Abstract
The degeneration of dopaminergic neurons in the course of Parkinson disease is largely blamed on oxidative damage in the brain. This study examined the potency of glutathione peroxidase-1 (GPX-1) to protect dopaminergic neurons against toxicity induced by the parkinsonian neurotoxin 6-hydroxydopamine (6-OHDA). We generated pLV-GPX1, a recombinant lentivirus vector carrying the coding sequence for human GPX-1, into the SK-N-MC neuroblastoma cell line. The pLV-GPX1–infected neurons showed an over 3-fold increase in enzyme expression and a 2.6-fold increase in enzyme activity compared to the pLV-EGFP–infected control cells. In the pLV-GPX1–infected cells, we also detected significantly increased neuronal survival and resistance to 6-OHDA–mediated toxicity compared to our controls (75 ± 4% versus 51 ± 7%, p < 0.001). To maximize this protection, the neurons were treated with conditioned medium taken from growing primary astrocytes (astro-CM). We found the treated pLV-GPX1–infected neurons even more significantly resistant to 6-OHDA toxicity compared to their untreated counterparts (86 ± 5% versus 75 ± 4%, p < 0.001). Concomitant with increased neuroprotection, co-presence of overexpressed GPX-1 and astro-CM significantly increased glutathione (GSH) levels compared to when either of the two was present (p < 0.001). Further analysis showed nearly 2.7-fold reduction, in the presence of astro-CM, of hydrogen peroxide (H2O2) levels released from the pLV-GPX1–infected neurons compared to control groups (p < 0.001). Finally, regression analysis between H2O2 levels and cell viability showed that co-presence of GPX-1 and astro-CM reduced 33% of cell death rate (p < 0.05). These data highlight the antioxidant properties of GPX-1 in protecting dopaminergic neurons and further emphasize the capacity of astrocytes in pumping growth-inducing factors that may synergize with GPX-1 to accelerate neuroprotection.
Introduction
Parkinson Disease (PD) is characterized by selective degeneration of midbrain dopaminergic (DAergic) neurons1 and disintegration of their nigrostriatal fibers.2,3 There is no known single factor that initiates DAergic neuron death in PD, but environmental toxins and genetic abnormalities are suspected causes.4
Oxidative stress plays an important role in PD development and progression.5 Various sources, including mitochondrial dysfunction and glutathione (GSH) reduction, cause oxidative stress by accelerating reactive oxygen species (ROS) production.6 Depletion of GSH levels over 40% reported in the substantia nigra (SN) of PD patients7,8 results in unlimited generation of ROS that damage mitochondrial complexes.9 A well-known role of GSH in preventing mitochondrial impairment is hydrogen peroxide (H2O2) removal via glutathione peroxidase and reductase.10
Glutathione peroxidase-1 (GPX-1) is a selenium-dependent antioxidant enzyme that converts H2O2 to water at the expense of oxidizing GSH to its disulfide form (GSSG).11 GPX-1 prevents perturbances to redox status and attenuates oxidation of lipid proteins, nicotinamide adenine dinucleotide phosphate (NADPH), and nicotinamide adenine dinucleotide (NADH).12 The antioxidant activities of GPX-1 documented in a diverse range of tissues and pathophysiological conditions have been reviewed by Lei et al.13
GPX-1 is largely found in microglia and to a lesser extent in neurons and astrocytes in the nervous system.14 The expression of GPX-1 is reportedly upregulated in PD brain, particularly in microglia,15 and GPX-1+ microglia increase around surviving DAergic neurons.16 Neurons are able to direct GPX-1 and other oxidative enzymes to specific regions of cell toxicity. In this case, the intracellular distribution of GPX-1 in degenerating DAergic neurons indicated that Lewy bodies producing high levels of H2O2 are fully surrounded by GPX-1.15 These observations indicate an active role for GPX-1 in protecting DAergic neurons in PD and further suggest that the enzyme protects other proteolytic enzymes from oxidative stress.
The detoxifying role of GPX-1 against ROS-mediated cytotoxicity in PD has been demonstrated by studies on GPX-1-transgenic animals that involved both GPX-1 overexpression and downregulation.17,18 Transgenic mice overexpressing GPX-1 showed increased resistance to paraquat-induced toxicity compared to their wild-type littermates, whereas the GPX-1 knockout mice were found more susceptible to such lethality.19 Likewise, GPX-1 overexpression attenuated 6-hydroxydopamine (6-OHDA) toxicity in DAergic neurons both in vitro and in vivo.20–22 In contrast, deletion of GPX-1 in mice rendered their nigral DAergic neurons more susceptible to 6-OHDA-induced injury.23,24
Astrocytes support neuronal maintenance and long-term survival. In the case of DAergic neurons, our in vitro studies clearly indicate that they survive parkinsonian toxicity better when co-cultured with astrocytes.25 The astrocytic cells engineered to oversecrete neurotrophic factors conferred an even higher level of neuronal protection.25 In the current study, we examined the effect of GPX-1 overexpression on DAergic neurons intoxicated with 6-OHDA. We found the enzyme to be protective of the neurons and that co-presence of GPX-1 and astro-CM significantly improves their survival rates.
