Abstract
Irritable bowel syndrome (IBS) is a common chronic disorder seen in gastroenterology and primary care practice. It is characterized by recurrent abdominal pain or discomfort associated with disturbed bowel function. It is a heterogeneous disorder with varying treatments, and in this regard physicians sometimes struggle with finding the optimal approach to management of patients with IBS. This disorder induces high health care costs and variably reduces health-related quality of life. IBS is in the class of functional gastrointestinal disorders, and results from dysregulation of central and enteric nervous system interactions. Psychosocial factors are closely related to their gut physiology, associated cognitions, symptom manifestations and illness behavior. Therefore, it is important for the physician to recognize the psychosocial issues of patients with IBS and in addition to build a good patient-physician relationship in order to optimize treatment. This review focuses on the interaction between psychological and physiological factors associated with IBS by using a biopsychosocial model. In this article, we describe (1) the predisposing psychological features seen in early life; (2) the psychological factors associated with life stress, the symptom presentation, and their associated coping patterns; (3) gut pathophysiology with emphasis on disturbances in motility, visceral hypersensitivity and brain-gut interactions; and finally (4) the clinical outcomes and effective treatments including psychotherapeutic methods.
Keywords: Irritable bowel syndrome, Pathophysiology, Psychology
Introduction
Irritable bowel syndrome (IBS) is a part of the larger group of functional gastrointestinal disorders (FGIDs), which have different bodily locations and symptom patterns, but which share disturbances in the regulation of peripheral, spinal and central pathways that are incompletely understood.1 IBS patients suffer from a chronic gastrointestinal (GI) disorder characterized by recurrent abdominal pain associated with altered bowel habits without obvious structural abnormalities seen on endoscopy or X-ray.2,3 The prevalence is high, affecting up to 20% of adults.4 When moderate to severe in intensity, patients struggle with severe pain or discomfort, abnormal bowel habit, impaired health-related quality of life (HRQOL) and disability.5 This can lead to high work absenteeism, physician visits and health care costs.6,7
Although highly prevalent in society, patients with IBS may not always receive optimal treatment. Physicians, including gastroenterologists, may feel unsure as to how to manage conditions without clear structural findings, since this is how traditionally physicians are trained. They may perceive this as "illness" without "disease." Furthermore, they may not fully understand the multiplicity of factors operative in understanding the pathophysiology which adheres to a brain-gut biopsychosocial model. As such, this disorder can be associated with major psychosocial distress that some physicians may feel ill equipped to manage. Early life experiences including major loss abuse and psychosocial trauma, family influences on illness behavior and maladaptive coping life-styles can influence the clinical expression and severity of IBS. These factors also produce complex interactions that affect biological, psychological and social systems for the individual with IBS. Thus abdominal symptoms as presented by IBS patients are only a part of the full understanding of full syndrome, perhaps an "alarm sign" of a complex of biopsychosocial factors.
In recent years scientific investigations have brought a greater understanding of the pathophysiological processes leading to the symptom complex of IBS and in the process have made these disorders clinically legitimate. The data have also led to improved clinical care and increased public awareness. This report provides a better understanding of these newly understood biopsychosocial determinants as part of an up-to-date synthesis of current research (Table 1).
Table 1.
Biopsychosocial Model and Treatment of Irritable Bowel Syndrome
Biopsychosocial Model
More recent scientific studies link the mind and the body as a part of a system where their dysregulation can produce illness. The term "bio-psycho-social" does not mean merely that psychosocial issues are important, or that psychosocial factors cause medical illness (psychogenic and psychosomatic), or that psychological symptoms result from a medical disorder (somatopsychic or psychologic overlay), or even that stress has physiologic effects (psychophysiologic), although all may be considered as plausible situations subsumed by the model.8-10
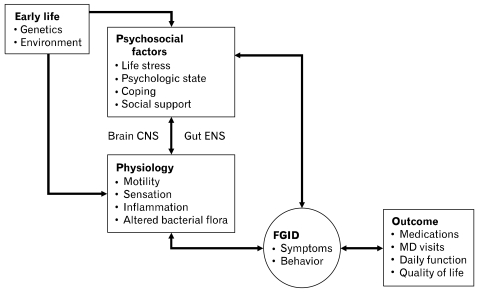
The biopsychosocial model of illness and disease, as described by Engel,8,10,11 has helped understanding the bi-directional relationship between mind and body and reconcileed the dualistic concepts that separated illness and disease. Figure illustrates this biopsychosocial understanding of the relationship between psychosocial and physiological factors associated with functional GI symptoms and the clinical outcome.12
Figure.
