Abstract
Aims
To examine the relationship between depressive symptomatology, diabetes-related distress and aspects of diabetes self-care in a cohort of individuals with Type 1 diabetes.
Methods
Individuals with Type 1 diabetes taking part in the Pittsburgh Epidemiology of Diabetes Complications Study completed the Beck Depression Inventory (BDI), the Center for Epidemiologic Studies Depression (CES-D) Scale and the Problem Areas in Diabetes (PAID) scale. Self-care was measured by physical activity in the past week and over the previous year, frequency of blood glucose/urine testing, smoking status and alcohol intake.
Results
Clinically significant levels of depressive symptomatology (i.e. scores ≥ 16) were reported by 14% of the study population on the BDI and by 18% on the CES-D. There were strong correlations between depressive symptoms and diabetes-related distress (PAID scores) and physical activity. Multivariate analyses indicated that depression was independently associated with diabetes-related distress scores and with physical activity, but not with frequency of blood glucose testing.
Conclusions
These findings have implications for clinical practice and treatment of both psychological morbidity and diabetes. There may be significant effects of depression on aspects of diabetes self-care. Further prospective studies are required to confirm these findings.
Keywords: depression, emotions, self-management, Type 1 diabetes
Introduction
Recently there has been increased interest in the role that psychological morbidity might play in the natural history of diabetes and its long-term complications [1-4]. Feeling depressed can impact on diabetes self-care, glycaemic control and overall quality of life [5-8]. To date, most studies have been conducted in individuals with Type 2 diabetes, with little research in those with Type 1 diabetes [9]. Whether or not psychological problems are of a general nature or are diabetes-related has important implications for treatment [10,11]. Emotional problems related to diabetes are associated with elevated levels of depression [12,13] and poorer self-care [5], but remain poorly understood. The aim of the current study was to examine the relationship between depressive symptomatology, diabetes-related distress and diabetes self-management in a cohort of individuals with Type 1 diabetes.
Patients and methods
The Pittsburgh Epidemiology of Diabetes Complications (EDC) study is a prospective investigation of people with Type 1 diabetes, diagnosed < 17 years of age, or seen within 1 year of diagnosis, at Children’s Hospital of Pittsburgh between 1950 and 1980, who were receiving insulin therapy upon initial discharge [14,15]. This cohort were first clinically assessed for the study at baseline between 1986 and 1988 (n = 658), and have subsequently been re-examined biennially, most recently at 18 years’ follow-up (2004–2006), when the current data were collected. The University of Pittsburgh Institutional Review Board approved the study protocol.
Prior to their clinic visit, participants completed postal questionnaires to gather information on demographic details and self-care of diabetes. The Beck Depression Inventory (BDI) [16] was completed; a validated 21-item self-complete scale, with scores ≥ 16 indicative of clinically significant levels of depressive symptomatology [16]. Participants also completed the Center for Epidemiological Studies of Depression Scale (CES-D) [17], and the Problem Areas in Diabetes (PAID) scale [18]. The CES-D is a 20-item self-complete scale measuring the frequency of symptoms of depression during the past week, with scores ≥ 16 indicative of clinically significant levels of depression [17]. The PAID is a 20-item self-complete questionnaire using a five-point Likert-type scale, which measures diabetes-related distress in individuals with diabetes [18,19]. A total PAID score of ≥ 50 is considered to be seriously elevated [19]. Information on use of antidepressant medications and smoking history were recorded. Glycated haemoglobin (HbA1c) was measured by the DCA® 2000 analyser (Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA) and converted to Diabetes Control and Complications Trial-aligned HbA1c values.
The presence of diabetes complications was also ascertained as described fully elsewhere [14]. Overt nephropathy (ON) was defined as an albumin excretion rate > 200 μg/min or > 300 mg/24 h in at least 2/3 validated timed urine collections. Coronary artery disease (CAD) was determined by EDC physician-diagnosed angina, or myocardial infarction confirmed by electrocardiogram or hospital records, coronary revascularization or coronary artery stenosis ≥ 50% or ischaemic ECG (Minnesota Coded). Onset of end-stage renal disease was defined as starting dialysis or undergoing renal transplantation. Retinopathy was assessed by stereo fundus photography using the Arlie House System of classification [20] or a history of laser treatment for proliferative disease.
Physical activity was indicated by total estimated energy expenditure in the past week (KCAL/WK = number of flights of stairs climbed + number of city blocks walked per day + hours of sporting activity per week), per week during the past year, by energy expenditure due to sporting activities participated in during the past week, and per week in the past year. Self-monitoring of blood glucose levels (SMBG) were measured by asking participants to indicate the number of times per week they checked their blood glucose levels.
