the most common procedure [15]. Prolonged postoperative rehabilitation, including hand therapy and splinting to improve the range of motion of the hand, is necessary to maintain the benefits of surgery [7, 15]. The potential benefits of surgery must be balanced with possible surgical complications, including injury to the tendon, digital nerve, or artery; potential loss of a finger; infection; hematoma; loss of grip strength or flexion; recurrence of contracture; complex regional pain syndrome; skin necrosis; wound healing complications; and joint stiffness [7, 14, 15]. A 20-year review of surgical complications associated with fasciectomy found overall surgical complication rates ranging from 3.6% to 39.1% [5].
Surgery to treat a hand symptom was used more commonly than needle aponeurotomies, and patients’ reports of treatment effectiveness were greater for surgery (80%) than for needle aponeurotomies (39%). Only 2% of the Dupuytren’s patients who had seen a doctor reported at least one needle aponeurotomy to correct a hand symptom. Percutaneous and needle fasciotomy are considered treatment options for Dupuytren’s; however, high-quality clinical studies of the effectiveness of these interventions are not readily available [18]. More recently, the results of studies using collagenase therapy, which involves injection of clostridial collagenase into cords, appear promising for the treatment of Dupuytren’s [1, 11, 21]. An effective nonsurgical treatment for Dupuytren’s such as this would avert surgery-related morbidity and complications. As this study was conducted prior to the widespread availability of clostridial collagenase as a therapeutic agent, it will be of interest in the future to determine how the availability of this nonsurgical treatment option may affect the overall treatment pattern for patients with Dupuytren’s disease.
The main strength of the current study is that it uses a large population-based sample representative of US adults to provide prevalence and incidence proportion estimates for a relatively rare condition, which were not previously available. Additional study strengths include a high response rate, the inclusion of many variables to describe treatment-seeking behavior and therapies received, real-life pictures to aid in detecting the presence of various hand symptoms, and utilization of different criteria to compute prevalence and incidence proportion estimates to allow for both conservative and more liberal estimates. Compared with the US Current Population Survey data [22], the phase 1 sample had older participants on average, had a higher percentage of whites, and had a higher percentage completing more than a high school level of education. To account for the potential influence of the demographic differences, statistical weights were applied when estimating the prevalence and incidence of Dupuytren’s.
Some caution should be used in interpreting these prevalence and incidence findings. The analyses are exclusively based on the use of self-reported data instead of clinical assessment of Dupuytren’s disease. Although the survey included real-life pictures of each symptom to help patients accurately assess the presence of these symptoms, there is potential for the symptoms included in the more liberal definitions of Dupuytren’s disease (e.g., definitions 4 and 5: hard bump, dimpling) to be confused with other conditions that are more prevalent, such as rheumatoid arthritis or osteoarthritis. Thus, it is possible that the corresponding prevalence estimates based on the less severe hand symptoms, such as hard bump and dimpling (definitions 4 and 5), may overestimate the true prevalence of Dupuytren’s disease.
Although the initial sample size for phase 1 was large, some of the data were based on small numbers of participants and/or rare events. In addition, all survey respondents who reported a diagnosis or surgical procedure (including needle aponeurotomies/injection procedures) in phase 1 were invited to participate in phase 2 (a random sample from those reporting symptoms only were selected to complete a survey). Oversampling was required to ensure that an adequate number of participants for rare events were included in the full survey. Thus, results may potentially be based on a patient sample more severe (on average) as compared with the general patient population. Therefore, the weighted average of the stratum-specific estimates was used to account for the oversampling. Nevertheless, the data obtained from the current study are rich and should provide a wealth of information in designing future studies and/or performing complex analyses.
Conclusions
We found the use of a Web-enabled panel an effective and efficient way to conduct a large, population-based survey of a rare medical condition. Data collected from this large-scale survey of a representative sample of participants in the general US population can provide critical information needed to improve understanding, recognition, and treatment of Dupuytren’s disease. The results from the current study indicate a number of unmet medical needs in this area, with particular emphasis on the need for better recognition of symptoms for both patients and clinicians, including education for non-hand specialists.
Acknowledgments
The authors would like to acknowledge Knowledge Networks for the use of the KnowledgePanel® and for administering the questionnaire. The authors would also like to thank Drs. Jianmin Wang and Cheryl D. Coon for their assistance in programming and statistical analyses, Dr. Catherine Johannes for her role as an epidemiology consultant, and Dr. Sheri E. Fehnel for her assistance in the design, analysis, and reporting of the study. Auxilium Pharmaceuticals, Inc provided funding to RTI Health Solutions for the conduct of this study, and to DesignWrite, LLC for writing and editorial assistance, provided by Jennifer Kent, PhD, and Lynanne McGuire, PhD.