A biopsychosocial conceptualization of the pathogenesis and clinical expression of irritable bowel syndrome (IBS). It shows the relationships between psychosocial and physiological factors, IBS symptoms and clinical outcome. CNS, central nervous system; ENS, enteric nervous system; FGID, functional gastrointestinal disorder. MD, medical doctor. Adapted from Drossman.12
Early Life
The natural history of symptoms and risk factors that contribute to IBS may begin in early life. Familial studies of IBS have demonstrated that reporting a first-degree relative with abdominal pain or bowel problems is significantly associated with reporting of IBS symptoms,13 suggesting a strong environmental basis for IBS. Heredity may explain the findings that IBS tends to run in families. Both the Australian twins study14 and the larger US study15 showed concordance rate for IBS between monozygotic twins was significantly higher than that between dizygotic twins. Genetic factors which may be associated with FGIDs may include serotonin transporter,16 IL-1017 and other genetic polymorphisms. Parental attitudes may also be important to the development or clinical expression of IBS. Levy et al15,18 indicated that children of patients with IBS had significantly more health care visits than those without IBS.19,20 Children whose mothers have IBS are prone to having more non-GI symptoms as well as GI symptoms. This may imply that parents tend to pay attention to trivial symptoms, sometimes referred to as "illness behavior".21 Trained parents who do not pay attention to their children's complaints produce fewer chronic functional abdominal symptoms than the parents who do show attention.22 Thus both a genetic and a social learning may contribute to the intergenerational transmission of IBS.
Psychosocial Factors
Life stress
Childhood experiences can affect psychological tendencies. As a child's transition into adulthood, genetics, early learning, custom and other environmental influences are integrated into each unique personality and behavior. They influence the determination of stress levels and their ability to cope with them. Chronic life stress includes divorce, relationship difficulties, serious illness (of self or other), lawsuits, business failures, housing difficulties and forced redundancies. One's perception of control over stressful unresolved life events, sensation-seeking status and degree of psychosocial assets may all mediate the impact of life stress.23
Young children, who suppress the feelings of anger and resentment, may also become conditioned to report somatic symptoms when distressed. They cannot recognize or communicate the association of symptoms with the stressful antecedents, because these antecedents are not acknowledged or attended to within the family.24 This can lead to higher pain scores, more physician visits, and poorer functioning.
A history of physical or sexual abuse also strongly influences the severity of the symptoms with poorer daily function, greater psychological distress, and poorer outcome.25 When compared with patients without abuse history, GI clinic patients who have a history of abuse reported more severe pain and greater psychological distress. They spent more days in bed in the previous 3 months with poorer health status, more often visits to physician in GI referral centers and more surgical procedures.25,26 A history of abuse is not always volunteered by patients. In fact, in 1 study only 17% of the abuse victims had informed their physicians, and 30% of the victims had not previously disclosed this history to anyone.27 The findings suggest that physicians should become aware of many risk factors among patients with chronic or severe refractory symptoms.
Psychological state
IBS symptoms are sometimes exacerbated by stress and can be associated with psychological co-morbidities. In patients with IBS, the most common comorbid psychiatric disorders seen include anxiety disorders (panic and generalized anxiety disorder), depression (including dysthymia), somatoform disorders (hypochondriasis and somatization disorder) and phobic disorders.28,29 Psychological distress also affects the somatic symptoms and its outcome; it lowers the pain threshold30,31 and influences health care seeking for patients with somatic symptoms.32 Psychological co-morbidities are seen between 42% and 61% of those patients with medical disorders and its presence often amplifies or modulates the clinical presentation and outcome.28,33 Psychosocial difficulties may not be recognized by the patient, and this pattern may develop early in life.34,35
Other psychiatric disturbance and personality (trait anger reactivity, neuroticism, extroversion and general hypochondriasis) were strongly related to the patient's behavior and overall severity of functional gut disturbance.36 An abuse history relates to its adverse clinical consequences. They include Axis I and Axis II psychiatric diagnoses of posttraumatic stress disorder, dissociation disorder and major depression with Axis II disorder of borderline personality disorder.8 Those can be associated with altered GI physiology. Drossman et al28 reported a high frequency of sexual and physical abuse history, 44% among women seen at a university-based gastroenterology practice.
