Abstract
Aim: The purpose of this study was to develop an improved method for collagen and protein assessment of fibrotic lungs while decreasing animal use. methods: 8-10 week old, male C57BL/6 mice were given a single intratracheal instillation of crocidolite asbestos or control titanium dioxide. Lungs were collected on day 14 and dried as whole lung, or homogenized in CHAPS buffer, for hydroxyproline analysis. Insoluble and salt-soluble collagen content was also determined in lung homogenates using a modified Sirius red colorimetric 96-well plate assay. results: The hydroxyproline assay showed significant increases in collagen content in the lungs of asbestos-treated mice. Identical results were present between collagen content determined on dried whole lung or whole lung homogenates. The Sirius red plate assay showed a significant increase in collagen content in lung homogenates however, this assay grossly over-estimated the total amount of collagen and underestimated changes between control and fibrotic lungs, conclusions: The proposed method provides accurate quantification of collagen content in whole lungs and additional homogenate samples for biochemical analysis from a single animal. The Sirius-red colorimetric plate assay provides a complementary method for determination of the relative changes in lung collagen but the values tend to overestimate absolute values obtained by the gold standard hydroxyproline assay and underestimate the overall fibrotic injury.
Keywords: Pulmonary fibrosis, hydroxyproline, Sirius red, asbestos, collagen quantification
Introduction
Collagen is the major structural protein of the acellular matrix of the lung [1]. The most common method for evaluating tissue fibrosis/ collagen deposition is hydroxyproline quantification [2]. Intratracheal instillation of fibrosis-stimulating agents like Bleomycin [3, 4] and asbestos [5], results in non-uniform dispersement and heterogeneous fibrosis, similar to that seen in human idiopathic pulmonary fibrosis (IPF)[6, 7]. Currently the best practices for biochemical and histological analysis of pulmonary fibrosis require that both lungs be used for hydroxyproline assessment of fibrosis and a second set of animals for biochemical and protein assays to ensure accurate assessment of fibrosis as one lung may be more effected than the other. This results in the need for large numbers of animals for accurate fibrosis analysis and additional biochemical analysis.
Tissues are composed of collagen types that can be categorized in two biochemical groups: 1) acid soluble collagens and 2) neutral-salt soluble or extractable collagens. Acid soluble collagens are composed of mature fibers with many intramolecular cross-links, while neutral-salt soluble collagen is thought to be an immature precursor (with fewer cross-links) to mature collagen [1, 8]. 4-Hydroxyproline is a major component of collagen, comprising around 13.5% of its amino acid composition[9]. The protein with the next highest content is elastin at < 2% hydroxyproline[9]. The basis of hydroxyproline quantification is that total collagen can be assessed by acid hydralization of proteins followed by measurement of hydroxyproline content. Only 1-5% of collagen in the adult lung is neutral salt soluble[1], suggesting that harsher techniques such as acid hydralization are required for accurate assessment of total collagen burden.
The Sirius red stain was first described by Junqueira et al. in 1979[10] and is a dye that binds to the [Gly-x-y] triple-helix structure found in all collagen fibers. This property of Sirius red stain can be utilized to assess collagen in lung and cardiac tissue sections under bright field and polarized light [10, 11] and in cell culture [12, 13]. It has also been used as a colorometric estimate of fibrosis in biochemical analysis Sirius red dye extraction from thick sections of rat liver compared to histological determination of Sirius red staining and hydroxyproline quantification [14]. However, the accuracy of this biochemical approach, which lacks tissue dissociation and the polarization required to increase the specificity of the signal, has not been directly compared to the gold standard hydroxyproline method to assess lung fibrosis and collagen burden.
The aim of this study was to create a simplified method for the accurate assessment of collagen burden and biochemical analysis of proteins in the lung during fibrosis using a single mouse for all assays. We applied the Sirius red staining assay principles and hydroxyproline quantification principles to develop a processing and analysis method that requires only a single mouse to assess multiple aspects of fibrosis and other biochemical parameters.
