Abstract
Background
The aim of this study was to investigate the relationship among apolipoprotein E (APOE) polymorphism, body mass index (BMI), and dyslipidemia and how these factors modify overall mortality in a cohort of hospitalized elderly patients.
Methods
Plasma concentrations of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), BMI, and APOE genotype were evaluated in 1,012 hospitalized elderly patients, who were stratified into three groups according to their baseline BMI and APOE allele status. Multivariate logistic regression analysis was used to assess whether APOE genotype, BMI, and dyslipidemia are associated with mortality, adjusting for potential confounders. Interaction analysis was also performed.
Results
Obese patients have significantly higher levels of TC and LDL-C compared to normal-weight and overweight subjects, for both sexes. APOE ε4 carriers have significantly higher levels of TC and LDL-C compared with ε2 and ε3 carrier both in males and females. Interaction analysis showed that women with TC < 180 mg/dL, LDL-C < 100 mg/dL, normal weight, and ε3 carrier (odds ratio [OR] = 3.42, 95% confidence interval [CI] 1.36–8.60) and men with LDL-C < 100 mg/dL, HDL-C < 40 mg/dL, and ε3 carrier (OR = 1.97, 95% CI 1.04–3.74) were at highest risk of mortality.
Conclusions
In elderly hospitalized patients, obesity and APOE genotype influence the lipid profile and mortality risk. A significant interaction among BMI, dyslipidemia, and APOE genotype was observed that could identify elderly patients with different risks of mortality.
Introduction
Excessive body weight is associated at all ages with detrimental changes in the lipid profile. Higher body mass index (BMI), a surrogate measurement of total body fat, is associated with higher plasma concentrations of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG).1 Data from several epidemiological studies have also shown that apolipoprotein E (APOE) polymorphism is associated with variation of serum TC and LDL-C concentrations.2,3 APOE is a multifunctional glycoprotein that plays a key role in regulating lipoprotein metabolism and cardiovascular risk.4 The APOE gene exhibits a genetic polymorphism with three common alleles (ε2, ε3, and ε4), which encode for three protein isoforms E2, E3, and E4.5 APOE polymorphism has been related to significant modifications of the lipoprotein profile, with the ε2 allele being associated with lower and the ε4 allele with higher TC and LDL-C levels than the ε3 allele.6,7 Isoform-specific effects include the association of APOE ε4 with increased risk for atherosclerosis, stroke, impaired cognitive function, and Alzheimer disease (AD).7,8–10 Moreover, some11,12 but not all, population-based studies13,14 have found a negative or positive association between mortality risk and APOE ε2 and APOE ε3 polymorphisms, respectively. However, to the best of our knowledge, no study has investigated the interaction between APOE polymorphism, BMI, and dyslipidemia in a large cohort of hospitalized elderly patients, in whom the aging process and co-morbidity may have profound effects on body composition and lipid metabolism.
The purpose of the present study was to evaluate the relationship among APOE polymorphism, adiposity, and dyslipidemia in a large group of elderly patients who were admitted to our hospital. A second aim was to study how these factors affect prognosis by modifying overall mortality.
Methods
Study design
From January, 2006, to March, 2007, all patients consecutively admitted to the Geriatrics Unit of the “Casa Sollievo della Sofferenza” Hospital, IRCCS, San Giovanni Rotondo, Italy, were screened for inclusion in the study. Inclusion criteria were: (1) Caucasian race, (2) age ≥65 years; (3) ability to provide an informed consent or availability of a proxy for informed consent. Exclusion criteria were: (1) Diagnosis of neoplasms; (2) medical/surgical hospitalization within 1 month before the study; (3) use of lipid-lowering drugs during the previous 4 weeks. Patients with AD were included in the study. Diagnoses of possible/probable AD were made according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association Work Group (NINDS-ADRDA).15 This study was approved by the Human Studies Committee of “Casa Sollievo della Sofferenza” Hospital, IRCCS, San Giovanni Rotondo, Italy, and all subjects gave informed consent before their participation.
All measurements were performed during the hospital admission examination. Height was measured without shoes to the nearest 0.1 cm. Body weight was obtained on a balance scale in the morning after subjects fasted for 12 h overnight. BMI was calculated by dividing body weight (in kilograms) by the square of height (in meters). According to the National Institutes of Health (NIH) guidelines, normal weight was defined as a BMI ≥18.5 and ≤24.9 kg/m2, overweight as a BMI ≥ 25 and ≤29.9 kg/m2, and obesity as a BMI ≥ 30 kg/m2.16
Information on the vital status of the participants was obtained at regular intervals from the municipal population registries located in the towns where the patients resided at the time of hospital admission. Mortality data were available up to January 31, 2009.
Laboratory analysis
A venous blood sample was taken to determine lipid and lipoprotein concentrations, and APOE genotypes after subjects had fasted for at least 12 h. Plasma levels of TC, TG, LDL-C, and high-density lipoprotein cholesterol (HDL-C) were measured by standardized enzymatic procedures and expressed in mg/dL.