Conflicts of interest
Dat Nguyen is an employee of Auxilium Pharmaceuticals. The other authors declare authors declare that they have no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am. 2007;32:767–774. doi: 10.1016/j.jhsa.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Bayat A, Cunliffe EJ, McGrouther DA. Assessment of clinical severity in Dupuytren’s disease. Br J Hosp Med. 2007;68:604–609. doi: 10.12968/hmed.2007.68.11.27683. [DOI] [PubMed] [Google Scholar]
- 3.Bergenudd H, Lindgarde F, Nilsson BE. Prevalence of Dupuytren’s contracture and its correlation with degenerative changes of the hands and feet and with criteria of general health. J Hand Surg Br. 1993;18B:254–257. doi: 10.1016/0266-7681(93)90123-w. [DOI] [PubMed] [Google Scholar]
- 4.Critchley EM, Vakil SD, Hayward HW, Owen VM. Dupuytren’s disease in epilepsy: result of prolonged administration of anticonvulsants. J Neurol Neurosurg Psychiatry. 1976;39:498–503. doi: 10.1136/jnnp.39.5.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denkler K. Surgical complications associated with fasciectomy for Dupuytren’s disease: a 20-year review of the English literature. J Plast Surg. 2010;10:116–133. [PMC free article] [PubMed] [Google Scholar]
- 6.Early PF. Population studies in Dupuytren’s contracture. J Bone Joint Surg Br. 1962;44B:602–613. [Google Scholar]
- 7.Eckhaus D, et al. Dupuytren’s disease. In: Clark GL, Shaw Wilgis EF, Aiello B, et al., editors. Hand rehabilitation: a practical guide. 2. New York: Churchill Livingstone; 1998. pp. 37–42. [Google Scholar]
- 8.Geoghegan JM, Forbes J, Clark DI, Smith C, Hubbard R. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423–426. doi: 10.1016/j.jhsb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Greenland S, Rothman KJ. Introduction to stratified analysis. In: Rothman KJ, Greenland S, editors. Modern epidemiology. 2. Philadelphia: Lippincott-Raven; 1998. pp. 253–279. [Google Scholar]
- 10.Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand (NY) 2009;4:256–269. doi: 10.1007/s11552-008-9160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 12.Khan AA, Rider OJ, Jayadev CU, Heras-Palou C, Giele H, Goldacre M. The role of manual occupation in the aetiology of Dupuytren’s disease in men in England and Wales. J Hand Surg Br. 2004;29:12–14. doi: 10.1016/j.jhsb.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Knowledge Networks. Methodological information about knowledge networks for reviewers. http://www.knowledgenetworks.com/ganp/reviewer-info.html, accessed 7 July 2009
- 14.Loos B, Puschkin V, Horch RE. 50 years experience with Dupuytren’s contracture in the Erlangen University Hospital—a retrospective analysis of 2919 operated hands from 1956 to 2006. BMC Musculoskelet Disord. 2007;8:60. doi: 10.1186/1471-2474-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarlane RM, MacDermid JC. Dupuytren’s disease. In: Mackin EJ, editor. Hunter–Mackin–Callahan rehabilitation of the hand and upper extremity. 5. Philadelphia: Mosby; 2002. pp. 971–988. [Google Scholar]
- 16.McGrouther DA, et al. Dupuytren’s contracture. In: Green DP, Hotchkiss RN, Pederson WC, et al., editors. Green’s operative hand surgery. 5. Philadelphia: Churchill Livingstone; 2005. pp. 159–185. [Google Scholar]
- 17.Rayan GM. Dupuytren’s disease: anatomy, pathology, presentation, and treatment. J Bone Joint Surg Am. 2007;89:189–198. doi: 10.2106/00004623-200701000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Rayan GM. Nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2008;33:1208–1210. doi: 10.1016/j.jhsa.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Rigby AJ, Ma J, Stafford RS. Women’s awareness and knowledge of hormone therapy post-Women’s Health Initiative. Menopause. 2007;14:853–858. doi: 10.1097/gme.0b013e3180333a33. [DOI] [PubMed] [Google Scholar]
- 20.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6:2133–2142. doi: 10.1111/j.1743-6109.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 21.Shaw RB, Jr, Chong AK, Zhang A, Hentz VR, Chang J. Dupuytren’s disease: history, diagnosis, and treatment. Plast Reconstr Surg. 2007;120:44e–54e. doi: 10.1097/01.prs.0000278455.63546.03. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Commerce, Bureau of the Census. Current population survey (CPS). http://www.census.gov/cps/, accessed 29 June 2009
- 23.US Department of Commerce, Bureau of the Census. Statistical abstract of the United States: 2010. 129th edition. U.S. Department of Commerce; U.S. Census Bureau; 2010.
- 24.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Granger AL, Fehnel SE, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11:32–43. doi: 10.1080/13697130701744696. [DOI] [PubMed] [Google Scholar]
- 25.Yost J, Winters T, Fett HC., Sr Dupuytren’s contracture: a statistical study. Am J Surg. 1955;90:568–571. doi: 10.1016/0002-9610(55)90537-7. [DOI] [PubMed] [Google Scholar]