Abstract
Background:
Chonemorpha grandiflora (Syn. Chonemorpha fragrans (Apocynaceae) is an endangered medicinal plant. It is used in different preparations, such as sudarsanasavam and kumaryasavam used in Kerala Ayurvedic system. C. grandiflora is used for the treatment of fever and stomach disorders. Phytochemical investigations have revealed the presence of steroidal alkaloids, such as chonemorphine and funtumafrine in C. grandiflora. Camptothecin, a well-known anticancer alkaloid has been detected in ethanolic extracts of stem with bark and callus cultures derived from C. grandiflora.
Methods:
Callus cultures of C. grandiflora were raised on Murashige and Skoog’s medium supplemented with 2, 4-D. Stem with bark and callus were used for phytochemical analysis mainly the alkaloids. Detection and identification of camptothecin was carried out using thin-layer chromatography (TLC), high-performance thin-layer chromatography, (HPTLC) and high-performance liquid chromatography (HPLC).
Results:
An important anticancer alkaloid, camptothecin was detected in ethanolic extracts of stem with bark and callus cultures of C. grandiflora. camptothecin content was 0.013 mg/g in stem with bark and 0.003 mg/g in callus.
Conclusion:
This is the first report on in vivo and in vitro production of camptothecin in C. grandiflora. Camptothecin is known to occur only in six plant sources so, alternative sources for camptothecin are needed. Thus of C. grandiflora could be a new promising alternative source of camptothecin.
Keywords: Apocynaceae, callus, camptothecin, Chonemorpha grandiflora
INTRODUCTION
Chonemorpha grandiflora(Roth) M. R. and S. M. Almeida (Syn. Chonemorpha fragrans) is a shrubby, latex-bearing climber belonging to the family Apocynaceae. It is a medicinal plant,[1,2] which has been assigned endangered status in Karnataka state and vulnerable in Kerala state.[3] It is used in different preparations, such as sudarsanasavam and kumaryasavam used in Kerala Ayurvedic system.[4] It is used for the treatment of fever and stomach disorders. Entire plant, roots, and root-bark are used for the treatment. The trade is mainly confined to Kerala state under the name Perumkurumba and the dried roots are sold commercially.[5] C. grandiflora is shown to possess antiparasitic and muscle relaxant properties.[6,7] Phytochemical investigations have revealed the presence of steroidal alkaloids, such as chonemorphine and funtumafrine.[8,9] There are so far no reports on phytochemical investigations of in vitro material of C. grandiflora. Thus, cultures of C. grandiflora were studied for the production of alkaloids and compared with in vivo material.
MATERIAL AND METHODS
Establishment of callus cultures
The plant material was obtained from Kerala (Thrissur district). The voucher specimens were identified and submitted at Botanical Survey of India, Western Circle, Pune, India. The plants were established by cutting and maintained in the Botanical garden, Department of Botany, University of Pune. Internodes from these plants were used for initiation and establishment of callus cultures in C. grandiflora. The intermodal segments were sterilized by using 0.1% HgCl2 and 70% alcohol and grown on Murashige and Skoog medium[10] supplemented with 4.52 μM 2,4-dichlorophenoxyacetic acid to raise callus cultures. Callus cultures were subcultured every fourth week.
Phytochemical analysis
The callus and the stem with bark was shade dried and used for phytochemical analysis. The plant material was powdered and used for the preparation of ethanolic extracts. Cold extraction was carried out using 50 g of the powder of the plant material and 200 mL ethanol for 48 h. The extracts were centrifuged at 9000 g for 5 min. The clear supernatant was passed through the membrane filter (cellulose nitrate, 0.20 μm, Pall Gellman, Bombay, India). The extracts were evaporated to dryness to get the residue. To the residue, 1 mL of methanol was added and these samples were used for thin-layer chromatography (TLC), high-performance thin-layer chromatography (HPTLC), and high-performance liquid chromatography (HPLC) analysis.