Genetic analysis
Genomic DNA was purified manually from peripheral blood by organic protein extraction and ethanol precipitation according to a standard method. The APOE genotypes were determined by polymerase chain reaction (PCR) and agarose gel electrophoresis as described previously.17 Briefly, a combination of four specific primers in three different pairs sharing the same stringent PCR conditions was used. Allele-specific primers were: ASP1, CGG ACA TGG AGG ACG TGT; ASP2, CGG ACA TGG AGG ACG TGC; ASP3, CTG GTA CAC TGC CAG GCG; and ASP4, CTG GTA CAC TGC CAG GCA, synthesized by Invitrogen (Invitrogen Corporation, Carlsbad, CA). The primer pair ASP-1/ASP-4 identifies the ε2 allele, the primer pair ASP-1/ASP-3 identifies the ε3 allele, and the primer pair ASP-2/ASP-3 identifies the ε4 allele. On an Applied Biosystems GeneAmp PCR System 9700, the amplification conditions were 94°C for 2 min, followed by 32 cycles at 96°C for 15 sec, 61°C for 30 sec, and 72°C for 30 sec. Reaction buffer included 1.5 U of Taq DNA polymerase (Platinum®Taq, Invitrogen, Carlsbad, CA), 10 pmoles of each primer, 100 picomoles (PM) nucleoside triphosphates (NTPs), and 1 mM MgCl2. The presence/absence of specific allele PCR products detected by electrophoresis analysis on a 2% agarose gel identified the six APOE genotypes. Patients were classified into the following three phenotype groups: APOE ε2, ε3, and ε4 groups. The ε3/ ε3 genotype was the reference category; subjects with the ε2/ ε2 or ε2/ ε3 genotypes were considered ε2+ carriers and subjects with ε3/ ε4 or ε4/ ε4 genotypes were considered ε4+ carriers. Subjects who were carriers of the ε2/ ε4 genotype were excluded from the analysis.
Statistical analysis
Patients' baseline characteristics were reported as mean ± standard deviation (SD) or frequencies and percentages for continuous and categorical variables, respectively. Baseline comparison between men and women was made using the chi-squared test for categorical variables and the Mann–Whitney U-test for continuous variables. Baseline differences according to BMI groups and APOE alleles groups were assessed with the analysis of variance (ANOVA) F-test or chi-squared test for continuous and categorical variables, respectively. p values for trend were estimated using the Mantel–Haenszel chi-squared test and ANOVA F-test for trend, appropriately. Genotype distribution according to the Hardy–Weinberg equilibrium was analyzed by the exact chi-squared test.
Multivariate logistic regression analysis was performed to investigate, separately, the association between BMI, dyslipidemia, APOE polymorphism, and all-cause mortality. The following cutoffs were used to dichotomize dyslipidemia variables: 180 mg/dL for TC, 100 mg/dL for LDL-C, 150 mg/dL for TG, 40 mg/dL for HDL-C in men, and 50 mg/dL for HDL-C in women. Results were shown and odds ratio (OR) along their 95% confidence interval (95% CI).
Furthermore, to evaluate interactions among covariates and identify distinct and homogeneous subgroups of patients in terms of mortality, the REPCAM method was used.18 This tree-based method integrates the advantages of main-effects logistic regression and tree-growing techniques. At each partitioning step, the method chooses the covariate and its best binary split to maximize the difference in the outcome of interest. The algorithm stops when user-defined conditions (stopping rules) are met. To obtain a more robust and stable split, a permutation approach was adopted to choose the best splitting variable. Global adjustment for age, diabetes, AD, and cardiovascular diseases was accounted for.
All analyses were performed separately for males and females. All analyses were performed using SAS® release 9.1. For the RECPAM analysis, we used an SAS macroroutine written by F. Pellegrini. p values <0.05 were considered significant.
Results
Study population
From January, 2006, to March, 2007, 1,129 patients were admitted consecutively to the Geriatric Unit of “Casa Sollievo della Sofferenza” Hospital in San Giovanni Rotondo (FG). Of the 1,129 patients assessed for eligibility, 72 patients were excluded because they were younger than 65 years, and 28 refused to participate. Because only 7 patients had a BMI value less than 18.5, they were excluded from the analysis. Thus, 1,022 patients (494 men and 528 women) with a mean age of 77.6 ± 6.7 years (range 65–100 years), were eligible for the present investigation. Table 1 reports the characteristics of patients included in the study, divided according to gender. Female patients had significantly higher BMI mean values, higher serum TC, LDL-C, and HDL-C concentrations, and a higher prevalence of hypertension than male patients (p = 0.0001). Age, serum TG concentration, and the prevalence of diabetes mellitus and AD were not significantly different between male and female patients. The distribution of the different APOE polymorphisms among all subjects was as follows: 11.5% for the ε2/ε3 genotype (n = 118), 71.2% for the ε3/ε3 genotype (n = 728), 15.3% for the ε3/ε4 genotype (n = 157), 0.88% for the ε4/ε4 genotype (n = 9), and 0.9% for the ε2/ε4 genotype (n = 10). No ε2/ε2 APOE genotypes were observed. Patients with the ε2/ε4 genotype (n = 10) were not considered in the analysis. The observed genotype frequency distributions did not show statistically significant differences compared with those expected under Hardy–Weinberg equilibrium (p = 0.106). Estimated allele frequencies were 0.062 for the ε2 allele, 0.846 for the ε3 allele, and 0.085 for the ε4 allele. The distribution of the APOE genotype frequencies was not significantly different between males and females (Table 1).
Table 1.
