Abstract
Background/Aims
There are few reports on the correlation between chewing gum and the gastrointestinal functions. But previous report showed use of chewing gum to be an effective method for controlling gastrointestinal symptoms. The aim of this study was to determine the correlation between chewing gum and gastric emptying using the continuous real time 13C breath test (BreathID system).
Methods
Ten healthy male volunteers participated in this randomized, 2-way crossover study. The subjects fasted overnight and were randomly assigned to chewing gum (Xylish, 2-3/1 tablet) for an hour following intake of a test meal (200 kcal/200 mL) or intake of the test meal alone. Gastric emptying was monitored for 4 hours after administration of the test meal by the 13C-acetic acid breath test performed continually using the BreathID system.
Results
No significant differences in the calculated parameters, namely, T1/2 (median, 111.82 vs 109.26 minutes; P = 0.575), Tlag (median, 53.28 vs 56.53 minutes; P = 0.333), gastric emptying coefficient (median, 3.58 vs 3.65; P = 0.285), regression-estimated constant β (median, 1.85 vs 1.80; P = 0.575) and regression-estimated constant κ (median, 0.61 vs 0.62; P = 0.959) were observed between the test meal alone group and the test meal and chewing gum group.
Conclusions
This study showed that chewing gum had no effect on the rate of gastric emptying. Therefore, since chewing gum did not enhance the speed of gastric emptying, it may ameliorate gastrointestinal symptoms through other mechanisms, such as saliva and autonomic nervous system.
Keywords: Breath test, Chewing gum, Gastric emptying
Introduction
In Japan, use of chewing gum has prevailed ever since World War II. There are few reports on the correlation between chewing gum and the gastrointestinal functions.
But, the previous study showed that consistently chewing gum increased the esophageal and pharyngeal pH.1 According to another study, chewing gum increased the salivary flow rate and controlled acid reflux and the related symptoms.2 Gastric emptying may cause retention of solid foods, gastric distention, resultant increase of gastric acid secretion and transient lower esophageal sphincter relaxation.3,4 We hypothesized that chewing gum may increase chewing activity, saliva production, gastric motility and thereby accelerate the rate of gastric emptying.
In this study, we investigated the physiologic effect of chewing gum on the rate of gastric emptying using a continuous real-time 13C breath test (BreathID system, Exalenz Bioscience Ltd, Modiin, Israel).5-13
Materials and Methods
Subjects
The subjects were 10 asymptomatic male volunteers, none of whom were habitual drinkers. None had a history of gastrointestinal disease or abdominal surgery, and all were non-smokers. None of the subjects were on any routine medication at the time of the study. The study was conducted in accordance with the declaration of Helsinki. The study protocol using the BreathID system was approved by the Ethics Committee of Yokohama City University School of Medicine.
13C-Acetic Acid Breath Test
Ten subjects participated in this randomized, 2-way crossover study. They were randomly assigned to receive a test meal and a chewing gum (Xylish, Meiji Seika, Tokyo, Japan; 1 pack [17 g/12 tablets] include: carbon 13.5 g, xylitol 6.7 g, 36 kcal, protein 0 g, lipid 0.1 g, Na 0 mg and sugar 0 g), or only a test meal. The 2 tests were conducted as follows; (1) experiment A: the subjects chewed gum for an hour just minutes after intake of test meal and (2) experiment B: the subjects only took a test meal without chewing gum. The 2 tests were conducted separately with a washout period of at least 7 days according to previous reports.6-10 In both the experiments, the tests were started after the patients had fasted overnight (at least 8 hours), and the breath test was performed while the subjects were seated.