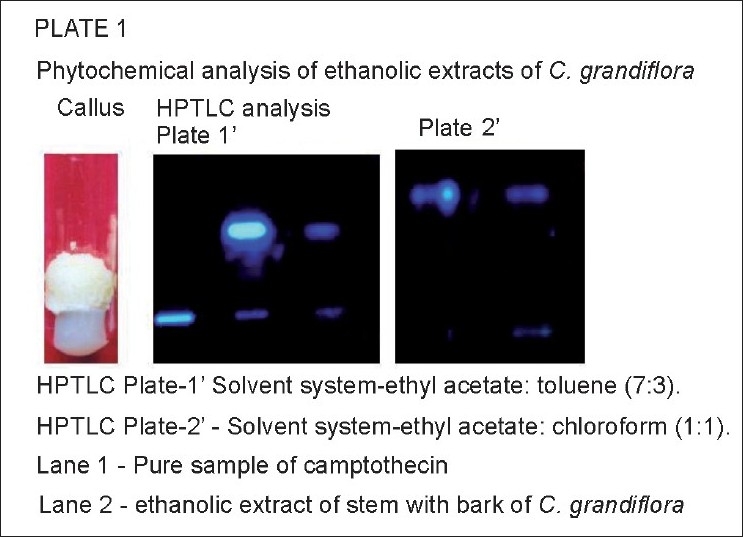
TLC was performed on silica gel 60 F254 precoated (20 × 20 cm; Merck, Darmstadt, Germany) plates, using protocol described by Fulzele et al. (2001).[11] A pure sample of camptothecin was procured from Sigma Aldrich, Bangalore. A standard sample of camptothecin was prepared by dissolving 40 μg camptothecin in dimethyl sulfoxide (DMSO):methanol (1:50) and run along with the extracts. Rf of standard camptothecin was recorded.
For HPTLC analysis, 500 μg of the extracts were loaded on HPTLC plates. The plates were run in duplicates in solvent systems (i) ethyl acetate:toluene (7:3) and (ii) chloroform:ethyl acetate (1:1). The chromatographs were scanned by Camag densitometric scanner and the peaks, peak areas, and the Rf of the spots were recorded. A pure sample of camptothecin was procured from Sigma Aldrich, Bangalore. A standard sample of camptothecin was prepared by dissolving 40 μg of camptothecin in 1 mL of DMSO:methanol (1:50) and run along with the extracts. Fluorescence was recorded at 366 nm. Rf of the standard camptothecin was recorded.
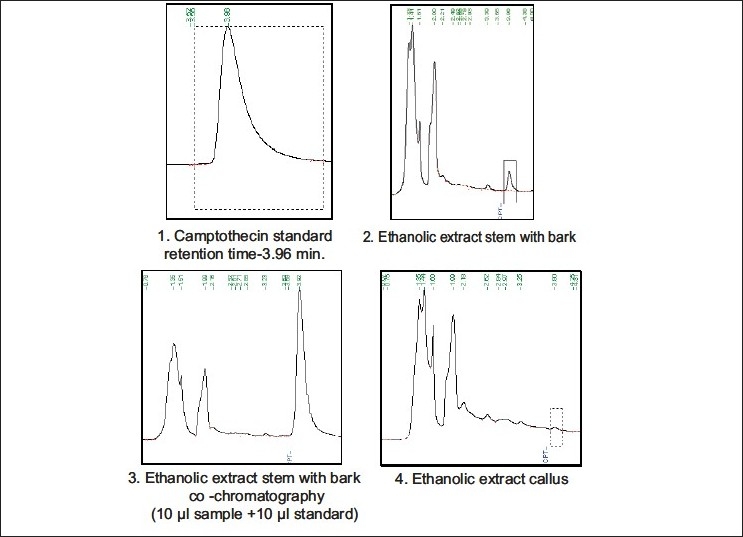
Isocratic analytical HPLC was carried out using RP-C18 column (Perkin Elmer, series 200, Switzerland, SPHERI-5, 5 mm, 250 × 4.6 mm). The mobile phase for alkaloid elution was acetonitrile:water (40:60), at a flow rate 1.6 mL/min with a sample size of 20 μL; and UV detection at 254 nm. A standard curve was obtained using authentic sample of camptothecin (Sigma Aldrich). The standard was prepared using DMSO:methanol (1:50 v/v). HPLC analysis of standard as well as extract yielded chromatogram with retention time of 3.85 min. Co-chromatography of the extracts was performed with authentic samples for confirmation. Validation of quantitative method was performed for samples in 5 replications. The results from the samples at two concentrations did not alter the retention time. The retention time proved that accuracy and reproducibility was excellent.
RESULTS
TLC analysis—Camptothecin showed a dark blue spot at 254 nm and Rf value was 0.46 in solvent system chloroform:ethyl acetate (1:1). In ethanolic extracts of stem with bark of C. grandiflora, a very faint spot with the same Rf and blue fluorescence at 254 nm were observed.
HPTLC analysis revealed the presence of a compound having same Rf as that of standard camptothecin in the ethanolic extract of stem with bark of C. grandiflora [Figure 1; Plate 1.].