Materials and Methods
Construction of recombinant lentivirus vector
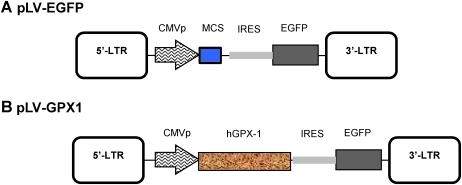
All three lentiviral vectors (transfer, pNL-EGFP/CMV-WPRE [pLV-EGFP]; packaging, pCD/NL-BH*ΔΔΔ; and envelope, pLTR-G) were kind gifts of Dr. J. Reiser.26 pLV-EGFP was digested with NheI/XhoI and blunted with the Klenow fragment. An internal ribosome entry site (IRES)–enhanced green fluorescent protein (EGFP) fragment was excised from pIRES2-EGFP (Clontech) using BglII/NotI restriction enzymes and ligated to the digested transfer plasmid to produce pLV-IRGFP. The pHIH-Ad2 plasmid containing human GPX-1 (hGPX-1) cDNA was kindly provided by Dr. J. Loscalzo.27 The NotI/HindIII GPX-1 fragment was isolated from pHIH-Ad2, blunt ended with Klenow fragment, and ligated to pLV-IRGFP already linearized with BamHI and blunt ended by Klenow fragment to generate pLV-GPX1 (Fig. 1).
FIG. 1.
Construction of pLV-GPX1. (A) pLV-EGFP was used both as the initial lentivirus transfer vector for subcloning steps and as an empty vector control throughout the study. (B) pLV-GPX1: the coding sequence for human glutathione peroxidase-1 (hGPX-1) was placed under the cytomegalovirus (CMV) promoter and in an internal ribosome entry site (IRES) with enhanced green fluorescent protein (EGFP) in pLV-EGFP. MCS, Multicloning site; CMVp, CMV promoter; LTR, long terminal repeat. (Color image available at www.liebertpub.com/rej).
Cell culture
The human embryonic kidney cell line 293T (HEK-293T) (ATCC CRL-11268) and the DAergic cell line SK-N-MC (ATCC HB-10) were both maintained and expanded in Dulbecco modified Eagle medium (DMEM) (GIBCO/Invitrogen) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. Primary cultures of ventral mesencephalon (VM) astrocytes were isolated from day-1 C57BL/6 mice (Pasteur Institute, Tehran) in accordance with the rules of Ethical Committee in Shahid Beheshti University of Medical Sciences and regulations set by Iran's Health Ministry. The procedure previously described25 was followed with minor changes. Briefly, VM tissue was dissected from freshly removed brains, mechanically dissociated, and then cultured at a density of 2 × 105 cells/mL in poly-D-lysine–coated six-well plates that contained DMEM supplemented with 10% FBS. All cell culture supplies were purchased from GIBCO/Invitrogen.
Recombinant lentivirus production and cell infection
HEK-293T cells were seeded in 10-cm-diameter cell culture dishes at a density of 2 × 106 cells/dish and transfected by standard DNA–calcium phosphate co-precipitation 24 h later. To produce mature recombinant lentiviruses, the packaging (10 μg) and the envelope (8 μg) vectors were used for co-transfection with each lentivirus transfer vector (pLV-EGFP control or pLV-GPX-1) (15 μg). We collected cell supernatant 24 and 48 h posttransfection, filtered it, and then applied it to Amicon columns-100K (Millipore) to obtain 500-μL volumes of concentrated virus stocks. For infection, we applied 100 μ of each stock directly into the medium of SK-N-MC cells seeded in six-well plates a day before. The infected cells were expanded either in T75 flasks for freezing or in 10-cm-diameter cell culture dishes for further analysis.
RNA extraction and reverse transcriptase polymerase chain reaction
Ninety six hours after cell infection, total RNA was extracted using a High Pure RNA Isolation Kit (Roche), DNase treated, and tested for its integrity by agarose gel electrophoresis. Two micrograms of each RNA sample was used for cDNA synthesis in a 30-min reaction at 42°C using murine leukemia virus (MuLV) reverse transcriptase (Fermentas, Lithuania) in the presence of random hexamers and RNase inhibitor. The reaction product was then visualized using gel electrophoresis. An equal amount of each cDNA sample was subjected to reverse transcriptase polymerase chain reaction (RT-PCR). The following primers were designed and synthesized based on the hGPX-1 coding sequence28 (GenBank accession no. M83094):
Forward, 5′-CTTATCGAGAATGTGGCGTCCC-3′, reverse, 5′-GCCCACCAGGAACTTCTCAAAG-3′.
To ensure of cDNA quality and sample equality, we also used glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers for each cDNA:
Forward, 5′-TTGCCATCAATGACCCCTTCA-3′, reverse, 5′-CGCCCCACTTGATTTTGGA-3′.
To amplify the 387-bp hGPX-1 fragment, the samples were first denatured at 95°C for 2 min. PCR reactions were carried out for 30 cycles and consisted of denaturation at 94°C for 45 sec, annealing at 58°C for 45 sec, and extension at 72°C for 45 sec. The PCR products were run on a 1.2% agarose gel electrophoresis, and the images were analyzed for the density of DNA bands using US-Scan-It Gel Analysis Software (Skill Scientific Inc. Orem, Utah). The RT-PCR analysis was repeated three times as three independent experiments.
Measurement of GPX-1 activity
Cells were lysed by repeated freeze/thawing. We adapted a method reported by St. Clair and Chow29 to determine the rate of NADPH oxidation as an indication of GPX-1 activity. This method involves two steps: (1) Hydroperoxides are reduced by GSH to the oxidized glutathione (GSSG), a reaction that is catalyzed by GPX-1; (2) GSSG is reduced back to GSH by GSH reductase, which uses the reducing equivalent of NADPH. We incubated up to 100 μL of each cell extract in 875 μL of a solution that contained 1 mM glutathione, 0.1 M sodium phosphate buffer (pH 7.2), 1 mM EDTA, and 1 U of glutathione reductase for 4 min. We then added 8.4 mL of NADPH and measured NADPH oxidation by determining its reduced absorbance at 340 nm over 3 min. After adding 160 μL of 1 mM cumene hydroperoxide, we measured the absorbance again for 3 min.