The test meal was a 200 kcal/200 mL liquid meal (Racol with milk flavor, Otsuka Pharmaceutical, Tokyo, Japan; 1 pack [200 mL] include protein 8.76 g, fat 4.46 g and carbon 31.24 g) containing 100 mg of 13C-acetic acid (Cambridge Isotope Laboratories, Boston, MA, USA),5-16 which the patients were requested to consume within 5 minutes. This protocol was Japanese standardized method by Japan society of smooth muscle research.5-9 After the meal intake, the subjects, participating in experiment A were asked to chew 2-3 chewing gums for an hour. On the other hand, the subjects participating in experiment B took the breath test without chewing gum after the meal intake. Breath samples were continuously collected via a nasal tube using the BreathID system (Exalenz Bioscience Ltd) at baseline before the test meal and following completion of the test meal ingestion (time 0) for up to 4 hours.5-13
Data analysis of the 13C-acetic acid breath test
The data from the 13C breath test were analyzed using Oridion Research Software (β version, Oridion Medical Ltd, Jerusalem, Israel). The time versus 13CO2 excretion rate curve was fitted to the conventional formula of z(t) = m(1-e-kt)β, and the regression-estimated constants (β and κ) were determined.17 After mathematical analysis, the time required for the emptying of 50% of the labeled meals (T1/2), the analog to the scintigraphy lag time for 10% emptying of the labeled meal (Tlag), the gastric emptying coefficient (GEC), and β and κ were calculated. A larger (smaller) β indicates slower (faster) emptying in the early phase, and a larger (smaller) κ indicates faster (slower) emptying in the later phase.
Statistical Methods
Statistical evaluation was carried out using the Wilcoxon's signed-rank test. The level of significance was P < 0.050. Statistical analyses were performed with the Stat View software (SAS Institute, Cary, NC, USA).
Results
13C-Acetic Acid Breath Test
Ten male subjects (mean age, 22; median age, 22; range, 20-28 years) completed this study. No adverse events occurred during the study. The subjects' mean height was 171.1 with median height of 170 cm (range, 165-178 cm), mean weight of 63.9 kg and median weight of 63 kg (range, 53-74 kg).
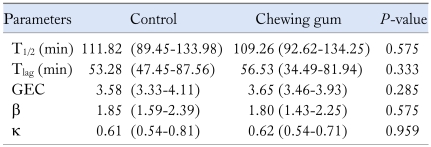
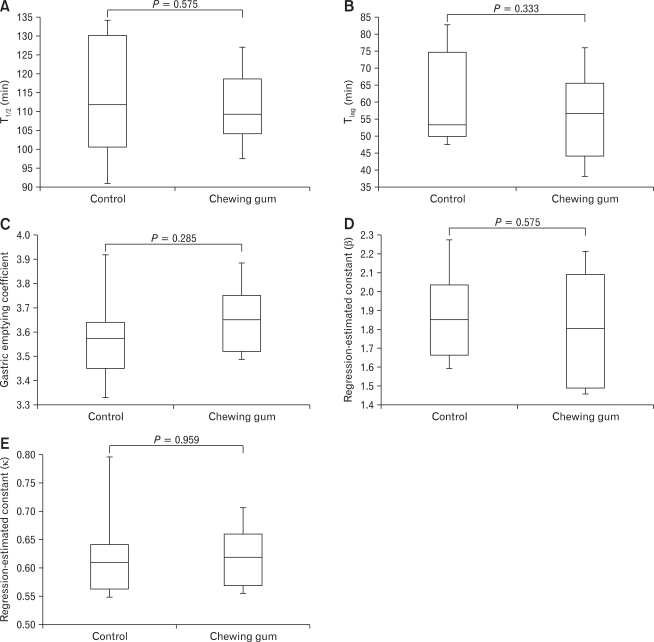
Table summarizes the chewing gum-induced changes in the breath test parameters. No significant differences were found in the T1/2 (median: range, 111.82: 89.45-133.98 vs 109.26: 92.62-134.25 minutes; P = 0.575) (Fig. 1A), Tlag (median: range, 53.28: 47.45-87.56 vs 56.53: 34.49-81.94 minutes; P = 0.333) (Fig. 1B), GEC (median: range, 3.58: 3.33-4.11vs 3.65: 3.46-3.93; P = 0.285) (Fig. 1C), β (median: range, 1.85: 1.59-2.39 vs 1.80: 1.43-2.25; P = 0.575) (Fig. 1D) and κ (median: range, 0.61: 0.54-0.81 vs 0.62: 0.54-0.71; P = 0.959) (Fig. 1E), between the control and chewing gum groups.
Table.
Comparison of the Breath Test Parameters Between the Control and Chewing Gum Groups
T1/2, time taken for emptying of 50% of the labeled meals; Tlag, the analog to the scintigraphy lag time for 10% emptying of the labeled meal; GEC, gastric emptying coefficient; β and κ, regression-estimated constant.
All values represent median values (range).
Figure 1.