Baseline Characteristics of Patients Divided According to Gender
| Total (n = 1022) | Men (n = 494) | Women (n = 528) | p | |
|---|---|---|---|---|
| Mean age (years, mean ± SD) | 77.6 ± 6.7 | 77.4 ± 6.7 | 77.7 ± 6.7 | 0.490 |
| BMIa (kg/m2, mean ± SD) | 27.5 ± 5.1 | 26.7 ± 4.3 | 28.3 ± 5.6 | 0.000 |
| Diabetes mellitusa | 206 (20.1) | 92 (18.6) | 114 (21.6) | 0.274 |
| Hypertensiona | 411 (40.2) | 161 (32.6) | 250 (47.3) | 0.000 |
| Ischemic cardiomiopathya | 87 (8.5) | 54 (10.9) | 33 (6.2) | 0.007 |
| Alzheimer dementia | 180 (17.8) | 83 (17.0) | 97 (18.5) | 0.532 |
| Total cholesterolb | 165.9 ± 45.7 | 158.4 ± 44.0 | 173.5 ± 45.9 | 0.000 |
| LDL-Cb | 100.9 ± 36.7 | 95.9 ± 36.7 | 105.6 ± 36.0 | 0.000 |
| HDL-Cb | 39.5 ± 14.9 | 37.1 ± 13.5 | 41.8 ± 15.8 | 0.000 |
| Triglycerides (TG)b | 119.1 ± 66.5 | 115.3 ± 62.9 | 122.6 ± 69.5 | 0.083 |
| Allele frequencies (%) | ||||
| ɛ3(%) | 84.6 | 84.6 | 85.4 | 0.657 |
| ɛ4(%) | 8.5 | 8.1 | 9.9 | 0.180 |
| ɛ2(%) | 6.2 | 7.1 | 5.4 | 0.134 |
| Genotype frequencies | ||||
| ɛ 3/ɛ 3a | 728 (71.2) | 349 (70.6) | 379 (71.7) | 0.749 |
| ɛ 2/ɛ 3a | 118 (11.5) | 65 (13.1) | 53 (10.0) | 0.148 |
| ɛ 4/ɛ 3a | 157 (15.3) | 73 (14.8) | 84 (15.9) | 0.688 |
| ɛ 4/ɛ 4a | 9 (0.88) | 1 (0.2) | 8 (1.5) | 0.140 |
| ɛ 2/ɛ 4a | 10 (0.9) | 6 (1.2) | 4 (0.7) | 0.615 |
| 1 year mortalitya | 161 (15.9) | 94 (19.3) | 67 (12.8) | 0.005 |
Data are N° (%).
Data are mg/dL.
SD, Standard deviation; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C high-density lipoprotein.
Table 2 shows the mean levels of serum lipids in patients, divided according to BMI and gender. The effect of BMI on serum lipid concentrations was studied by using BMI classes (normal weight, overweight, and obese). The mean value of BMI in the general population is 27.5 ± 5.1 kg/m2; 336 (33.2%) patients were normal weight, 399 (39.4%) were overweight, and 277 (27.4) were obese according to their baseline BMI. In both male and female patients, BMI was significantly associated with serum concentrations of TC and LDL-C, but not with HDL-C. BMI was significantly associated with serum concentration of TG only in males.
Table 2.
Lipid Levels in Patients Divided According to BMI Classes
| |
Men (n = 488) |
Women (n = 524) |
||||||
|---|---|---|---|---|---|---|---|---|
| Normal weight (n = 180) | Overweight (n = 208) | Obese (n = 100) | p | Normal weight (n = 156) | Overweight (n = 191) | Obese (n = 177) | p | |
| TC | 152.1 ± 42.4 | 159.1 ± 44.5 | 165.9 ± 44.3 | 0.038 a | 164.3 ± 43.8 | 179.9 ± 46.0 | 174.3 ± 47.3 | 0.007 a |
| TG | 101.2 ± 47.7 | 122.3 ± 66.0 | 123.1 ± 70.1 | 0.001b | 115.7 ± 73.4 | 117.6 ± 60.1 | 131.8 ± 69.1 | 0.054b |
| HDL-C | 38.2 ± 14.7 | 36.3 ± 13.7 | 36.9 ± 10.5 | 0.392 | 43.2 ± 17.4 | 41.1 ± 13.5 | 41.6 ± 16.9 | 0.462 |
| LDL-C | 91.1 ± 35.9 | 96.3 ± 38.5 | 103.1 ± 36.7 | 0.033c | 96.8 ± 35.8 | 111.2 ± 35.6 | 107.6 ± 34.8 | 0.001c |
Data are mean ± standard deviation (SD), mg/dL.
Men: normal weight vs, obese, p = 0.036; women: normal weight vs. overweight, p = 0.005.
Men: normal weight vs. overweight, p = 0.002; normal weight vs, obese, p = 0.013.
Men: normal weight vs. obese, p = 0.028; women: normal weight vs. overweight, p = 0.001; normal weight vs. obese, p = 0.020.