Cell treatment with astro-CM
At 72–96 h after infection, half of the cell medium was replaced with a 48-h-old medium of 2-week-old growing primary astrocytes (astro-CM). The infected cells were allowed to grow for another 24 h before refeeding them with their normal medium and 48 h before starting 6-OHDA treatment.
6-OHDA treatment
A 5-mM stock of 6-OHDA (Sigma) containing 0.9% saline and 0.1% ascorbate was prepared and filter-sterilized before storing it in aliquots at −20°C. A day before 6-OHDA treatment, SK-N-MC cells were seeded in 96-well plates at the density of 3,000/well. To determine the median lethal dose (LD50) of SK-N-MC cells, we treated the cells with serial dilutions of 6-OHDA in serum-free DMEM for 48 h before testing their viability. Triplicate wells were set up for each 6-OHDA dilution, and the entire treatment was repeated three times as independent experiments. Once the LD50 was determined, we applied it to the rest of experiments in which 6-OHDA treatment was needed.
MTT reduction assay
A 0.5-mg/mL stock of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was prepared in PBS and stored at 4°C until use. From this stock, we added 20 μL to 180 μL serum-free DMEM to each well after removing the 6-OHDA medium; the cells were incubated at 37°C with 5% CO2 for 3 h. Finally, we replaced this medium with 200 μL of dimethylsulfoxide (DMSO), incubated the cells for 5 min, and read the absorbance at 580 nm.
Measurement of GSH and GSSG content
Protein lysates prepared by repeated freeze/thawing of cell samples and precipitated with 5% (wt/vol) sodium metaphosphoric acid were used to determine reduced GSH content as described by Browne and Armstrong.30 Briefly, the supernatant was incubated with o-phthalaldehyde (OPT) for 15 min before reading fluorescence at 420 nm at an excitation wavelength of 350 nm. To determine GSSG levels, the samples were incubated with N-ethylmaleimide for 20 min and then with OPT for 15 min before reading fluorescence. The results for each sample were expressed as nmole/mg protein in the original cell extract and used to calculate GSH/GSSG ratios. Standard curves were generated by applying reduced GSH and GSSG (Sigma) as described.30
H2O2 assay
The Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen) was used to prepare stock solutions, draw standard curves, and measure H2O2 in our cell media. Briefly, for the H2O2 assay, we loaded serial dilutions of H2O2 from 0 to 10 μM and our diluted samples each in 50 μL into individual wells on a microplate. To initiate reactions, we added 50 μL of Amplex Working Solution (consisting of 50 μL of 10 mM Amplex Red Stock Solution, 100 μL of 10 U/mlL HRP Stock Solution, 4.45 mL of 1 × Reaction Buffer) to each well and incubated the plate at room temperature in dark for 30 min. At the end, we measured the absorbance at 560 nm using a microplate reader and corrected the data for the no-H2O2 background.
Statistical analysis
Data in the figures are represented as the mean ± standard error of the mean (SEM) of three or more separate experiments carried out in triplicate. We used an unpaired Student t-test to analyze differences between two groups. Differences between three or more groups were analyzed by one-way analysis of variance (ANOVA), followed by a post hoc Duncan multiple-comparisons test in SPSS version 16. We considered a value of p < 0.05 (a, b, d, f) as statistically significant, and p < 0.01 (aa, ff) or p < 0.001 aaa, bbb, ccc, ddd, eee, or fff) as highly significant.
Results
Virus production and GPX-1 overexpression
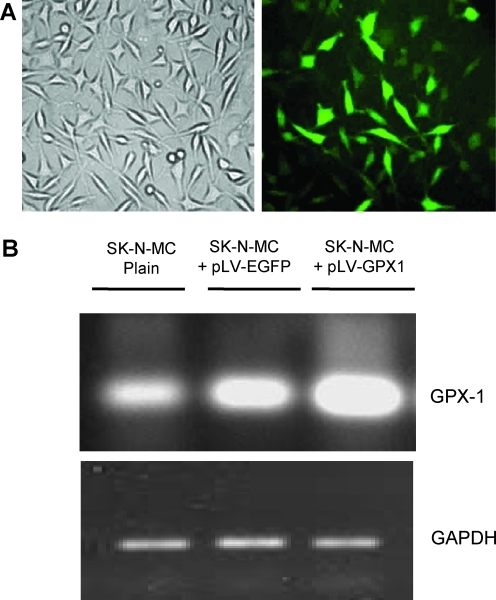
We modified pLV-EGFP (Fig. 1A) to generate our final construct pLV-GPX1 in which the cytomegalovirus (CMV) promoter drove expression of GPX-1 placed in the IRES with EGFP (Fig. 1B). We used a fraction of each lentivirus stock to infect SK-N-MC cells, an established neuroblastoma cell line that expresses tyrosine hydroxylase and produces dopamine. Hereafter, we will designate plain and pLV-EGFP–infected SK-N-MC cells as the control groups and pLV-GPX1–infected cells as the test group. Infected cells in both groups began expressing EGFP 48 h postinfection (Fig. 2A) that reached its saturation in many individual cells by 72 h. At this time point, 100% of cells were detected to be positive for EGFP. RT-PCR analysis of infected cells showed an over 3-fold increase of GPX-1 mRNA expression in the test group compared to pLV-EGFP controls (Fig. 2B). Unexpectedly, these control cells expressed slightly more GPX-1 than uninfected plain cells.
FIG. 2.