No significant differences were found in the T1/2 (A), Tlag (B), gastric emptying coefficient (C), regression-estimated constant β (D) or regression-estimated constant κ (E) between the 2 study condition, indicating that chewing gum had no significant effect on the rate of gastric emptying.
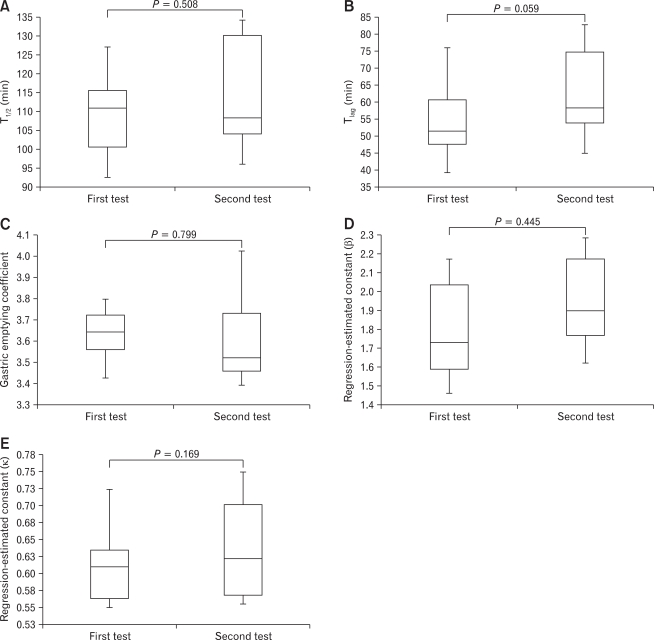
Because carry over or order effect was estimated, we carried out statistical tests between the first and second test. No significant differences were found in the T1/2 (median: range, 110.78: 92.44-134.25 vs 108.28: 89.45-133.98 minutes; P = 0.508) (Fig. 1A), Tlag (median: range, 51.405: 34.49-81.94 vs 58.065: 41.97-87.56 minutes; P = 0.059) (Fig. 1B), GEC (median: range, 3.64: 3.33-3.84 vs 3.52: 3.33-4.11; P = 0.799) (Fig. 1C), β (1.73: 1.43-2.25 vs 1.90: 1.49-2.39; P = 0.445) (Fig. 1D) and κ (median: range, 0.61: 0.54-0.81 vs 0.62: 0.54-0.78; P = 0.169) (Fig. 1E), between the control and chewing gum groups.
Discussion
These study examined changes in the rate of gastric emptying during the first 4 hours after gum chewing for an hour in healthy subjects.
The 13C-acetic acid breath test is a noninvasive and well-established test for measuring the rate of gastric emptying of liquid meals and has been shown to be significantly correlated with the results of scintigraphy.5-16 The subject ingests 13C-labeled acetic acid, which passes through the stomach and is absorbed into the duodenum and upper small bowel. The 13C-labeled acetic acid is then metabolized in the liver and excreted from the lung as 13CO2. This pathway enables gastric emptying to be measured in a noninvasive manner.5-21
The BreathID system allows continuous evaluation of gastric emptying. For patients, it can serve as a real-time breath analysis. It also decreases the examination time and alleviates patient discomfort. Continuous analysis also provides quick, immediate results.5-13
We hypothesized that chewing of gum increases the chewing activity, saliva production, gastric motility, thereby accelerating the rate of gastric emptying. However, in this study, chewing gum did not affect the rate of gastric emptying. We were not able to demonstrate why chewing gum did not affect the rate of gastric emptying. Our results could be related to the form of our test meals, the volume of meals, the starting time of chewing gum and the duration of chewing.
In this study, we used a 200 kcal/200 mL liquid meal (Racol with milk flavor, Otsuka Pharmaceutical, Tokyo, Japan; 1 pack [200 mL] include protein 8.76 g, fat 4.46 g and carbon 31.24 g) as the test meal.5-16 Although this protocol was Japanese standardized method by Japan society of smooth muscle research,5-9 the protocol was not used in major studies for reasons of small volume, light calorie and liquid meal. In Japan, we usually chew gum before meal or after heavy rich meal. We should research during fasting status or after heavy test meal.