BMI, body mass index; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
APOE polymorphism, plasma lipid, lipoprotein concentrations, and BMI
The bioclinical characteristics according to APOE polymorphisms are summarized in Table 3. No difference in the mean age of the patients across the APOE polymorphisms was observed. In both male and female patients, the mean concentrations of TC and LDL-C were APOE genotype dependent; APOE ε4 carriers had significantly higher levels of TC and LDL-C compared to ε2 and ε3 carriers. In contrast, there were no significant differences among APOE genotypes in plasma concentrations of HDL-C and TG. The effect of APOE alleles on BMI was studied by using BMI as a continuous variable or by using BMI classes (normal weight, overweight, and obese). In both male and female patients, there was no significant difference among APOE genotypes between normal-weight, overweight, and obese patients (Table 3).
Table 3.
Serum Lipid Levels and BMI Distribution in Patients Divided According to Their APOE Allele Status
| |
Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| ɛ2/ (n = 65) | ɛ3/ɛ3 (n = 349) | ɛ4/ (n = 74) | p | ɛ2/ (n = 53) | ɛ3/ɛ3 (n = 379) | ɛ4/ (n = 92) | p | |
| Age | 77.9 ± 6.9 | 77.2 ± 6.6 | 78.3 ± 6.6 | 0.360 | 77.2 ± 6.5 | 77.9 ± 6.7 | 77.4 ± 6.5 | 0.671 |
| TCa (mg/dL) | 141.8 ± 42.5 | 159.4 ± 42.2 | 164.9 ± 50.1 | 0.004b | 164.8 ± 51.4 | 172.1 ± 45.1 | 183.4 ± 46.0 | 0.039 |
| TGa (mg/dL) | 116.8 ± 71.9 | 115.9 ± 57.6 | 106.8 ± 69.8 | 0.489 | 116.5 ± 57.1 | 118.9 ± 63.1 | 136.9 ± 87.7 | 0.061 |
| HDL-Ca (mg/dL) | 36.5 ± 11.9 | 36.6 ± 13.4 | 39.8 ± 15.0 | 0.183 | 40.8 ± 15.2 | 42.4 ± 14.5 | 40.3 ± 21.7 | 0.472 |
| LDL-Ca (mg/dL) | 80.7 ± 34.9 | 97.7 ± 36.2 | 100.8 ± 38.0 | 0.001c | 90.8 ± 33.9 | 106.9 ± 34.7 | 109.5 ± 35.8 | 0.006c |
| BMIa (kg/m2) | 26.9 ± 3.7 | 26.8 ± 4.3 | 26.1 ± 4.3 | 0.419 | 28.9 ± 5.6 | 28.3 ± 5.6 | 27.9 ± 6.2 | 0.639 |
| Normalweightd | 19 (29.2) | 129 (37.0) | 32 (43.2) | 0.088 | 15 (28.3) | 111 (29.3) | 30 (32.6) | 0.534 |
| Overweightd | 33(50.8) | 146 (41.8) | 29 (39.2) | 0.178 | 17 (32.1) | 141 (37.2) | 33 (35.9) | 0.755 |
| Obesed | 13 (20.0) | 74 (21.2) | 13 (17.6) | 0.698 | 21 (39.3) | 127 (33.5) | 29 (31.5) | 0.358 |
Data are mean ± standard deviation.
Men: ε2 vs. ε3/ ε3, p = 0.009; ε2 vs. ε4, p = 0.006.
Men: ɛ2 vs. ɛ3/ɛ3, p = 0.002; ɛ2 vs. ɛ4, p = 0.004; women: ɛ2 vs. ɛ3/ɛ3, p = 0.008; ɛ2 vs. ɛ4, p = 0.009.
Data are N° (%).
BMI, body mass index; APOE, apolipoprotein E; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Mortality data
A total of 161 deaths (15.9%) occurred during the follow up. Males had significantly higher mortality rates than females (19.3% vs. 12.8%, p = 0.005). Female patients who died had significantly lower mean BMI than female patients who survived (BMI = 26.8 ± 5.2 vs. 28.5 ± 5.7, p = 0.018); this difference was not found in male patients (BMI = 26.1 ± 4.3 vs. 26.8 ± 4.2, p = 0.118). Mortality was significantly higher in normal weight patients than obese patients both in males (40.4% vs. 20.2%, p for trend = 0.001) and female (41.8% vs. 25.4%; p for trend = 0.001). Male patients who died had a significantly lower level of LDL-C (85.8 ± 38.5 vs. 98.2 ± 35.9; p = 0.003) and HDL-C (34.5 ± 15.4 vs. 37.7 ± 12.9; p = 0.041) than patients who survived, whereas TC (151.1 ± 47.0 vs. 159.5 ± 43.1; p = 0.095) and TG levels (118.4 ± 67.4 vs. 113.8 ± 60.2; p = 0.540) did not differ significantly. In female patients, levels of TC (147.6 ± 47.7 vs. 177.16 ± 44.7; p = 0.000), LDL-C (92.8 ± 36.8 vs. 107.6 ± 35.4; p = 0.002), and HDL-C (37.3 ± 14.7 vs. 42.6 ± 15.9; p = 0.016) were significantly lower in patients who died compared to survivors. The APOE ε2 carriers had lower mortality compared with APOE ε3 and ε4 carriers both in males (8.5% vs. 75.5% vs. 16%) and females (6% vs. 80.6% vs. 13.4%) (Table 4). No significant differences in the prevalence of AD were observed between patients who died versus survivors.
Table 4.