Enhanced green fluorescent protein (EGFP) and glutathione peroxidase-1 (GPX-1) overexpression in SK-N-MC cells. (A) Cells that began expressing EGFP 48 h postinfection with pLV-GPX1. (B) Agarose gel image of reverse transcriptase polymerase chain reaction (RT-PCR) samples. Total RNA was collected from infected cells and applied for RT-PCR reactions using hGPX-1 primers. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also amplified as a control and loaded onto a separate gel. (Color image available at www.liebertpub.com/rej).
Enhanced GPX-1 activity
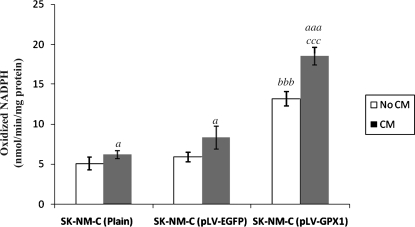
We used NADPH oxidation as a standard method to determine GXP-1 enzymatic activity in infected cells. As shown in Fig. 3, NADPH oxidation in the absence of astro-CM increased from 5.0 ± 0.8 and 5.9 ± 0.6 in plain and pLV-EGFP controls, respectively, to 14.8 ± 0.9 in the test group (Fig. 3, both controls versus pLV-GPX1, p < 0.001; unpaired Student t-test). NADPH oxidation in the presence of astro-CM increased from 6.3 ± 0.5 and 8.4 ± 1.4 in plain and pLV-EGFP controls, respectively, to 18.5 ± 1.1 in the test group (Fig. 3, both controls versus test group, p < 0.001). We also observed an increase in GPX-1 activity in all astro-CM–treated cell groups compared to their untreated counterparts. This increase was significant in both controls (p < 0.05), but highly significant in the test group (p < 0.01).
FIG. 3.
Measurement of glutathione peroxidase-1 (GPX-1) enzymatic activity. Results are expressed as mean of three or more observations in nmol/min per mg of protein. Statistical differences in nicotinamide adenine dinucleotide phosphate (NADPH) activity between untreated and astro-CM (CM)-treated cells within each group are indicated by a (P < 0.05) or by aaa (p < 0.001), whereas statistical differences between control groups (plain or pLV-EGFP cells) and test group (pLV-GPX1 cells) are indicated by bbb before treatment with CM and by ccc after treatment with CM (p < 0.001).
GPX-1-mediated protection of SK-N-MC cells
Using serial concentrations of 6-OHDA, we determined 100 μM as the LD50 for the SK-N-MC cell line and applied it to the rest of our experiments. We treated the test group in parallel with the controls with 100 μM 6-OHDA to examine their viability (Fig. 4A). In the presence of 6-OHDA, only 53.2 ± 5% plain and 51.1 ± 7% pLV-EGFP control cells survived. In comparison, an average 75.2 ± 4% pLV-GPX1–infected cells were found alive (Fig. 4B, both controls versus pLV-GPX1, p < 0.001; Duncan multiple-comparisons test). Despite their upregulated expression of GPX-1 shown earlier in Fig. 2, pLV-EGFP control cells did not show different susceptibility to 6-OHDA toxicity than plain cells.
FIG. 4.
Glutathione peroxidase-1 (GPX-1)–mediated protection of SK-N-MC cells against 6-hydroxydopamine (6-OHDA) toxicity. (A) Infected cells were treated with 200 μM 6-OHDA for 48 h and their images were collected. A fraction of pLV-GPX1–infected cells were also fed with astro-CM (CM). (B) 6-OHDA–treated cells in both the absence and presence of CM were tested for their viability using the MTT reduction assay. Each column represents the average of three independent experiments carried out in triplicate. Statistical differences in cell survival rate between untreated and CM-treated cells within the test group (pLV-GPX1 cells) are indicated by aa (p < 0.01), whereas statistical differences between control groups (plain cells or pLV-EGFP cells) and test group (pLV-GPX1 cells) are indicated by bbb before treatment with CM and by ccc after treatment with CM (p < 0.001).
Contribution of astro-CM to neuroprotection
When we added astro-CM from growing primary astrocytes, this improved cell survival rate in both controls and the test group against 6-OHDA toxicity (Fig. 4B). Our plain and pLV-EGFP controls were protected by 57.2 ± 5% and 58.4 ± 8%, respectively, upon astro-CM treatment. These figures showed a slight and statistically insignificant increase compared to those in the untreated counterparts of the cells (Fig. 4B). However, 86.5 ± 5% of the test cells survived toxicity upon astro-CM treatment that was highly significant compared to 75.2 ± 4% achieved before treatment (Fig. 4B, p < 0.01; Duncan multiple-comparisons test). Compared to both controls, this figure was also highly significant (p < 0.001; Student t-test).
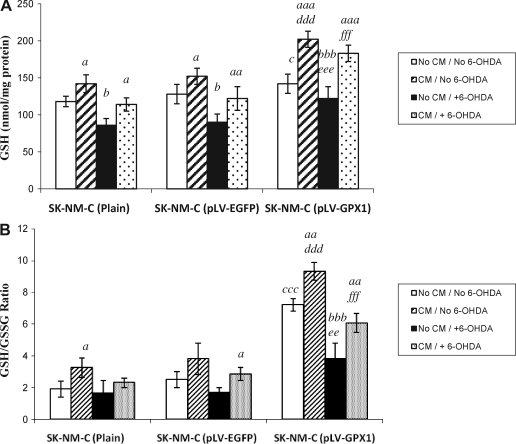
Elevated GSH levels in the co-presence of GPX-1 and astro-CM
As shown in Fig. 5A, all three SK-N-MC cell groups showed comparable levels of GSH in the absence of any treatment, and GPX-1 overexpression per se did not significantly affect GSH content. Addition of astro-CM, however, resulted in significant increase in GSH levels in both control groups (p < 0.05; Duncan multiple-comparisons test). 6-OHDA treatment alone resulted in significantly diminished levels of GSH in both control groups (p < 0.001 for plain and 0.01 for pLV-EGFP). These data reflect the failure of treated cells to replenish the lost levels of GSH to its normal level, observations consistent with similar reports in vivo.31,32 Interestingly, astro-CM partially compensated the lost levels of GSH in these cells that was significant compared to their untreated counterparts (p < 0.05 for plain and p < 0.01 for pLV-EGFP cells).