The previous study showed that gum chewing consistently increased the esophageal and pharyngeal pH.1 According to another study, when gastroesophageal reflux occurs, the refluxate volume and acid clearance from the esophagus depend on 2 major mechanisms. Peristalsis initiates esophageal clearance, and swallowed saliva neutralizes the residual acid.2 A common dietary stimulant for the production of saliva is chewing gum. Chewing gum increases the salivary flow rate.2 Use of chewing gum has been shown as a comparatively safe and effective method of controlling acid reflux and the related symptoms.2 Various type of foods has been shown to promote reflux either by decreasing the lower esophageal sphincter pressure, stimulating gastric secretion or noxiously interacting directly with the oesophageal mucosa.3 Over time, gastric emptying may cause retention of solid foods, gastric distention, resultant increase of gastric acid secretion and transient lower esophageal sphincter relaxation.3,4 Therefore, the esophageal and pharyngeal pH values are increased after chewing.
Sugar content has been suggested to influence the osmotic detector in the duodenum and delayed gastric emptying. However in this study, the chewing gum used as the test food contained little sugar.
The parasympathetic activity is reduced in patients who are unable to masticate and swallow food, resulting in adverse effects on the gastric motor and excretion functions. Mastication and swallowing not only prepare food for passage from the oral cavity into the esophagus, but are also important in terms of subsequent events that occur in the stomach. It has been proposed that autonomic nervous activity might be involved in mastication and swallowing.22,23 Therefore, we thought that chewing of gum might accelerate gastric emptying. We guess that the form of test meals, their volume, the starting time of chewing gum, duration of chewing, gum volume and the relatively small number of subjects might have influenced the results of our study.
Lunding suggested that in functional dyspepsia patients, but not healthy volunteers, the motility index increased and intragastric volume tended to increase following sham feeding.24 In functional dyspepsia patients, vagal stimulation by sham feeding improved the antral motility in response to a soup meal. Similar results were obtained in healthy volunteers, such as in our study.
In conclusion, our study showed that chewing of gum had no significant effect on the rate of gastric emptying. The physiological implications of our results remain unclear, and further investigation is needed.
Figure 2.
No significant differences were found in the T1/2 (A), Tlag (B), gastric emptying coefficient (C), regression-estimated constant β (D) or regression-estimated constant κ (E) between the 2 study condition, indicating that carry over had no significant effect on the rate of gastric emptying.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Smoak BR, Koufman JA. Effects of gum chewing on pharyngeal and esophageal pH. Ann Otol Rhinol Laryngol. 2001;110:1117–1119. doi: 10.1177/000348940111001206. [DOI] [PubMed] [Google Scholar]
- 2.Moazzez R, Bartlett D, Anggiansah A. The effect of chewing sugar-free gum on gastro-esophageal reflux. J Dent Res. 2005;84:1062–1065. doi: 10.1177/154405910508401118. [DOI] [PubMed] [Google Scholar]
- 3.Avidan B, Sonnenberg A, Schnell TG, Sontag SJ. Walking and chewing reduce postprandial acid reflux. Aliment Pharmacol Ther. 2001;15:151–155. doi: 10.1046/j.1365-2036.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 4.Kimura Y, Nomura M, Sawada Y, Muraoka N, Kohno N, Ito S. Evaluation of the effects of mastication and swallowing on gastric motility using electrogastrography. J Med Invest. 2006;53:229–237. doi: 10.2152/jmi.53.229. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka H, Inamori M, Fujisawa N, et al. Two cases of pyloduodenal stenosis: the efficiency of gastric emptying evaluation using 13C continuous breath test (BreathID system) Digestion. 2006;74:238. doi: 10.1159/000100968. [DOI] [PubMed] [Google Scholar]
- 6.Inamori M, Akiyama T, Akimoto K, et al. Early effects of peppermint oil on gastric emptying: a crossover study using continuous real time 13C breath test (BreathID system) J Gastroenterol. 2007;42:539–542. doi: 10.1007/s00535-007-2067-3. [DOI] [PubMed] [Google Scholar]