Baseline Characteristics of Men and Women by Vital Status
| |
Men |
Women |
||||
|---|---|---|---|---|---|---|
| Survivors (n = 394) | Decedents (n = 94) | p | Survivors (n = 457) | Decedents (n = 67) | P | |
| BMIa (kg/m2) | 26.8 ± 4.2 | 26.1 ± 4.3 | 0.118 | 28.5 ± 5.7 | 26.8 ± 5.2 | 0.018 |
| Normal weightb | 142 (36.0) | 38 (40.4) | 128 (28.0) | 28 (41.8) | ||
| Overweightb | 171 (43.4) | 37 (39.4) | 169 (37.0) | 22 (32.8) | ||
| Obeseb | 81 (20.6) | 19 (20.2) | p for trend 0.001 | 160 (35.0) | 17 (25.4) | p for trend 0.003 |
| Alzheimer diseaseb | 73 (18.5) | 10 (10.6) | 0.219 | 88 (19.3) | 9 (13.4) | 0.082 |
| ε2 carrierb | 57 (14.5) | 8 (8.5) | 0.127 | 49 (10.7) | 4 (6) | 0.228 |
| ε3 carrierb | 273 (70.6) | 71 (75.5) | 0.337 | 325 (71.1) | 54 (80.6) | 0.105 |
| ε4 carrierb | 59 (15) | 15 (16) | 0.811 | 83 (18.2%) | 9 (13.4) | 0.342 |
| TCa (mg/dL) | 159.5 ± 43.1 | 151.1 ± 47.0 | 0.095 | 177.16 ± 44.7 | 147.6 ± 47.7 | 0.000 |
| LDL-Ca (mg/dL) | 98.2 ± 35.9 | 85.8 ± 38.5 | 0.003 | 107.6 ± 35.4 | 92.8 ± 36.8 | 0.002 |
| HDL-Ca (mg/dL) | 37.7 ± 12.9 | 34.5 ± 15.4 | 0.004 | 42.6 ± 15.9 | 37.3 ± 14.7 | 0.016 |
| TGa (mg/dL) | 113.8 ± 60.2 | 118.4 ± 67.4 | 0.540 | 120.9 ± 64.8 | 128.4 ± 85.4 | 0.394 |
Data are mean ± standard deviation.
Data are N° (%).
BMI, Body mass index; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides.
Multivariate logistic regression analysis, adjusted for several confounding factors, indicates that obesity (OR = 0.486, 95% CI 0.255–0.927, p = 0.028), TC > 180 mg/dL (OR = 0.350, 95% CI 0.187–0.657, p = 0.001), and LDL-C > 100 mg/dL (OR = 0.456, 95% CI 0.235–0.882; p = 0.020) are factors significantly associated with a lower risk of mortality in female patients. These associations were not observed in male patients. No significant association between APOE genotype, higher TG, lower HDL-C level, and 1-year mortality was observed (Table 5).
Table 5.
Risk Factor for 1-Year Mortality According to Gender
| |
OR (95% CI) |
|
|---|---|---|
| Men | Women | |
| Normal weight | 1 (reference) | 1 (reference) |
| Overweight | 0.809 (0.488–1.339) | 0.595 (0.325–1.088)a |
| Obese | 0.877 (0.474–1.621) | 0.486 (0.255–0.927)a |
| ε2 carrier | 0.598 (0.270–1.104) | 0.323 (0.094–1.119)b |
| ε3 carrier | 1 (reference) | 1 (reference) |
| ε4 carrier | 1.024 (0.542–1.936) | 0.883 (0.404–1.931)b |
| Hypercholesterolemia | 0.819 (0.488–1.374) | 0.350 (0.187–0.657)c |
| High LDL-C | 0.860 (0.496–1.492) | 0.456 (0.235–0.882)c |
| High TG | 1.197 (0.673–2.130) | 1.316 (0.675–2.568)d |
| Lower HDL-C | 1.640 (0.778–3.458) | 1.338 (0.768–2.330)e |
Data were adjusted for age, diabetes, dyslipidemia, and cardiovascular disease.
Data were adjusted for total-cholesterol and LDL-C levels and Alzheimer disease.
Data were adjusted for age, BMI, cardiovascular disease; APOE genotype.
Data were adjusted for age, BMI, and diabetes.
Data were adjusted for age and cardiovascular disease.
OR, Odds ratio; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; APOE, apolipoprotein E.
Interactions
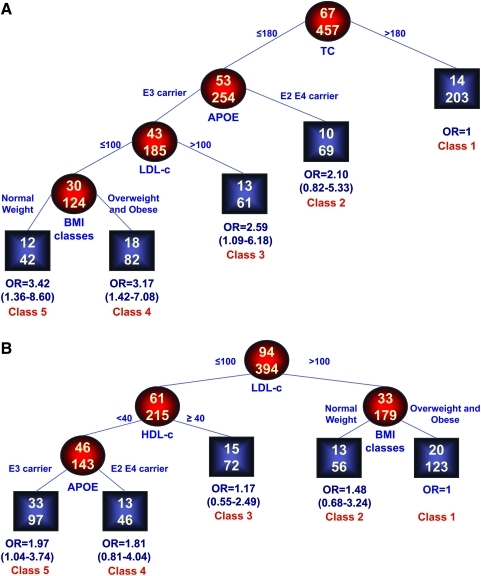
To investigate the interaction between APOE genotype, BMI, lipid levels, and mortality, a RECPAM analysis was performed separately for males and females leading to the identification of five classes of patients at different risk of mortality (Fig. 1A,B). The reference category was represented by the subgroup with the lowest mortality. Thus, the ORs for all the other subgroups were estimated with respect to the reference class.