FIG. 5.
Measurement of glutathione (GSH) content in neuronal cell extracts. (A) GSH levels in each sample, and (B) GSH/oxidized glutathione (GSSG) ratio calculated upon measurement of both reduced and oxidized glutathione (see Methods). Each column represents the average of at least three independent experiments carried out in triplicate. Statistical differences in GSH levels and GSH/GSSG ratios between untreated cells and their astro-CM (CM)-treated counterparts within each cell group are shown by a (p < 0.05), aa (p < 0.01), or aaa (p < 0.001). Statistical differences between healthy cells and their 6-hydroxydopamine (6-OHDA)–treated counterparts are shown by b (p < 0.05) or bbb (p < 0.001). On the other hand, differences between control groups (plain or pLV-EGFP) and test group (pLV-GPX1) before any treatment are shown by c (p < 0.05) or ccc (p < 0.001). Differences between the controls and test group after CM treatment but before 6-OHDA treatment are shown by ddd (p < 0.001), whereas differences between controls and test group before CM treatment but after 6-OHDA treatment are shown by ee (p < 0.01) or by eee (p < 0.001). Finally, differences between the controls and the test group after treatment with both CM and 6-OHDA are shown by fff (p < 0.001).
Addition of astro-CM alone to our test group significantly increased GSH levels (p < 0.001). Although 6-OHDA alone highly and significantly reduced GSH levels within the controls groups, the intoxicated test group cells did not show outstanding changes in GSH levels compared to their untreated counterparts. We indeed found significant difference in GSH levels between the pLV-EGFP control and test groups upon 6-OHDA treatment (p < 0.001; Student t-test). More importantly, astro-CM treatment of the same intoxicated test group cells augmented GSH content to the level comparable to that in their healthy counterparts. This level of GSH was significantly higher than that observed in pLV-EGFP control counterparts (p < 0.001; Student t-test).
Proportional changes in GSH/GSSG ratio were also detected in each cell group (Fig. 5B). This ratio was significantly higher in healthy test cells compared to both control groups (p < 0.001; Student t-test) both before and after astro-CM treatment. Addition of astro-CM significantly increased the ratio in healthy plain (p < 0.05; Duncan multiple-comparisons test) and test groups (p < 0.01) compared to their nontreated counterparts. In pLV-EGFP control cells, a significant increase of GSH/GSSG ratio occurred only when both astro-CM and 6-OHDA were applied (p < 0.05).
Although 6-OHDA highly reduced the GSH/GSSG ratio within test cells (p < 0.001; Duncan multiple-comparisons test), they still showed significantly higher ratio than their control counterparts (p < 0.01). Addition of astro-CM partially but significantly compensated this reduction (p < 0.01; Student t-test), an increase that was highly significant compared to control groups (p < 0.001).
Reduction of free radical generation in infected neurons
To monitor changes in ROS generation, H2O2 levels were measured in our cell samples. Before astro-CM treatment, we detected 108 ± 7 and 115 ± 4 μM H2O2 in intoxicated plain and pLV-EGFP controls, respectively. This figure stood at 54 ± 3 μM for the test group that showed over 2-fold reduction compared to controls (Fig. 6, both controls versus test group, p < 0.001; unpaired Student t-test).
FIG. 6.
Measurement of hydrogen peroxide (H2O2) levels in cell samples. Each column represents the average of three independent experiments carried out in triplicate. Statistical differences in H2O2 levels between untreated and astro-CM (CM)-treated cells within each group are shown by aa (p < 0.01) or by aaa (P < 0.001). Statistical differences between control groups (plain or pLV-EGFP) and the test group (pLV-GPX1) are shown by bbb before treatment with CM (p < 0.001) and by ccc after treatment with CM (p < 0.001).
Treatment with astro-CM significantly reduced H2O2 levels in all cell groups compared to their untreated counterparts: In plain cells from 108 ± 7 to 94 ± 3 μM (Fig. 6, p < 0.01; Duncan multiple-comparisons test), in pLV-EGFP cells from 115 ± 4 to 99 ± 6 μM (p < 0.01), and in pLV-GPX1 from 54 ± 3 to 37 ± 4 μM (p < 0.001), nearly 2.7-fold down from pLV-EGFP (Fig. 6, p < 0.001; Student t-test).
Neuroprotection in the co-presence of GPX-1 and astro-CM
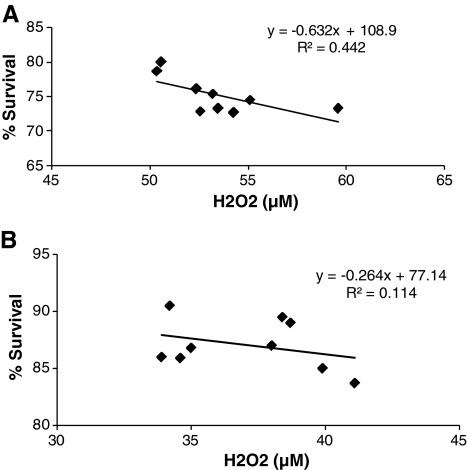
Finally, we carried out regression analysis between H2O2 levels (Fig. 6) and neuronal cell survival (Fig. 4B). This analysis showed that following 6-OHDA treatment H2O2 killed 44% of the pLV-GPX1–infected cells in the absence of astro-CM, whereas the figure was reduced to 11% when astro-CM was added to the cells (Fig. 7). Therefore, the co-presence of prosurvival GPX-1 and astro-CM reduced cell death rate by 33%, and this reduction was significant (p < 0.05). We also carried out the same analysis between plain control groups in Figs. 4B and 6, and between pLV-EGFP–infected control groups of the two figures but did not find any significant reduction in cell death rates in either group (data not shown).