Catastrophizing, which has been defined as a cognitive variable associated with exaggerated intestinal pain, emotional distress, a morbid pessimism about the condition, perceived helplessness and functional limitations,37,38 is also associated with more difficult interpersonal relationships, and can contribute to greater worry and suffering in patients with IBS.39 The ability to become consciously aware of one's own feelings is believed to be a cognitive skill requiring certain developmental processes. Ineffective coping patterns, such as abdominal pain and discomfort, sometimes particularly induce to catastrophize. Changed cognitions with impaired memory and intrusive thoughts, hypervigilance to bodily sensations, feelings of ineffectiveness and vulnerability and lack of trust finally alter physiological and even structural effects on the brain. Recent brain imaging studies have shown that there are morphological40 and functional changes in brain imaging among individuals with abuse history.41 The flashbacks and intrusive traumatic memories seen in abuse victims may result from stress-mediated alterations in noradrenergic neurons projects to the hippocampus, the brain area involved with the encoding and retrieval of memory.36,42 Therefore, consistent with the biopsychosocial model, psychosocial trauma may lead both to physiological and even anatomic alterations.
The above features also interfere with the patient's ability to interact with family, peer groups, and physicians, or to engage in a treatment plan. It is important for physicians to recognize these maladaptive patterns and maintain clear boundaries of medical care. They may refer to clinical skills in the mental health professional, when feeling the need of particular specific interviewing skills or to assess multiple complex variables.43 Psychological distress in IBS patients can usually be reduced with appropriate treatment, whereas patients with IBS who have more marked personality features are much less amenable to treatment.42 However, there is no personality profile unique to IBS.28,32
Physiology
Abnormal motility and sensitivity
Patients with IBS show abnormal GI motility and visceral hypersensitivity. Colonic segmental contractions and activity are exaggerated during stress in IBS patients.44 IBS patients are also likely to indicate lower pain thresholds to balloon distention of the colon compared with healthy controls.45 Recent studies may explain the physiological abnormalities observed in IBS. A number of enterochromaffin cells which contain serotonin in the gut mucosa are more increasing in patients with IBS than controls.46 IBS patients demonstrated that mast cell proximity to enteric sensory nerves correlated with the severity and frequency of abdominal pain or discomfort.47,48
Brain-gut interaction
As the physiological abnormalities in IBS are closely correlated with psychological stress, the combined functioning of GI intestinal motor, sensory and CNS activity is thought to be an important factor on development of IBS symptoms, which is termed as the brain-gut interactions.49 The brain-gut axis is a bidirectional and integrated system in which thoughts, feelings, memories and environmental influences can lead to neurotransmitter release that affects sensory, motor, endocrine, autonomic, immune and inflammatory function.50,51 In IBS patients, it may result from dysregulation of central and enteric nervous system, which induces the dysmotility or visceral sensitivity, and is modified by psychosocial processes. In the end, they determine the experience of the illness.52 Therefore, the concepts of the brain-gut interactions help us to understand pathophysiology of IBS.
Psychological distress is generated by a network comprised of integrative brain structures, in particular, subregions of the hypothalamus, amygdala, and medial thalamus and anterior cingulate cortex. They form an integral part of the limbic system, which is regarded as the "visceral" or "emotional" brain.53-55 In the limbic system and the cerebral cortex, there are a lot of histamine H1 binding sites.56 The H1 receptor blockage induced shortening of cerebral evoked potential latencies after electrical painful stimulation of the rectum, suggesting that the brain histamine or its receptors may play an important role for nociceptive process in the brain.57 Stress-induced damage to the hippocampus resulted in long-lived hyperactivity of corticotropin-releasing hormone (CRH). CRH is also an important factor on central responses to stressful situations.58 CRH is distributed in the whole brain with dense localization in the paraventricular nucleus of the hypothalamus.59 CRH stimulates pituitary ACTH secretion, which releases glucocorticoids from the adrenal glands. It has been reported that CRH aggravated visceral sensorimotor function as well as ACTH response in patients with IBS.58 Increased hypothalamic-pituitary-adrenal responsiveness to stress produces the glucocorticoids, which in turn, increase the expression of inflammation and induce the production of cytokines.