Data were analysed using spss version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Stepwise linear regression analyses were conducted to examine the independent correlates of depressive symptoms, and the independent correlates of physical activity and frequency of SMBG.
Results
Two hundred and sixty-four individuals (40% of the baseline cohort; 60% of those still alive and locally resident) had complete information collected for the 18-year clinical examination, as shown in Table 1. Overall, only 35 individuals (12%) reported currently taking antidepressant medications, where BDI scores ranged from 0 to 40 and CES-D scores from 0 to 46. Twenty-six percent of those with high BDI scores were taking antidepressant medications, while 16% of those with high CES-D scores reported taking antidepressant medications at the current time. Of those with extremely high PAID scores, 12.5% reported taking antidepressant medications, 64% had BDI scores ≥ 16 and 80% had CES-D scores ≥ 16.
Table 1.
Depression and diabetes-related distress scores (mean, median, sd) of the study population at 18-year follow-up of the EDC study
| Total sample (n = 264) |
Male (n = 131) |
Female (n = 133) |
|
|---|---|---|---|
| Age at 18-year follow-up (years) | 45.0, 44.5, 7.5 | 44.6, 44.6, 7.6 | 45.3, 44.5, 7.4 |
| Duration of diabetes at 18-year follow-up (years) | 36.7, 35.4, 7.1 | 36.9, 35.4, 7.2 | 36.6, 35.5, 7.0 |
| Age of onset of diabetes (years) | 8.2, 8.3, 4.2 | 7.7, 7.2, 4.3 | 8.6, 9.0, 4.1 |
| HbA1c (%) | 7.5, 7.4, 1.4 | 7.6, 7.4, 1.4 | 7.5, 7.4, 1.4 |
| BDI | 7.9, 6.0, 7.5 | 7.1, 5.0, 7.2) | 8.4, 7.0, 7.4 |
| % with BDI ≥ 16 | 14 (n = 35) | 16 (n = 21) | 11 (n = 14) |
| CES-D* | 9.2, 6.0, 9.2 | 7.8, 6.0, 7.6 | 10.2, 6.5, 10.5 |
| % with CES-D ≥ 16 | 18 (n = 47) | 11 (n = 14) | 25 (n = 33) |
| PAID Scale | 17.0, 11.3, 16.5 | 14.7, 10.0, 14.0 | 19.3, 15.0, 18.4 |
| % with severe distress (PAID ≥ 50) | 6 (n = 16) | 5 (n = 6) | 8 (n = 10) |
| Frequency of SMBG per week | 27.7, 28.0, 15.7 | 26.7, 25.0, 15.4 | 28.7, 28.0, 16.1 |
| Energy expenditure in the past week (kcal) | 1431.9, 899.5, 1557.7 | 1609.2, 1176.0, 1493.3 | 1254.6, 728.0, 1605.7 |
| Coronary artery disease | 24% (n = 64) | 26% (n = 34) | 22% (n = 30) |
| Proliferative retinopathy | 36% (n = 96) | 36% (n = 48) | 36% (n = 48) |
| Overt nephropathy | 12% (n = 33) | 16% (n = 21) | 9% (n = 12) |
| Renal failure | 7% (n = 20) | 8% (n = 10) | 7% (n = 10) |
Gender difference significantly different: P < 0.01.
EDC, Epidemiology of Diabetes Complications; BDI, Beck Depression Inventory; CES-D, Centre for Epidemiologic Studies of Depression Scale; SMBG, self-monitoring of blood glucose; PAID, Problem Areas in Diabetes Scale.
Neither current age nor age at onset of diabetes were significantly correlated with the BDI, CES-D or PAID. Duration of diabetes was not significantly correlated with the PAID, but was correlated with the BDI (r = 0.17; P < 0.01) and the CES-D (r = 0.23; P < 0.01). A significantly greater proportion of women had high CES-D scores compared with men (25% vs. 11%; P < 0.01). Excluding those on antidepressant medications did not alter these results.
Overall a strong correlation was observed between the CES-D and the PAID (Fig. 1), and also between the BDI and the PAID (r = 0.49; P < 0.01). A similar pattern was observed when men and women were analysed separately (data not shown) and when the data were analysed excluding those on antidepressant medications.
FIGURE 1.
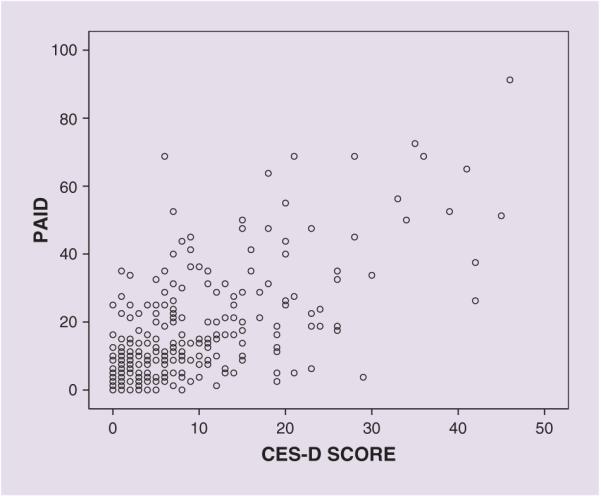
Correlation between depression (CES-D scores) and diabetes-related distress (PAID) scores.