FIG. 7.
Regression analysis between SK-N-MC cell survival and hydrogen peroxide (H2O2) levels. The pLV-GPX1–infected cells were intoxicated with 6-hydroxydopamine (6-OHDA) in the absence (A) and presence of astro-CM (B). Each analysis included data from an average of three independent experiments carried out in triplicate.
Discussion
The role of GPX-1 as an antioxidant enzyme has been well documented. Here, we studied its function in protecting DAergic neurons against selective neurotoxicity. Transduction of SK-N-MC cells with our lentivirus construct carrying the human GPX-1 coding sequence resulted in increased GPX-1 protein expression and its enzymatic activity. These events inhibited elevation of intracellular ROS generation that eventually conferred neuroprotection against 6-OHDA–induced cytotoxicity. GPX-1–overexpressing cells were also better protected in the presence of a medium conditioned by primary astrocytes. Analysis of GSH content revealed that GSH molecules reached their maximum levels when astro-CM treatment coincided with GPX-1 overexpression.
Our pLV-GPX1 construct drove over 3-fold overexpression of GPX-1 mRNA in SK-N-MC cells that was highly significant compared to its expression in our controls (Fig. 2B). The strong presence of GPX-1 correlated well with its enzymatic overactivity (Fig. 3). Addition of astro-CM further significantly enhanced GPX-1 activity in both controls and test groups (Fig. 3), suggesting additional GPX-1 and/or GSH molecules may have been provided by astrocytes.
GPX-1 overactivity increased the survival rate of our test group against 6-OHDA–mediated cell death. This increase was highly significant compared to that in both controls (Fig. 4B). To clarify the mechanism behind GPX-1–mediated neuroprotection, the effect of GPX-1 activity on the levels of H2O2 generation induced by 6-OHDA was examined. Our test cells exhibited reduced levels of H2O2 compared to controls, and this reduction was highly significant (Fig. 6). In line with previous reports, our data indicate that increased GPX-1 activity suppresses excessive H2O2 generation and so protects the neurons from 6-OHDA–mediated cytotoxicity.
We did not fully understand the reason behind GPX-1 overexpression in our pLV-EGFP control, although it might reflect cellular response to stress induced by viral infection and subsequent genome modifications. This change did not, however, have a major inducing impact on GPX-1 activity or cell survival, suggesting a threshold for GPX-1 activity below which the enzyme will only have normal contribution to cell maintenance.
Because astrocytes secrete various growth factors and antioxidants in support of neurons, we assumed that their conditioned medium would further improve neuronal survival. Addition of astro-CM significantly reduced H2O2 production in all cell groups (Fig. 6). However, it was only in pLV-GPX1–infected cells where astro-CM contributed to a significant increase in survival rate against 6-OHDA toxicity (Fig. 4B). In other words, addition of astro-CM per se was unable to induce protection; strong presence of GPX-1 was also needed to potentiate cell survival. In fact, it was only in the presence of GPX-1 overactivity that addition of astro-CM before 6-OHDA treatment could suppress H2O2 to the levels nearly close to its levels in healthy counterpart cells (Fig. 6).
Interestingly, the regression analysis between H2O2 levels and survival rate (Figs. 4B and 6) indicated that astro-CM added to control group cells did not cause significant cell death reduction. However, it reduced 33% of cell death rate among pLV-GPX1–infected neurons, and this reduction was statistically significant (Fig. 7). Therefore, to exert its pro-survival effects, astro-CM more likely requires cooperation with another protecting agent such as GPX-1 that by itself plays a pivotal role in neuroprotection (Fig. 4B). These observations imply that additive and/or synergistic interactions might be happening between GPX-1 and growth factors present in astro-CM.
To further clarify cooperation between GPX-1 and astro-CM, we measured the GSH content of the cells. Addition of astro-CM significantly increased GSH levels in all our cell groups. Reports indicate that astrocytes secrete GSH precursors, the components that can be readily taken up by neuronal cells for reconstitution of GSH molecules.33 Our data suggest that astro-CM treatment has provided our cells with such an opportunity to increase their glutathione content.
Co-presence of astro-CM and overexpressed GPX-1 caused further increase of GSH levels. This co-presence was also able to compensate significantly for the lost levels of GSH in 6-OHDA–intoxicated cells. These GSH elevations indeed paralleled H2O2 reductions in GPX-1–overexpressing cells treated with astro-CM (Fig. 6). Therefore, the co-presence of astro-CM and GPX-1 overactivity simultaneously enhances GSH levels and attenuates H2O2 levels, changes that translate to elevated rates of SK-N-MC cell survival against 6-OHDA–mediated cell death.