- 7.Inamori M, Iida H, Endo H, et al. Aperitif effects on gastric emptying: a crossover study using continuous real-time 13C breath test (BreathID system) Dig Dis Sci. 2009;54:816–818. doi: 10.1007/s10620-008-0427-3. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda T, Inamori M, Fujisawa N, et al. Effects of body positions on gastric emptying with enteral nutrition: a crossover study using a continuous real time 13C breath test (BreathID system) Hepatogastroenterology. 2008;55:1905–1907. [PubMed] [Google Scholar]
- 9.Akimoto K, Inamori M, Iida H, et al. Does postprandial coffee intake enhance gastric emptying?: a crossover study using continuous real time 13C breath test (BreathID system) Hepatogastroenterology. 2009;56:918–920. [PubMed] [Google Scholar]
- 10.Mawatari H, Inamori M, Fujita K, et al. The continuous real-time 13C-octanoate breath test for patients with nonalcoholic steatohepatitis using the BreathID system. Hepatogastroenterology. 2009;56:1436–1438. [PubMed] [Google Scholar]
- 11.Shimoyama Y, Kusano M, Kawamura O, et al. High-viscosity liquid meal accelerates gastric emptying. Neurogastroenterol Motil. 2007;19:879–886. doi: 10.1111/j.1365-2982.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- 12.Zai H, Kusano M, Hosaka H, et al. Monosodium L-glutamate added to a high-energy, high-protein liquid diet promotes gastric emptying. Am J Clin Nutr. 2009;89:431–435. doi: 10.3945/ajcn.2008.26180. [DOI] [PubMed] [Google Scholar]
- 13.Zai H, Kusano M. Investigation of gastric emptying disorders in patients with functional dyspepsia reveals impaired inhibitory gastric emptying regulation in the early postcibal period. Digestion. 2009;79(suppl 1):13–18. doi: 10.1159/000167861. [DOI] [PubMed] [Google Scholar]
- 14.Mossi S, Meyer-Wyss B, Beglinger C, et al. Gatric emptying of liquid meals measured noninvasively in humans with [13C]acetate breath test. Dig Dis Sci. 1994;39(12 suppl):107S–109S. doi: 10.1007/BF02300386. [DOI] [PubMed] [Google Scholar]
- 15.Sanaka M, Anjiki H, Yamamoto T, Kuyama Y. Rabeprazole delays gastric emptying of a nutrient liquid. J Gastroenterol Hepatol. 2007;22:1806–1809. doi: 10.1111/j.1440-1746.2006.04763.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanaka M, Yamamoto T, Anjiki H, Nagasawa K, Kuyama Y. Effects of agar and pectin on gastric emptying and post-prandial glycaemic profiles in healthy human volunteers. Clin Exp Pharmacol Physiol. 2007;34:1151–1155. doi: 10.1111/j.1440-1681.2007.04706.x. [DOI] [PubMed] [Google Scholar]
- 17.Futagami S, Iwakiri K, Shindo T, et al. The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying. J Gastroenterol. 2010;45:413–421. doi: 10.1007/s00535-009-0173-0. [DOI] [PubMed] [Google Scholar]
- 18.Shindo T, Futagami S, Hiratsuka T, et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion. 2009;79:65–72. doi: 10.1159/000205740. [DOI] [PubMed] [Google Scholar]
- 19.Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640–1647. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- 20.Anjiki H, Sanaka M, Kuyama Y. Dual effects of rabeprazole on solid-phase gastric emptying assessed by the 13C-octanoate breath test. Digestion. 2005;72:189–194. doi: 10.1159/000088465. [DOI] [PubMed] [Google Scholar]
- 21.Sanaka M, Anjiki H, Tsutsumi H, et al. Effect of cigarette smoking on gastric emptying of solids in Japanese smokers: a crossover study using the 13C-octanoic acid breath test. J Gastroenterol. 2005;40:578–582. doi: 10.1007/s00535-005-1591-2. [DOI] [PubMed] [Google Scholar]
- 22.Wøjdemann M, Treberg P, Stadil F, et al. Effect of sham feeding and acute suppression of acid secretion on human gastric lipase secretion. Am J gastroenterol. 1998;93:244–248. doi: 10.1111/j.1572-0241.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 23.Helman CA. Chewing gum is as effective as food in stimulating cephalic phase gastric secretion. Am J gastroenterol. 1988;83:640–642. [PubMed] [Google Scholar]
- 24.Lunding JA, Nordström LM, Haukelid AO, Gilja OH, Berstad A, Hausken T. Vagal activation by sham feeding improves gastric motility in functional dyspepsia. Neurogastroenterol Motil. 2008;20:618–624. doi: 10.1111/j.1365-2982.2007.01076.x. [DOI] [PubMed] [Google Scholar]