(c) HPLC analysis also showed the presence of a peak having same retention time as that of pure camptothecin in the ethanolic extracts of stem with bark and callus of C. grandiflora [Figure 2; Plate 2]. The amount of camptothecin in the samples was calculated considering the following values: (1) peak area shown by standard camptothecin sample, (2) peak area of peak in plant extracts showing the same retention time as that of standard camptothecin, (3) total volume of the extract prepared, and (4) dry weight of the plant material used to prepare the extract. Percentage of camptothecin was calculated for the samples on dry weight basis (mg/g). The stem with bark yielded 0.013 mg/g camptothecin, whereas internode callus yielded 0.003 mg/g camptothecin [Figure 2 Plate 2].
Figure 1.
HPTLC analysis of ethanolic extracts of Chonemorpha grandiflora.
Figure 2.
HPLC analysis of ethanolic extracts of Chonemorpha grandiflora.
DISCUSSION
Camptothecins are one of the most important anticancer alkaloids of the 21st century because of their clinical applications against cancer[12,13] and HIV.[14] They have been found to be active against parasitic trypanosomes, Leishmania,[15] and falciparum malaria.[16] Camptothecin is known to occur in different unrelated genera, including Camptotheca acuminata,[17] Nothapodytes nimmoniana,[18,19] Tabernaemontana heyneana,[20] and Ophiorrhiza rugosa var. prostrata.[21] Camptothecin was detected and identified in ethanolic extracts of stem with bark and callus derived from C. grandiflora using TLC, HPTLC, and HPLC. Thus, on the basis of the present investigations, we propose C. grandiflora as a new source of camptothecin.
The yield of camptothecin calculated for C. acuminata was 400-5000 mg/g,[17] for N. nimmoniana 0.23%-0.33%,[19] and for T. heyneana stem bark 0.00013%.[20] Ever increasing worldwide demand for camptothecin from pharmaceutical industries and subsequent pressure on the wild populations of N. nimmoniana and C. acuminata has endangered the plants. Thus, there is an urgent need to find alternative plant sources of camptothecin. Although the amount of camptothecin reported by us in stem with bark in C. grandiflora is low as compared with that reported in C. acuminata and N. nimmoniana, it is more as compared with that reported in T. heyneana. Thus, C. grandiflora could be a new promising alternative source of camptothecin.
In vitro cultures of C. acuminata, N. nimmoniana, and Ophiorrhiza pumila have been established for camptothecin production. Undifferentiated callus cultures[22] and suspension cultures, usually produce significantly low amount of camptothecin, for example, C. acuminata[23] and N. nimmoniana (0.0003%-0.01%).[24] In callus cultures of N. foetida, camptothecin levels reported were 100- to 1000-fold lower than in the intact plant.[25,26] Root and hairy root cultures of O. pumila have been successfully employed for camptothecin production.[27] Presence of camptothecin has been detected from callus cultures of C. grandiflora. Although the amount reported is low, it could be enhanced by using biotic and abiotic elicitors. Thus, our results indicate C. grandiflora callus, a new and promising source of camptothecin useful in drug development.
Acknowledgments
The first author (AVK) is thankful to UGC, India, for teacher fellowship, and to the Head, Department of Botany, University of Pune, Principal, S.P. College, Pune, Head, Dept. of Microbiology, MES Garware College, Pune, for providing facilities. Thanks are due to Dr. Tessey Joseph, for providing plant material and Dr. Anuradha Upadhye for HPTLC analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Varier VS. 19. Vol. 2. Madras: Orient Longman; 1994. Indian Medicinal Plants: A Compendium of 500 Species. [Google Scholar]
- 2. Available from: http://www.envformizo.in/forest/medicinal_inuse.htm Medicinal plants in Mizoram. [Last accessed on 2010 Apr 06]
- 3.Khan S, Karnat NM, Darshan S. India’s foundation for revitalization of local health traditions, Pioneering In situ conservation strategies for medicinal plants and local cultures. Herbalgram. 2005;68:34–45. [Google Scholar]
- 4. Available from: http://www.oilbath.com [Last accessed on 2010 Apr 06]
- 5. Available from: http://envis://frlht.org.in.cfragrans.htm [Last accessed on 2010 Apr 06]