All four physical activity (energy expenditure) variables were significantly and negatively correlated with the BDI (r between −0.20 and −0.27; P < 0.01), the CES-D (r between −0.16 and −0.33; P < 0.01) and the PAID (r between −0.14, P < 0.05, and −0.23, P < 0.01). Overall, HbA1c and PAID scores were significantly correlated (r = 0.16; P < 0.01), but this was not the case when adjusted for sex. Depression and PAID scores did not differ significantly according to smoking status or alcohol intake.
Those who had CAD at 18-year follow-up had significantly higher depression and poorer diabetes-related distress scores compared with those who had not developed CAD (CES-D: 11.6 ± 10.8 vs. 8.3 ± 8.3, P = 0.009; BDI: 10.9 ± 9.3 vs. 6.9 ± 6.4, P = 0.000; PAID: 21.8 ± 20.2 vs. 15.3 ± 14.7, P < 0.01). Mean CES-D and BDI, but not PAID scores, were significantly lower in those without any major complications at 18-year follow-up compared with those with one and those with two μ three major complications (CES-D: 8.3 ± 8.6 vs. 9.8 ± 10.2 vs. 13.4 ± 10.5, P = 0.024; BDI: 6.9 ± 6.7 vs. 9.2 ± 9.2 vs. 11.1 ± 7.5, P = 0.007).
Linear regression analyses demonstrated that higher PAID scores (P < 0.001), longer duration of diabetes (P < 0.001) and lower household income (P = 0.028) were all significant independent correlates of depression (CES-D) (r2 = 0.43). Substituting BDI scores for CES-D as the dependent variable did not change the model. Further linear regression models were calculated in order to identify the significant independent correlates of self-care. Lower HbA1c (P < 0.001), CAD (P = 0.004) and ON (P = 0.005) were significantly independently correlated with SMBG (r2 = 0.14). Significant independent correlates of physical activity, with energy expenditure during the past week (KCAL/WK) used as the dependent variable, were CES-D (P = 0.002) and smoking (P = 0.017) (r2 = 0.10). If energy expenditure in the past year was used as the dependent variable, HbA1c (P = 0.003) and duration of diabetes (P = 0.025) were significant along with CES-D (P < 0.001). PAID scores did not enter the model except when CES-D scores were removed or when BDI scores were substituted for CES-D scores. Substituting number of major complications for specific complications did not alter any of these models.
Discussion
This study has demonstrated a strong association between depressive symptomatology and diabetes-related distress, independent of other potential covariates including complication status, duration of diabetes and gender. These data are cross-sectional, however, and no causal link can be inferred. Similar rates of moderate—severe depressive symptoms and an increased rate among women have been observed previously [1,12,21-23]. Of concern is the relatively low proportion of individuals with high depression scores who reported taking antidepressant medication. Although we do not have data on the use of other treatments, this suggests that depression continues to be both underdiagnosed as well as undertreated. The overall mean scores on both the BDI and the CES-D, and the proportion of individuals reporting severe diabetes-related distress, might be considered to be fairly low, which could reflect a healthy survivor effect as the majority of those participating in this 18th year of follow-up are individuals who have survived a relatively long period of time without developing severe diabetes complications. It is therefore difficult to apply these findings to a shorter duration Type 1 diabetes population.
Depression scores were the strongest correlate of physical activity, regardless of duration, complication status, or diabetes-related distress, in contrast to the model for SMBG. Although physical activity might not be the optimal indicator for successful self-care, our measure did include day-to-day exercise rather than only sporting activity, which might be more problematic in those with major complications. As in many studies, a limitation of our research is the reliance on self-report to measure self-care.
The presence of depression, distress, or a combination of these may lead to greater barriers to appropriate self-management of diabetes, and has important implications for treatment. Our study highlights the importance of individually tailored diabetes care in order to reduce the risk of poor self-care, poor glycaemic control and increased risk of diabetes complications.
Acknowledgements
This research was supported by National Institutes of Health grant number DK034818.
Abbreviations
- BDI
Beck Depression Inventory
- CAD
coronary artery disease
- CES-D
Center for Epidemiological Studies of Depression Scale
- EDC
Epidemiology of Diabetes Complications
- HbA1c
glycated haemoglobin
- ON
overt nephropathy
- PAID
Problem Areas in Diabetes
- SMBG
self-monitoring of blood glucose
Footnotes
Competing interests
Nothing to declare.