FIG. 1.
Identification of subgroups at different risks for mortality: Results of RECPAM analysis. REPCAM analysis identified patient subgroups at different risks for mortality. The tree-growing algorithm modeled odds ratios (OR) after a logistic regression with age, diabetes, and ischemic cardiomyopathy as global variables. Splitting variables are shown between branches, whereas a condition sending patients to left or right sibling is on a relative branch. Class 1 with lowest mortality was the reference category (OR = 1). Circles indicate subgroups of patients; squares indicate the patient subgroup REPCAM class. Numbers inside circles and squares represent the number of events (top) and the number of nonevents (bottom), respectively. (A) Females. Global adjustment variables: Age OR = 1.11 (1.06–1.16, diabetes OR = 1.15 (0.57–2.34, ischemic cardiomyopathy OR = 0.71 (0.28–1.81. (B) Males. Global adjustment variables: Age OR = 1.03 (1.00–1.07), diabetes OR = 1.59 (0.81–3.08), ischemic cardiomyopathy OR = 0.52 (0.26–1.03).
In females, the most important variable in discriminating the risk of death was represented by the TC, with the lowest mortality in patients with values of TC greater than 180 mg/dL. On the opposite side of the regression tree, patients APOE ε3 carriers, LDL-C values lower than 100 mg/dL and with a normal weight represented the subgroup with the highest mortality (OR = 3.42, 95% CI = 1.36–8.60). The class of patients with TC lower than 180 mg/dL APOE ε3 carrier but with LDL-C greater than 100 mg/dL showed a significant risk of mortality (OR = 2.59, 95% CI 1.09–6.18). For patients showing a TC lower than 180 mg/dL, APOE ε3 carrier, a LDL-C lower than 100 mg/dL but overweight or obese, the estimated OR = 3.17 (95% CI 1.42–7.08). Patients' age (p < 0.001) and presence of diabetes (p = 0.70), AD, and cardiovascular diseases (p = 0.47) were included in the model as global adjustment variables.
In males, the class of patients with lowest mortality were those with LDL-C higher than 100 mg/dL and overweight or obese. On the opposite side of the regression tree, patients with LDL-C lower than 100 mg/dL, HDL lower than 40 mg/dL, and APOE ε3 carrier showed the highest mortality (OR = 1.97, 95% CI 1.04–3.74). Patients' age (p = 0.057), presence of diabetes (p = 0.175), AD, and cardiovascular disease (p = 0.062) were included in the model as global adjustment variables.
Discussion
The detection of gene–environment interactions can provide key information on the biological mechanisms of diseases and can lead to new insights into the prevention, diagnosis, and treatment of chronic diseases in specific subgroups of the population. In this study, we evaluated the relationship among APOE polymorphism, BMI, and dyslipidemia and how these factors modify overall mortality risk. In agreement with previous studies,19,20 we found that the mean concentrations of TC and LDL-C were APOE genotype and BMI dependent in both male and female elderly patients. In this study, TC and LDL-C levels were significantly higher in obese than in normal weight patients, and in subjects carrying ε4 compared to subjects carrying ε2 and ε3 in both males and females patients. In contrast, we did not find a significant association between APOE alleles and serum HDL-C concentrations in both men and women. A significant association between APOE polymorphism and HDL-C has been reported in some studies21 but not in others.22 Discrepancies among different studies may be due to other factors that regulate the gene–environment interaction affecting HDL-C levels, such as physical activity, alcohol consumption, and smoking.
In this population, no significant association between APOE polymorphism and obesity was found. Only a few studies have examined the relationship between APOE polymorphisms and obesity. Although a significant association between APOE ε4 allele status and obesity was reported in children and young adults,23 other studies failed to find a significant difference in the APOE allele and genotype frequencies between obese and nonobese subjects.24 In addition, none of these studies included hospitalized elderly patients. A possible explanation for the lack of association between BMI and APOE polymorphism in our population is that BMI may be a poor indicator of obesity in elderly persons because body composition varies with age.
In addition, we found that, unlike in nonelderly patients, higher levels of TC in females and LDL-C in males are associated with a lower risk of mortality. This paradoxical result is in line with previous reports showing that hypercholesterolemia is associated with lower mortality in elderly patients.25–27 There are several potential explanations for these findings. It is possible that subjects with higher cholesterol levels died before recruitment and that the sample represents a group of healthy survivors with traits that make them less susceptible to disease caused by high cholesterol levels. Moreover, it is well known that cholesterol levels decrease with age, and it has been suggested that low cholesterol levels in the elderly represent a surrogate marker of frailty or subclinical disease.28
In the present study, multivariate regression analysis shows a negative relationship between BMI and mortality; and the interaction analysis revealed that overweight and obesity are protective in both males and females, even if with a different grade of interaction, as suggested by the different composition of the RECPAM tree-based algorithm, adjusted for age, diabetes, and AD or cardiovascular disease. These findings are in agreement with previous studies.29–31 Several hypotheses have been made to explain this effect of BMI on mortality in the elderly: (1) The relationship among BMI, body fat, and fat distribution weaken with aging; (2) lean mass and fat mass act as important nutritional reserve during prolonged illness and may be particularly important in old age as the incidence and significance of illness and disease increase; (3) individuals susceptible to the ill effects of elevated BMI may have already died, determining a selective survival.