In our previous report, we applied astrocytes as minipumps and showed that GSH secreted by them has neuroprotective effects.25 We also showed that astrocytic GSH acts in synergy with glial cell line–derived neurotrophic factor (GDNF) to protect DAergic neurons from 6-OHDA toxicity. Our current data similarly suggest synergy between GPX-1 and GSH, and probably between GPX-1 and growth factors supplied by astro-CM. This is in line with the report by Chao and Lee that indicates GDNF is capable of inducing GPX-1 activity.34 In fact, our preliminary observations indicate that co-presence of GPX-1 overexpressed by neurons and GDNF oversecreted by astrocytes protects the neurons even more strongly than when either factor is present, suggesting a synergistic effect is being shaped by the two.35 Our current data indicate that plain astro-CM, by supplying a range of growth factors at basal levels, confers only modest levels of protection. We have previously applied DAergic neuron- and astrocyte-specific promoters to target transgenes separately in either cell type, indicating that the two protecting factors could individually be overexpressed in a mixed culture.25,36 We are currently pursuing such a cell type-specific, but simultaneous, model of overexpression that will allow dissection of possible GDNF-GPX-1 synergy and astrocyte-DAergic neuron cross-talks both in vitro and in vivo.
Conclusion
In this study, we generated recombinant lentiviruses overexpressing GPX-1 for neuroprotection and showed that the strong presence of GPX-1 can protect DAergic neurons from parkinsonian degeneration by significantly reducing free radical levels. We also showed that this protection can be improved by addition of a medium conditioned by astrocytes. Saving over 86% of DAergic neurons from toxicity-mediated cell death is a significant goal that we achieved via such a combination. Our data also suggest synergistic effects between GPX-1 strongly present in DAergic neurons and factors secreted by astrocytes, even at their basal levels, leading to increased DAergic neuroprotection. Further studies can dissect such possible interactions in vitro to develop more effective neuroprotection approaches for PD. Our hypothesis can also be tested by transferring the pLV-GPX1 construct to DAergic neurons of the midbrain in animal models of PD where GPX-1 can directly interact with astrocytic factors in vivo.
Acknowledgments
We are grateful to Dr. J. Reiser of Louisiana State University for his kind gifts of lentivirus vectors. We also thank Dr. J. Loscalzo in Harvard Medical School for sending us the pHIH-Ad2-hGPX-1 plasmid. We appreciate Y. Panahi and E. Gharib of NRC for their technical assistance. Our special thanks go to Dr. M. Zaefizadeh of the Department of Plant Breeding, IAU-Ardebil for his generous help with statistical analyses. M. Gholami has contributed to this work during his studies for his Master of Science degree in Biology in The School of Biology, College of Science, The University of Tehran. This work was supported in part by a grant (Project-348) from National Institute of Genetic Engineering and Biotechnology (NIGEB) and one from Neuroscience Research Center (NRC), Shahid Beheshti University of Medical Sciences (Project-134).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hirsch EC. Faucheux B. Damier P. Mouatt-Prigent A. Agid Y. Neuronal vulnerability in Parkinson's disease. J Neural Transm Suppl. 1997;50:79–88. doi: 10.1007/978-3-7091-6842-4_9. [DOI] [PubMed] [Google Scholar]
- 2.Ziv I. Barzilai A. Offen D. Nardi N. Melamed M. Nigrostriatal neuronal death in Parkinson's disease-a passive or an active genetically-controlled process? J Neural Transm Suppl. 1997;49:69–76. doi: 10.1007/978-3-7091-6844-8_7. [DOI] [PubMed] [Google Scholar]
- 3.Lang AE. Obeso J. Challenges in Parkinson's disease: Restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 2004;3:309–316. doi: 10.1016/S1474-4422(04)00740-9. [DOI] [PubMed] [Google Scholar]
- 4.Greenamyre JT. Hastings TG. Parkinson's—Divergent causes, convergent mechanisms. Science. 2004;304:1120–1122. doi: 10.1126/science.1098966. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C. Huang Y. Przedborski S. Oxidative stress in Parkinson's disease: A mechanism of pathogenic and therapeutic significance. Ann NY Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 7.Jenner P. Dexter DT. Sian J. Schapira AHV. Marsden CD. Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. Ann Neurol. 1992;32:S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- 8.Sofic E. Lange KW. Jellinger K. Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson's disease. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 9.Heales SJR. Davies SEC. Bates TE. Clark JB. Depletion of brain glutathione is accompanied by impaired mitochondrial function and decreased N-acetyl aspartate concentration. Neurochem Res. 1995;20:31–38. doi: 10.1007/BF00995149. [DOI] [PubMed] [Google Scholar]
- 10.Wullner U. Loschmann PA. Schulz JB. Schmid A. Dringen R. Eblen F. Turski L. Klockgether T. Glutathione depletion potentiates MPTP and MPP+ toxicity in nigral dopaminergic neurones. Neuroreport. 1996;7:921–923. doi: 10.1097/00001756-199603220-00018. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Biologically relevant metal ion-dependent hydroxyl radical generation. FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- 12.Cheng W. Fu YX. Porres JM. Ross DA. Lei XG. Selenium-dependent cellular glutathione peroxidase protects mice against a pro-oxidant-induced oxidation of NADPH, NADH, lipids, and protein. FASEB J. 1999;13:1467–1475. doi: 10.1096/fasebj.13.11.1467. [DOI] [PubMed] [Google Scholar]