Postinfectious IBS (PI-IBS) model may be a good example of brain and gut interaction. Psychological factors may partly explain the increased reports of PI-IBS,60-62 a condition in which at least a subgroup of IBS patients reported onset of their IBS symptoms after a resolution of acute GI infection. Prospective studies have found that anxiety and depression are significant and independent risk factors of PI-IBS.42,60,61,63 However, visceral sensation thresholds or motility were not different between PI-IBS and those who recover from infection without developing IBS.64-66 These support a better understanding of brain-gut interaction to relate to PI-IBS, with visceral sensitization and high levels of psychological distress.
Outcome
IBS status shows an aspect of illness. "Illness" is defined as the personal experience of the medical condition, which is evident from the person's symptom reports, perceptions, and behavior. "Disease" is abnormalities in the structure and function of organs and tissues. It is externally verifiable by evidence from a pathological state. Illness can be inappropriately dichotomized to a disease, or organic disorder, which presumably distinguish medical (organic) from psychological (functional) illness or relegates functional illness to a condition with no cause or treatment.8
Appropriate coping and social supports may manage the effects of life stress, abuse, and morbid psychological factors on the illness and its outcome. Coping has been defined as "problem solving efforts, both action-oriented and intrapsychic, to manage external and internal demands and conflicts that are apprised as taxing or exceeding a person's resources".67 In general, emotion-focused coping, although possibly adaptive for acute overwhelming stresses, is not effective for chronic stressors. Whereas problem-focused coping strategies (eg, seeking social support or reappraising the stressor) involves efforts by finding out one's response to the stressor and learning skills to manage the problems, that is more effective for chronic illness. Social supports through family, supportive relationships, social participation and social networks can have similar benefits in reducing the impact of stressors on physical and mental illness, thereby improving ability to cope with the illness.68 Therefore, IBS patients, who have severe and chronic life threats, may lack of satisfactory interpersonal support. They also cannot improve the symptom intensity over time and reduce the HRQOL.28,69
Treatment
To control IBS symptoms, some medications based on the pathophysiology are often used. Motility and pain modifying medications, such as enteric agents, fiber, prokinetics, are useful, in particular, for mild IBS.70 However, physicians may treat patients with more severe symptoms whose medications are not working as well as hoped. Because psychological factors often adversely affect IBS, psychopharmacological or psychological treatments can be employed to reduce their impact on the GI symptoms. Furthermore, good psychosocial treatment must also rest on good relationships between physician and patients - the patient-physician relationship.
Approach to the Patient
In the course of caring and evaluating for patients, different perspectives between patients and physicians may occur with regard to the role of psychosocial factors in the illness. Certainly psychosocial factors may influence symptoms and behaviors in ways that are not readily treatable by disease-specific methods.8 Some patients, for example those who have experienced severe traumatic events such as sexual abuse, may initially be unwilling to accept the role of psychosocial factors in the illness. It is important for the physician to develop an effective patient-physician interaction, to improve the therapeutic outcome. This can be established with attention to several issues: (1) make an effort to understand and offer empathy when patients report the experience of the illness; (2) obtain the history and acknowledge the role of the patient's psychological factors; (3) clarify any misunderstandings; (4) provide education how to manage the illness, including psychosocial function; and (5) make a plan of treatment with the patient.29,71
Physicians should also obtain histories about the associations between bowel symptoms and psychosocial factors. Obtaining information that relates the daily pattern of the symptoms to aggravating factors such as the timing of menstruation, dietary or life-style changes and stressors can provide the basis for behavioral modification or even cognitive-behavioral strategies. Thus, the therapeutic plan should be individualized and established on the relationships between the patient and physician.
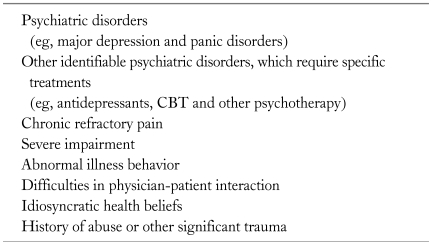
It is preferable for gastroenterologists and primary care doctors to work closely with mental health professional, if available. In particular, this should occur if the patient shows (1) a severe depression, which may be accompanied by suicidal ideas, (2) chronic refractory pain, (3) severe disability, (4) maladaptive illness behavior, (5) difficulties in physician-patient interaction, (6) idiosyncratic health beliefs and (7) other identifiable psychiatric difficulties (somatization disorder, post-traumatic stress disorder and severe anxiety), history of abuse that leads to continuing distress and/or marked distress (Table 2).28,72
Table 2.