This study has limitations. First, our study evaluated the interaction among APOE polymorphisms, BMI, dyslipidemia, and mortality risk, but did not evaluate the serum concentrations of APOE, which has been shown to influence the levels of circulating lipids.32 Second, in our study, we used BMI as marker of obesity, instead of waist circumference or trunk fat mass measured by dual-energy X-ray absorptiometry (DEXA), which are better predictors of mortality in old age.33 Finally, the use of hospitalized elderly patients does not allow us to exclude that demographic selection could play a role in the reported differences. In conclusion, in elderly hospitalized patients, obesity and APOE genotype influence the lipid profile and mortality risk. A significant interaction among BMI, dyslipidemia, and APOE genotype was observed that could identify elderly patients with different risks of mortality.
Acknowledgments
This research was supported by grants from Ministero della Salute and IRCCS Research Program 2008-2010, Line 2: “Malattie di rilevanza sociale” and was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Author Disclosure Statement
None of the authors has any conflict of interest associated with the work presented in this manuscript. All authors had access to the data and played a role in writing this manuscript.
References
- 1.Denke MA. Sempos CT. Grundy SM. Excess body weight: An under-recognized contributor to dyslipidemia in white American women. Arch Intern Med. 1994;154:401–10. doi: 10.1001/archinte.154.4.401. [DOI] [PubMed] [Google Scholar]
- 2.Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding in cell biology. Science. 1998;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 3.Grönroos P. Raitakari OT. Kähönen M. Hutri-Kähönen N. Marniemi J. Viikari J. Lehtimäki T. Influence of apolipoprotein E polymorphism on serum lipid and lipoprotein changes: a 21-year follow-up study from childhood to adulthood. The Cardiovascular Risk in Young Finns Study. Clin Chem Lab Med. 2007;45:592–598. doi: 10.1515/CCLM.2007.116. [DOI] [PubMed] [Google Scholar]
- 4.Ward H. Mitrou PN. Bowman R. Luben R. Wareham NJ. Khaw KT. Bingham S. APOE genotype, lipids, and coronary heart disease risk: A prospective population study. Arch Intern Med. 2009;169:1424–1429. doi: 10.1001/archinternmed.2009.234. [DOI] [PubMed] [Google Scholar]
- 5.Zannis VI. Genetic polymorphism in human apolipoprotein E. Methods Enzymol. 1986;95:2358–2367. doi: 10.1016/0076-6879(86)28109-4. [DOI] [PubMed] [Google Scholar]
- 6.Bennet AM. Di Angelantonio E. Ye Z. Wensley F. Dahlin A. Ahlbom A. Keavney B. Collins R. Wiman B. de Faire U. Danesh J. Association of apoliprotein E genotype with lipid levels and coronary risk. JAMA. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 7.Corbo RM. Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE e4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63:301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 8.Sudlow C. Martinez Gonzalez NA. Kim J. Clark C. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and meta-analysis of 31 studies among 5961 cases and 17965 controls. Stroke. 2006;37:364–370. doi: 10.1161/01.STR.0000199065.12908.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orsitto G. Seripa D. Panza F. Franceschi M. Cascavilla L. Placentino G. Matera MG. Paris F. Capurso C. Solfrizzi V. Dallapiccola B. Pilotto A. Apolipoprotein E genotypes in hospitalized elderly patients with vascular dementia. Dement Geriatr Cogn Dis. 2007;23:327–333. doi: 10.1159/000100972. [DOI] [PubMed] [Google Scholar]
- 10.Seripa D. Panza F. Franceschi M. D'Onofrio G. Solfrizzi V. Dallapiccola B. Pilotto A. Non-apolipoprotein E and apolipoprotein E genetics of sporadic Alzheimer's disease. Ageing Res Rev. 2009;8:214–236. doi: 10.1016/j.arr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Fillenbaum GG. Burchett BM. Lee JH. Blazer DG. Mortality and apolipoprotein E in African-American, and White elders: An attempted replication. Am J Med Genet. 2003;119A:141–146. doi: 10.1002/ajmg.a.20146. [DOI] [PubMed] [Google Scholar]
- 12.Hayden KM. Zandi PP. Lyketsos CG. Tschanz JT. Norton MC. Khachaturian AS. Pieper CF. Welsh-Bohmer KA. Breitner JC. Cache County Investigators. Apolipoprotein E genotype and mortality: findings from the Cache County Study. J Am Geriatr Soc. 2005;53:935–942. doi: 10.1111/j.1532-5415.2005.53301.x. [DOI] [PubMed] [Google Scholar]
- 13.Slooter AJ. Cruts M. Van Broeckhoven C. Hofman A. van Duijn CM. Apolipoprotein E and longevity: The Rotterdam Study. J Am Geriatr Soc. 2001;49:1258–1259. doi: 10.1046/j.1532-5415.2001.49251.x. [DOI] [PubMed] [Google Scholar]