- 13.Lei XG. Cheng W-H. McClung JP. Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr. 2007;27:41–61. doi: 10.1146/annurev.nutr.27.061406.093716. [DOI] [PubMed] [Google Scholar]
- 14.Lindenau J. Noack H. Asayama K. Wolf G. Enhanced cellular glutathione peroxidase immunoreactivity in activated astrocytes and in microglia during excitotoxin induced neurodegeneration. Glia. 1998;24:252–256. [PubMed] [Google Scholar]
- 15.Power JH. Blumbergs PC. Cellular glutathione peroxidase in human brain: cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:63–73. doi: 10.1007/s00401-008-0438-3. [DOI] [PubMed] [Google Scholar]
- 16.Damier P. Hirsch EC. Zhang P. Agid Y. Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 17.Mirault ME. Tremblay A. Beaudoin N. Tremblay M. Overexpression of seleno-glutathione peroxidase by gene transfer enhances the resistance of T47D human breast cells to clastogenic oxidants. J Biol Chem. 1991;266:20752–10760. [PubMed] [Google Scholar]
- 18.Taylor SD. Davenport LD. Speranza MJ. Mullenbach GT. Lynch RE. Glutathione peroxidase protects cultured mammalian cells from the toxicity of adriamycin and paraquat. Arch. Biochem. Biophys. 1993;305:600–605. doi: 10.1006/abbi.1993.1467. [DOI] [PubMed] [Google Scholar]
- 19.Cheng WH. Ho YS. Valentine BA. Ross DA. Combs GFJ. Lei XG. Cellular glutathione peroxidase is the mediator of body selenium to protect against paraquat lethality in transgenic mice. J Nutr. 1998;128:1070–1076. doi: 10.1093/jn/128.7.1070. [DOI] [PubMed] [Google Scholar]
- 20.Ridet JL. Bensadoun JC. Déglon N. Aebischer P. Zurn AD. Lentivirus-mediated expression of glutathione peroxidase: Neuroprotection in murine models of Parkinson's disease. Neurobiol Dis. 2006;21:29–34. doi: 10.1016/j.nbd.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Bensadoun JC. Mirochnitchenko O. Inouye M. Aebischer P. Zurn AD. Attenuation of 6-OHDA-induced neurotoxicity in glutathione peroxidase transgenic mice. Eur J Neurosci. 1998;10:3231–3236. doi: 10.1046/j.1460-9568.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- 22.Thiruchelvam M. Prokopenko O. Cory-Slechta DA. Buckley B. Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. J Biol Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- 23.Klivenyi P. Andreassen OA. Ferrante RJ. Dedeoglu A. Mueller G. Lancelot E. Bogdanov M. Andersen JK. Jiang D. Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4- phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J. Graham DG. Montine TJ. Ho YS. Enhanced Nmethyl- 1,2,3,6-tetrahydropyridine toxicity in mice deficient in CuZn-superoxide dismutase or glutathione peroxidase. J Neuropathol Exp Neurol. 2000;59:53–61. doi: 10.1093/jnen/59.1.53. [DOI] [PubMed] [Google Scholar]
- 25.Sandhu JK. Gardaneh M. Iwasiow R. Lanthier P. Gangaraju S. Ribecco-Lutkiewicz M. Tremblay R. Kiuchi K. Sikorska M. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6-OHDA cytotoxicity. Neurobiol Dis. 2009;33:405–414. doi: 10.1016/j.nbd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Pluta K. Luce ML. Bao L. Agha-Mohammadi S. Reiser J. Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters. J Gene Med. 2005;7:803–817. doi: 10.1002/jgm.712. [DOI] [PubMed] [Google Scholar]
- 27.Weiss N. Zhang YY. Heydrick S. Bierl C. Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc Natl Acad Sci USA. 2001;98:12503–12508. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moscow JA. Morrow CS. He R. Mullenbachalln GT. Cowan KH. Structure and function of the 5′-flanking sequence of the human cytosolic selenium-dependent glutathione peroxidase gene (hgpxl) J Biol Chem. 1992;267:5949–5958. [PubMed] [Google Scholar]
- 29.St. Clair DK. Chow CK. Glutathione peroxidase: Activity and steady-state level of mRNA. In: Punchard NA, editor; Kelly FJ, editor. Free Radicals. IRL Press; Oxford: 1996. pp. 227–240. [Google Scholar]
- 30.Browne RW. Armstrong D. Reduced glutathione and glutathione disulfide. In: Armstrong D, editor. Free Radical and Anti-oxidant Protocols. Humana Press; Totowa N: 1998. pp. 347–352. [DOI] [PubMed] [Google Scholar]
- 31.Tariq M. Ahmad Khan H. Al Moutaery K. Al Deeb S. Protective effect of quinacrine on striatal dopamine levels in 6-OHDA and MPTP models of Parkinsonism in rodents. Brain Res Bullet. 2001;54:77–82. doi: 10.1016/s0361-9230(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 32.Garia JC. Remires D. Leiva A. Gonzalez R. Depletion of brain glutathione potentiates the effect of 6-hydroxydopamine in a rat model of parkinson's disease. J Mol Neurosci. 2000;14:147–153. doi: 10.1385/JMN:14:3:147. [DOI] [PubMed] [Google Scholar]
- 33.Dringen R. Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 34.Chao CC. Lee EHY. Neuroprotective mechanism of glial cell line-derived neurotrophic factor on dopamine neurons: role of anti-oxidation. Neuropharmachology. 1999;38:913–916. doi: 10.1016/s0028-3908(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 35.Gardaneh M. Shojaei S. Saafi R. Panahi P. Gholami M. Synergy between glial cell line-derived neuotrophic factor and glutathione peroxidase-1 maximizes protection of dopaminergic neurons against parkinsonian toxicity (abstract). Annual Meeting of Society for Neuroscience; Nov 14–17;2010 ; San Diego, CA. [Google Scholar]
- 36.Gardaneh M. O'Malley K. Rat tyrosine hydroxylase promoter directs tetracycline-inducible foreign gene expression in dopaminergic cell types. Mol Brain Res. 2004;126:173–180. doi: 10.1016/j.molbrainres.2004.04.008. [DOI] [PubMed] [Google Scholar]