Consultation to Mental Health Professionals
CBT, cognitive behavioral therapy.
Psychopharmacology
Psychopharmacologic agents work on neurotransmitter receptors in the brain-gut regulatory pathways that target serotonergic, dopaminergic, opioidergic and noradrenergic receptor sites in the brain. Their effects may be to reduce central modulation of visceral afferents, moderate visceral pain, and improve underlying psychiatric conditions, such as anxiety, depression, nausea, and loss of appetite.70 Prescribing psychopharmacologic agents is best accomplished in the context of a strong physician-patient relationship, where these agents are complementary to an overall multicomponent treatment plan. The physician is required to explain the possible side effects, and expected benefits from the medication and address to ensure consistent treatment at an appropriate dose level over a longer period of time (2-3 months) than change rapidly from one drug to another. The tricyclic antidepressants (TCAs), which block transporters of norepinephrine and serotonin, in relatively low doses can reduce chronic pain.73 Selective serotonin reuptake inhibitors (SSRI) are used to reduce anxiety and are particularly helpful for psychiatric comorbid conditions, including major depression, panic disorder and other high-anxiety conditions.74 However, SSRIs have little antinociceptive agents and their prokinetic effect which produce cramping, nausea and diarrhea, the patient with abdominal pain, diarrhea or nausea would do well with a TCA. The serotonin-norepinephrine reuptake inhibitors and the noradrenergic and specific serotonergic antidepressant are relatively new classes of antidepressants that have substantial serotonergic and noradrenergic effects (unlike the SSRIs) to be particularly helpful for the treatment of painful conditions, but without the antihistaminic and anticholinergic effects of the TCAs that lead to most of the side effects, including dry mouth, difficulty urinating, constipation, decrease in sexual ability, nausea, increased heart rhythms, migraine, appetite change, and weight gain.72,75-77
Anxiolytic agents, particularly the benzodiazepines, are effective for reducing anxiety in the short term, particularly if the anxiety is associated with stress induced flare-ups of bowel disturbance.70 With considering the long-term basis of CNS depressant effect, the potential benefit should be balanced with the long-term risks of sedation, drug interactions, habituation and rebound after withdrawal.
In order to avoid side effects of these drugs, psychopharmacologic medication would be started with low doses and then gradually increased. Some side effects often go away after the first few weeks of drug medication. Medication should be stopped with intolerable side effects. Other strategies may exist to manage psychopharmacologic-induced dysfunction, including waiting, reducing, use of adjunctive pharmacotherapy, and switching to another drugs.
Opiates have little role in treating patients with chronic pain or psychosocial disturbance and often induce narcotic bowel syndrome. A narcotic help the pain at first, but the progressive and paradoxical increase in abdominal pain is followed despite continued or escalated dosages of narcotic prescribed in an effort to relieve the pain.30,78 It can be seen in the patient who often visits emergency department or the clinical service with no diagnosis or meaningful treatment plan and is prescribed with opiates.71
The placebo response rate in IBS trials is high, which is approximately 47%,79 and providing placebo treatment with defined positive physician-patient relationships showed higher clinical outcome.80 An effective physician-patient relationship would help to manage a consistent plan of narcotic withdrawal coupled with the initiation of effective alternative treatments to manage the pain and bowel symptoms.
Psychotherapies
Psychological treatments (eg, cognitive behavioral therapy, dynamic psychotherapy and hypnotherapy) are often appropriate for IBS patients.81 Cognitive behavioral therapy helps IBS patients to learn new ways of thinking and behaving skills for taking a more proactive role in controlling symptoms, coping with their emotional anxieties, and improving illness beliefs in chronic pain.82,83 Dynamic psychotherapy consists of close relationships between the patient and therapist, which induce the patient to overcome internal resistance and learn how to respond in such relationship.83 Hypnotherapy, which alters the conscious state, requires specialized training to be used in IBS.57,84 Psychological treatments are, as a class of interventions, effective in reducing symptoms compared with a pooled group of control conditions.85 The most important aspect of treatment is the patient's acceptance of the need for treatment and his or her motivation to engage in it. This can be indicated if the gastroenterologist and psychologist/psychiatrist help the patient to accept the treatment as a necessary part of an overall plan of care.