- 14.Heijmans BT. Slagboom PE. Gussekloo J. Droog S. Lagaay AM. Kluft C. Knook DL. Westendorp RG. Association of APOE epsilon2/epsilon3/epsilon4 and promoter gene variants with dementia but not cardiovascular mortality in old age. Am J Med Genet. 2002;107:201–208. doi: 10.1002/ajmg.10142. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G. Drachman D. Folstein M. Katzman R. Price D. Stadlan EM. Clinical diagnoses of Alzheimer's disease: Report of NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Heart, Lung and Blood Institute; 1998. [Google Scholar]
- 17.Seripa D. Signori E. Gravina C. Matera MG. Rinaldi M. Fazio VM. Simple and effective determination of apolipoprotein E genotypes by positive/negative polymerase chain reaction products. Diagn Mol Pathol. 2006;15:180–185. doi: 10.1097/01.pdm.0000213451.99655.1d. [DOI] [PubMed] [Google Scholar]
- 18.Ciampi A. Negassa A. Lou Z. Tree-structured prediction for censored survival data and the Cox model. J Clin Epidemiol. 1995;48:675–689. doi: 10.1016/0895-4356(94)00164-l. [DOI] [PubMed] [Google Scholar]
- 19.Marques-Vidal P. Bongard V. Ruidavets JB. Fauvel J. Hanaire-Broutin H. Perret B. Ferrières J. Obesity and alcohol modulate the effect of apolipoprotein E polymorphism on lipids and insulin. Obes Res. 2003;11:1200–1206. doi: 10.1038/oby.2003.165. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan SR. Ehnholm C. Elkasabany A. Berenson GS. Apolipoprotein E polymorphism modulates the association between obesity and dyslipidemias during young adulthood: The Bogalusa Heart Study. Metabolism. 2001;50:696–702. doi: 10.1053/meta.2001.23299. [DOI] [PubMed] [Google Scholar]
- 21.Ikewaki K. Rader DJ. Zech LA. Brewer HB., Jr In vivo metabolism of apolipoproteins A-I and E in patients with abetalipoproteinemia: Implications for the roles of apolipoproteins B and E in HDL metabolism. J Lipid Res. 1994;35:1809–1819. [PubMed] [Google Scholar]
- 22.Martin LJ. Connelly PW. Nancoo D. Wood N. Zhang ZJ. Maguire G. Quinet E. Tall AR. Marcel YL. McPherson R. Cholesteryl ester transfer protein and high density lipoprotein responses to cholesterol feeding in men: Relationship to apolipoprotein E genotype. J Lipid Res. 1993;34:437–446. [PubMed] [Google Scholar]
- 23.Srivastava N. Achyut BR. Prakash J. Agarwal CG. Pant DC. Mittal B. Association of cholesteryl ester transfer protein (TaqIB) and apolipoprotein E (HhaI) gene variants with obesity. Mol Cell Biochem. 2008;314:171–177. doi: 10.1007/s11010-008-9778-5. [DOI] [PubMed] [Google Scholar]
- 24.Eto M. Watanabe K. Ishii K. Apolipoprotein E polymorphism and yperlipoproteinemia in obesity. Int J Obes. 1989;13:433–440. [PubMed] [Google Scholar]
- 25.Tikhonoff V. Casiglia E. Mazza A. Scarpa R. Thijs L. Pessina AC. Staessen JA. Low-density lipoprotein cholesterol and mortality in older people. J Am Geriatr Soc. 2005;53:2159–2164. doi: 10.1111/j.1532-5415.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 26.Schupf N. Costa R. Luchsinger J. Tang MX. Lee JH. Mayeux R. Relationship between plasma lipids and all cause mortality in non demented elderly. J Am Geriatr Soc. 2005;53:219–226. doi: 10.1111/j.1532-5415.2005.53106.x. [DOI] [PubMed] [Google Scholar]
- 27.Chyou P. Eaker ED. Serum cholesterol concentrations and all cause mortality in older people. Age Ageing. 2000;29:69–74. doi: 10.1093/ageing/29.1.69. [DOI] [PubMed] [Google Scholar]
- 28.Schalk BW. Visser M. Deeg DJ. Bouter LM. Lower lvels of serum albumin and total cholesterol and future decline in functional performance in older persons: The Longitudinal Aging Study Amsterdam. Age Ageing. 2004;33:266–272. doi: 10.1093/ageing/afh073. [DOI] [PubMed] [Google Scholar]
- 29.Adams KF. Schatzkin A. Harris TB. Kipnis V. Mouw T. Ballard-Barbash R. Hollenbeck A. Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 30.Janssen I. Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 31.Janssen I. morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity. 2007;15:1827–1840. doi: 10.1038/oby.2007.217. [DOI] [PubMed] [Google Scholar]
- 32.Haddy N. De Bacquer D. Chemaly MM. Maurice M. Ehnholm C. Evans A. Sans S. Do Carmo Martins M. De Backer G. Siest G. Visvikis S. The importance of plasma apolipoprotein E concentration in addition to its common polymorphism on inter-individual variation in lipid levels: Results from APO Europe. Eur J Hum Gen. 2002;10:841–850. doi: 10.1038/sj.ejhg.5200864. [DOI] [PubMed] [Google Scholar]
- 33.Price GM. Uauy R. Breeze E. Bulpitt CJ. Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]