- 6.Chatterji DK, Iyer N, Ganguli BN. Anti-amoebic activity of chonemorphine a steroidal alkaloid in experimental models. Parasitol Res. 1987;74:30–3. doi: 10.1007/BF00534928. [DOI] [PubMed] [Google Scholar]
- 7.Roy RK, Ray NM, Das AK. Skeletal muscle relaxant, effect of Chonemorpha macrophylla in experimental animals. Indian J Pharmacology. 2005;37:116–9. [Google Scholar]
- 8.Chatterji DK, Banerji J. Occurrence of Funtumafrine in Chonemorpha macrophylla.G. Don. (C. fragrans, Moon) Indian J Chem. 1972;10:1197. [Google Scholar]
- 9.Banerji J, Chatterji A. A new steriodal alkaloid from Chonemorpha fragrans (Moon) Alston. Indian J Chem. 1973;56:1056. [Google Scholar]
- 10.Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tissue culture. Physiol Plantarum. 1962;15:473–97. [Google Scholar]
- 11.Fulzele DP, Satdive RK, Pol BB. Growth and production of camptothecin by cell suspension Culture of Nothapodytes foetida. Planta Med. 2001;67:150–2. doi: 10.1055/s-2001-11519. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi A, Dohashi K, Fujimoto S, Tanaka K, Suzuki M, Terashima Y, et al. A late phase II study of CPT-II in uterine, cervical cancer and ovarian cancer. Jpn J Cancer Chemother. 1991;8:1661–89. [PubMed] [Google Scholar]
- 13.Potsmesil M. Camptothecins: From Bench Research to Hospital Wards. Cancer Res. 1994;54:1431–39. [PubMed] [Google Scholar]
- 14.Priel SD, Showalter, Blair DG. Inhibition of human immunodeficiency virus (HIV-l) replication in vitro by non-cytotoxic doses of camptothecin. A topoisomerase inhibitor. Aids Res Hum Retrovirus. 1991;7:65–8. doi: 10.1089/aid.1991.7.65. [DOI] [PubMed] [Google Scholar]
- 15.Bodley AL, Shapiro TA. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc Natl Acad Sci USA. 1995;92:3726–30. doi: 10.1073/pnas.92.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodley AL, Cumming JN, Shapiro TA. Effect of camptothecin, a topoisomerase inhibitor, on Plasmodium falciparum. Biochem Pharmacol. 1998;55:709–11. doi: 10.1016/s0006-2952(97)00556-x. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Meyer V RM, Mc-Knight TD, Nessler CL. Sustained harvest of camptothecin from the leaves of Camptotheca acuminata. J Nat Prod. 1997;60:618–9. doi: 10.1021/np9700228. [DOI] [PubMed] [Google Scholar]
- 18.Aiyama R, Hisako N, Nokata K, Shinohara C, Sawada S. A camptothecin derivative from Nothapodytes nimmoniana. Phytochemistry. 1988;27:36634. [Google Scholar]
- 19.Padmanabh BV, Chandrashekhar M, Ramesh BT, Hombegowda HC, Gunaga RP, Suhas S, et al. Pattern of accumulation of camptothecin an anticancer alkaloids in Nothapodytes nimmoniana, Graham in Western Ghats, India, implications for high yielding sources of alkaloids. Curr Sci. 2006;90:95–100. [Google Scholar]
- 20.Gunasekera SP, Badawi MM, Cordell GA, Farnsworth NR, Chitnis M. Plant anticancer agents and Isolation of Camptothecin and 9 methoxy Camptothecin from Ervatamia heyneana. J Nat Prod. 1979;42:475–7. doi: 10.1021/np50005a006. [DOI] [PubMed] [Google Scholar]
- 21.Gharpure G, Chavan B, Lele U, Hastak A, Bhave, Malpure N, et al. Camptothecin accumulation in Ophiorrhiza rugosa var. prostrata from northern Western Ghats. Curr Sci. 2010;98:302–4. [Google Scholar]
- 22.Van Hengel AJ, Harkes MP, Wichers HJ, Hesselink PG, Buitelaar RM. Characterization of callus formation and camptothecin production by cell lines of Camptotheca acuminata. Plant Cell Tiss Org Cult. 1992;28:11–8. [Google Scholar]
- 23.Sakato K, Tanaka H, Mukai N, Misawa M. Isolation and identification of camptothecin from Camptotheca acuminata suspension cultures. Agri Bio Chem. 1974;38:217–8. [Google Scholar]
- 24.Roja G, Heble MT. The quinone alkaloids and 9-methoxycamptothecin from tissue cultures and mature trees of Nothapodytes foetida. Phytochemistry. 1994;36:65–6. [Google Scholar]
- 25.Ciddi V, Shuler ML. Camptothecin from callus cultures of Nothapodytes foetida. Biotech Lett. 2000;22:129–32. [Google Scholar]
- 26.Thengane SR, Kulkarni DK, Shrikhande VA, Joshi SP, Sonawane KB, Krishnamurthy KV. Influence of medium composition on callus induction and camptothecin accumulation in Nothapodytes foetida. Plant Cell Tiss Org Cult. 2003;72:247–51. [Google Scholar]
- 27.Saito K, Sudo H, Yamazaki M, Koseki NM, Kitajima M, Takayama H, et al. Feasible production of camptothecin by hairy root culture of Ophiorrhiza pumila. Plant Cell Rep. 2001;20:267–71. [Google Scholar]