Conclusion
IBS is often explained as a "functional" disorder, meaning the inclusion of both physiological and psychological factors. When an IBS patient is first seen, he or she often mainly complains about chronic abdominal symptoms, such as pain, discomfort, and diarrhea. While some physicians may focus primarily on "abdominal" symptoms, from the point of view of biopsychosocial model, stress can play an important part in the full clinical expression and outcome of the disorder. Therefore, physicians should focus on integrating the medical components of the illness with the patient's psychological aspects and try to build a more satisfactory treatment on this understanding.
Using the concept of biopsychosocial model may be a good way to explain the interaction between psychological and physiological factors, which may exist in patients with IBS. However, research methods of brain and gut, or stress and cognition are limited, thus further studies are awaited.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. Rome II. The functional gastrointestinal disorders. Diagnosis, pathophysiology and treatment: a multinational consensus. 2nd ed. McLean, VA: Degnon Associates; 2000. pp. 1–764. [Google Scholar]
- 2.Drossman DA, Ritcher JE, Talley NJ, et al. Functional gastrointestinal disorders. Boston: Little Brouwn; 1994. pp. 1–174. [Google Scholar]
- 3.Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–1508. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 4.Gwee KA, Bak YT, Ghoshal UC, et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189–1205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]
- 5.Drossman DA, Patrick DL, Whitehead WE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 6.Longstreth GF, Drossman DA. Severe irritable bowel and functional abdominal pain syndromes: managing the patient and health care costs. Clin Gastroenterol Hepatol. 2005;3:397–400. doi: 10.1016/s1542-3565(05)00084-4. [DOI] [PubMed] [Google Scholar]
- 7.Ananthakrishnan AN, McGinley EL, Saeian K. Length of office visits for gastrointestinal disease: impact of physician specialty. Am J Gastroenterol. 2010;105:1719–1725. doi: 10.1038/ajg.2010.172. [DOI] [PubMed] [Google Scholar]
- 8.Drossman DA. Presidential address: gastrointestinal illness and the biopsychosocial model. Psychosom Med. 1998;60:258–267. doi: 10.1097/00006842-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Halpert A, Drossman D. Biopsychosocial issues in irritable bowel syndrome. J Clin Gastroenterol. 2005;39:665–669. doi: 10.1097/01.mcg.0000174024.81096.44. [DOI] [PubMed] [Google Scholar]
- 10.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 11.Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137:535–544. doi: 10.1176/ajp.137.5.535. [DOI] [PubMed] [Google Scholar]
- 12.Drossman DA. The functional gastrointestinal disorders and the Rome III process. In: Drossman DA, Corazziari E, Delvaux M, Spiller R, Talley NJ, Thompson WG, Whitehead WE, et al., editors. Rome III: the functional gastrointestinal disorders. 3rd ed. McLean, VA: Degnon Associates; 2006. pp. 1–30. [Google Scholar]
- 13.Locke GR, 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ., 3rd Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907–912. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 14.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 15.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 16.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonsalkorale WM, Perrey C, Pravica V, Whorwell PJ, Hutchinson IV. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut. 2003;52:91–93. doi: 10.1136/gut.52.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy RL, Whitehead WE, Walker LS, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol. 2004;99:2442–2451. doi: 10.1111/j.1572-0241.2004.40478.x. [DOI] [PubMed] [Google Scholar]
- 19.Levy RL, Whitehead WE, Von Korff MR, Feld AD. Intergenerational transmission of gastrointestinal illness behavior. Am J Gastroenterol. 2000;95:451–456. doi: 10.1111/j.1572-0241.2000.01766.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanazawa M, Endo Y, Whitehead WE, Kano M, Hongo M, Fukudo S. Patients and nonconsulters with irritable bowel syndrome reporting a parental history of bowel problems have more impaired psychological distress. Dig Dis Sci. 2004;49:1046–1053. doi: 10.1023/b:ddas.0000034570.52305.10. [DOI] [PubMed] [Google Scholar]
- 21.Levy RL, Whitehead WE, Walker LS, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol. 2004;99:2442–2451. doi: 10.1111/j.1572-0241.2004.40478.x. [DOI] [PubMed] [Google Scholar]