References
- 1.Anderson R, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 2.De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd CE, Kuller LH, Becker DJ, Ellis D, Wing RR, Orchard TJ. Coronary artery disease in IDDM: gender differences in risk factors, but not risk. Arterioscler Thromb Vasc Biol. 1996;16:720–726. doi: 10.1161/01.atv.16.6.720. [DOI] [PubMed] [Google Scholar]
- 4.Kinder LS, Kamarck TW, Baum A, Orchard TJ. Depressive symptomatology and coronary heart disease in Type 1 diabetes: a study of possible mechanisms. Health Psychol. 2002;21:542–552. doi: 10.1037//0278-6133.21.6.542. [DOI] [PubMed] [Google Scholar]
- 5.Lin EHB, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 6.Lustman PJ, Anderson RJ, Freedland KE, De Groot M, Carney RM, Clouse RE. Depression and poor glycemic control. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd CE, Wing RR, Matthews KM, Orchard TJ. Psychosocial factors and the complications of insulin-dependent diabetes mellitus: the Pittsburgh Epidemiology of Diabetes Complications Study—VIII. Diabetes Care. 1992;15:166–172. doi: 10.2337/diacare.15.2.166. [DOI] [PubMed] [Google Scholar]
- 8.Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression and quality of life. Diabetes Care. 2004;27:1066–1070. doi: 10.2337/diacare.27.5.1066. [DOI] [PubMed] [Google Scholar]
- 9.Barnard K, Skinner T, Peveler R. The prevalence of co-morbid depression in adults with Type 1 diabetes: systematic literature review. Diabet Med. 2006;23:445–448. doi: 10.1111/j.1464-5491.2006.01814.x. [DOI] [PubMed] [Google Scholar]
- 10.Katon W, Von Korkk M, Ciechanowski P, Russo J, Lin E, Simon G, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 11.Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. How to screen for depression and emotional problems in patients with diabetes: comparison of screening characteristics of depression questionnaires, measurement of diabetes-specific emotional problems and standard clinical assessment. Diabetologia. 2006;49:469–477. doi: 10.1007/s00125-005-0094-2. [DOI] [PubMed] [Google Scholar]
- 12.Pouwer F, Skinner TC, Pibernik-Okanovic M, Beekman ATF, Cradock S, Szabo S, et al. Serious diabetes-specific emotional problems and depression in a Croatian-Dutch-English survey from the European Depression in Diabetes (EDID) Research Consortium. Diabetes Res Clin Pract. 2005;70:166–173. doi: 10.1016/j.diabres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Pibernik-Okanović M, Begić D, Peroš K, Szabo S, Metelko Ž . Psychosocial factors contributing to persistent depressive symptoms in Type 2 diabetic patients: a Croatian survey from the European Depression in Diabetes Research Consortium. J Diabetes Complications. 2008;22:246–253. doi: 10.1016/j.jdiacomp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, et al. Prevalence of complications of IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 15.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Ellis D, LaPorte RE, et al. Factors associated with the avoidance of severe complications after 25 years of insulin dependent diabetes mellitus: Pittsburgh Epidemiology of Diabetes Complications Study—I. Diabetes Care. 1990;13:741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Garbin MG. Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 17.Radloff LS. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18.Polonsky WH, Anderson BJ, Lohrer PA, Welch GW, Jacobson AM, Aponte JE. Assessment of diabetes related distress. Diabetes Care. 1995;18:754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 19.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale. An evaluation of its clinical utility. Diabetes Care. 1997;20:760–766. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- 20.Manual of Operations. University of Maryland School of Medicine; Baltimore, MD: 1980. Early Treatment of Diabetic Retinopathy Study Coordinating Center. [Google Scholar]
- 21.Gendelman N, Snell-Bergeon JK, McFann K, Kinney G, Wadwa RP, Bishop F, et al. Prevalence and correlates of depression in individuals with and without Type 1 diabetes. Diabetes Care. 2009;32:575–579. doi: 10.2337/dc08-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snoek FJ, Pouwer F, Welch GW, Polonsky WH. Diabetes related emotional distress in Dutch and U.S. diabetic patients. Diabetes Care. 2000;23:1305–1309. doi: 10.2337/diacare.23.9.1305. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd CE, Zgibor J, Wilson RR, Barnett AH, Dyer PH, Orchard TJ. Cross-cultural comparisons of anxiety and depression in adults with Type 1 diabetes. Diabetes Metab Res Rev. 2003;19:401–407. doi: 10.1002/dmrr.394. [DOI] [PubMed] [Google Scholar]