- 22.Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani TA. Parent attention versus distraction: impact on symptom complaints by children with and without chronic functional abdominal pain. Pain. 2006;122:43–52. doi: 10.1016/j.pain.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 24.Kirmayer LJ, Young A. Culture and somatization: clinical, epidemiological, and ethnographic perspectives. Psychosom Med. 1998;60:420–430. doi: 10.1097/00006842-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Drossman DA, Li Z, Leserman J, Toomey TC, Hu YJ. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology. 1996;110:999–1007. doi: 10.1053/gast.1996.v110.pm8613034. [DOI] [PubMed] [Google Scholar]
- 26.Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med. 1995;123:782–794. doi: 10.7326/0003-4819-123-10-199511150-00007. [DOI] [PubMed] [Google Scholar]
- 27.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–833. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 28.Drossman DA, Creed FH, Olden KW, Svedlund J, Toner BB, Whitehead WE. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999;45(suppl 2):II25–II30. doi: 10.1136/gut.45.2008.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drossman DA, Chang L. Psychosocial factors in the care of patients with GI disorders. In: Yamada T, editor. Textbook of gastroenterology. Philadelphia: Lippincott-Raven; 2003. pp. 636–654. [Google Scholar]
- 30.Drossman DA, Ringel Y, Vogt BA, et al. Alterations of brain activity associated with resolution of emotional distress and pain in a case of severe irritable bowel syndrome. Gastroenterology. 2003;124:754–761. doi: 10.1053/gast.2003.50103. [DOI] [PubMed] [Google Scholar]
- 31.Drossman DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology. 1997;112:2120–2137. doi: 10.1053/gast.1997.v112.agast972120. [DOI] [PubMed] [Google Scholar]
- 32.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin North Am. 2000;84:1313–1327. doi: 10.1016/s0025-7125(05)70288-1. [DOI] [PubMed] [Google Scholar]
- 34.Walker EA, Roy-Byrne PP, Katon WJ, Li L, Amos D, Jiranek G. Psychiatric illness and irritable bowel syndrome: a comparison with inflammatory bowel disease. Am J Psychiatry. 1990;147:1656–1661. doi: 10.1176/ajp.147.12.1656. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98(5 Pt 1):1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 36.Bennett EJ, Piesse C, Palmer K, Badcock CA, Tennant CC, Kellow JE. Functional gastrointestinal disorders: psychological, social, and somatic features. Gut. 1998;42:414–420. doi: 10.1136/gut.42.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lackner JM, Gurtman MB. Pain catastrophizing and interpersonal problems: a circumplex analysis of the communal coping model. Pain. 2004;110:597–604. doi: 10.1016/j.pain.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Cano A, Leonard MT, Franz A. The significant other version of the Pain Catastrophizing Scale (PCS-S): preliminary validation. Pain. 2005;119:26–37. doi: 10.1016/j.pain.2005.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lackner JM, Quigley BM, Blanchard EB. Depression and abdominal pain in IBS patients: the mediating role of catastrophizing. Psychosom Med. 2004;66:435–441. doi: 10.1097/01.psy.0000126195.82317.46. [DOI] [PubMed] [Google Scholar]
- 40.Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 42.Creed F. The relationship between psychosocial parameters and outcome in irritable bowel syndrome. Am J Med. 1999;107:74S–80S. doi: 10.1016/s0002-9343(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 43.Porter M, Gorman D. Approaches to somatisation. BMJ. 1989;298:1332–1333. doi: 10.1136/bmj.298.6684.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukudo S, Nomura T, Muranaka M, Taguchi F. Brain-gut response to stress and cholinergic stimulation in irritable bowel syndrome. A preliminary study. J Clin Gastroenterol. 1993;17:133–141. doi: 10.1097/00004836-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990;43:377–386. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- 46.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 48.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 49.Paquette V, Lévesque J, Mensour B, et al. "Change the mind and you change the brain": effects of cognitive-behavioral therapy on the neural correlates of spider phobia. Neuroimage. 2003;18:401–409. doi: 10.1016/s1053-8119(02)00030-7. [DOI] [PubMed] [Google Scholar]
- 50.Tougas G. The autonomic nervous system in functional bowel disorders. Gut. 2000;47(suppl 4):iv78–iv80. doi: 10.1136/gut.47.suppl_4.iv78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology and psychosomatic medicine: back to the future. Psychosom Med. 2002;64:15–28. doi: 10.1097/00006842-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45(suppl 2):II1–II5. doi: 10.1136/gut.45.2008.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tucker DM, Luu P, Pribram KH. Social and emotional self-regulation. Ann N Y Acad Sci. 1995;769:213–239. doi: 10.1111/j.1749-6632.1995.tb38141.x. [DOI] [PubMed] [Google Scholar]
- 54.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59:489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- 55.Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 56.Kanba S, Richelson E. Histamine H1 receptors in human brain labelled with [3H]doxepin. Brain Res. 1984;304:1–7. doi: 10.1016/0006-8993(84)90856-4. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe S, Hattori T, Kanazawa M, Kano M, Fukudo S. Role of histaminergic neurons in hypnotic modulation of brain processing of visceral perception. Neurogastroenterol Motil. 2007;19:831–838. doi: 10.1111/j.1365-2982.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- 58.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrusz P, Merchenthaler I. The corticotropin-releasing factor system. In: Nemeroff CB, editor. Neuroendocrinology. Boca Raton, Florida: CRC Press; 1992. pp. 129–183. [Google Scholar]
- 60.Gwee KA, Graham JC, McKendrick MW, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 61.Marshall JK, Thabane M, Garg AX, et al. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605–611. doi: 10.1136/gut.2009.202234. [DOI] [PubMed] [Google Scholar]
- 62.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 63.Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410–413. doi: 10.1136/gut.51.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 65.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drossman DA. Mind over matter in the postinfective irritable bowel. Gut. 1999;44:306–307. doi: 10.1136/gut.44.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer-Verlag; 1984. pp. 117–139. [Google Scholar]
- 68.Berkman LF. Assessing the physical health effects of social networks and social support. Annu Rev Public Health. 1984;5:413–432. doi: 10.1146/annurev.pu.05.050184.002213. [DOI] [PubMed] [Google Scholar]
- 69.Jones MP, Wessinger S, Crowell MD. Coping strategies and interpersonal support in patients with irritable bowel syndrome and inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:474–481. doi: 10.1016/j.cgh.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 70.Camilleri M, Bueno L, de Ponti F, Fioramonti J, Lydiard RB, Tack J. Pharmacological and pharmacokinetic aspects of functional gastrointestinal disorders. Gastroenterology. 2006;130:1421–1434. doi: 10.1053/j.gastro.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 71.Drossman DA. Psychosocial sound bites: exercises in the patient-doctor relationship. Am J Gastroenterol. 1997;92:1418–1423. [PubMed] [Google Scholar]
- 72.Levy RL, Olden KW, Naliboff BD, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–1458. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 73.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Creed F. How do SSRIs help patients with irritable bowel syndrome? Gut. 2006;55:1065–1067. doi: 10.1136/gut.2005.086348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–747. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- 76.Friedrich M, Grady SE, Wall GC. Effects of antidepressants in patients withirritable bowel syndrome and comorbid depression. Clin Ther. 2010;32:1221–1233. doi: 10.1016/j.clinthera.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Yin J, Wang W, Winston JH, Zhang R, Chen JD. Ameliorating effects of mirtazapine on visceral hypersensitivity in rats with neonatal colon sensitivity. Neurogastroenterol Motil. 2010;22:1022–1028. e267. doi: 10.1111/j.1365-2982.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 78.Rogers M, Cerda JJ. The narcotic bowel syndrome. J Clin Gastroenterol. 1989;11:132–135. [PubMed] [Google Scholar]
- 79.Patel SM, Stason WB, Legedza A, et al. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332–340. doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 80.Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 82.Toner BB. Cognitive-behavioral treatment of functional somatic syndromes: integrating gender issues. Cogn Behav Pract. 1994;1:157–178. [Google Scholar]
- 83.Levy RL, Olden KW, Naliboff BD, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–1458. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 84.Hattori T, Watanabe S, Kano M, Kanazawa M, Fukudo S. Differntial responding of autonomic function to histamine H1 antagonism in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:1284–1291. e335. doi: 10.1111/j.1365-2982.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 85.Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. 2004;72:1100–1113. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]