Methods
Animal experiments
Animal protocols were approved by the University of Pittsburgh IACUC. Wild-type C57BL/6, males at 8 weeks of age, were randomized into two groups of 10 mice each and administered a single intratracheal injection of 0.14 mg crocidolite asbestos (NIEHS, Bethesda, MD) or 0.14 mg titanium dioxide (inert control particle, sigma) in sterile saline, as previously described [15-17]. Animals were euthanized on day 14 after treatment and lungs were collected for processing. Both lungs of the first group of control- and asbestos-treated mice (n=5 per treatment) were removed and dried at 110°C in glass vacuoles for hydroxyproline analysis (see below). For the second group of mice (n=5 per treatment), the lungs were immediately homogenized in CHAPS homogenization buffer, as described below.
Tissue processing
Both lungs were homogenized on ice in 3 ml of CHAPS detergent buffer (50mmol/L Tris-HCl, pH7.4, 150 mM NaCl, 10 mM CHAPS) with protease inhibitors (10 mM Dichloro-isocoumarin, 3m M EDTA, and 100 mM E-64 (trans-Epoxysuccinyl-leucylamido-[4-guanidino] butane), Sigma). One half of the tissue homogenate (1.5 ml) was placed in glass vacuoles for hydroxyproline analysis. For the Sirius red assay, 100 ml was set-aside immediately after homogenization for analysis. The remaining homogenate samples were rotated for 2 hours at 4°C, followed by sonication and centrifugation, and the supernatants were collected. 100 ml of this supernatant was set aside for the Sirius red plate assay. All samples were stored at −80°C until further use.
Hydroxyproline analysis
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Thermo-Fisher (Waltam, MA) unless otherwise noted. The hydroxyproline assay has been previously described [2, 4, 18, 19]. The whole lung tissue and one half the volume of the homogenized samples (value later utilized in equation 1:C below) were dried in glass vacuoles (Thermo-Fisher) in a 110 °C oven for 48 hours. Acid hydrolysis was completed by adding 2 ml of 6M HCl to each sample. The vials were vacuumed to remove oxygen, which was replaced with nitrogen gas, sealed and incubated under anoxic conditions for 24 hours at 110°C. The vials were broken open and the acid was allowed to evaporate for 24 hours at 110°C. Each sample was reconstituted with 2 ml of sterile PBS (See equation 1: B, below), sealed with parafilm and incubated for 1 hour in a 60°C water bath. Each sample was centrifuged and the supernatants were analyzed for hydroxyproline.
4-hydroxy-L-proline standards, ranging from 0-5 mg/ml hydroxyproline, were created from a 10 mg/ml hydroxyproline stock solution. All of the standards and sample dilutions (1:40 for whole lung samples and 1:20 for homogenate samples) were created using PBS as the diluent in a final volume of 1ml. Both the standards and the processed tissue samples were incubated with 0.5 ml of chloramine-T solution (50mM chloramine-T, 30% v/v Ethylene glycol mono-methyl ether, 50% (v/v) hydroxyproline buffer (0.26M citric acid, 1.46M sodium acetate, 0.85M sodium hydroxide, 1.2% v/v glacial acetic acid), distilled H2O for the remaining volume) for 20 minutes at room temperature, followed by 0.5ml of 3.15M perchloric acid for 5 minutes at room temperature. Samples were mixed well after each addition. P-dimethylamino-benzaldehyde solution (1.34M p-dimethylamino-benzaldehyde dissolved in Ethylene glycol monomethyl ether) was added to each (0.5ml) and incubated for 20 minutes at 60°C for color development. The standards and samples were read in a 96-well plate at 557nm on a spectro-photometer (SpectraMax plus, Molecular Devices, Sunnyvale, CA) with results reported as mg hydroxyproline/ml (See equation 1: A, below). To determine the total hydroxyproline (equation 2: D) and collagen per total lung (equation 2: X), the following calculations were completed:
Equation 1: Calculation for mg hydroxyproline per total lung:
A = 96-well plate raw data (mg hydroxyproline/ ml); B = Hydroxyproline sample volume in PBS (2 ml); C = One half the lung homogenate dried for hydroxyproline; D = mg hydroxyproline/total lung.
Equation 2: Conversion to Total collagen per total lung:
D = mg hydroxyproline / total lung; Collagen conversion factor = 13.5; × = mg collagen / total lung.
Neuman et al. reported that approximately 13.5% of collagen is composed of hydroxyproline [9], thus this value was used as the conversion factor.
Sirius red colorimetric plate assay
The Sirius red 96-well plate assay was modified from similar assays described for cell culture supernatants [13]. Collagen I (Sigma) was used for the collagen standard control. The collagen standards ranged from 20-200mg collagen I / well and dilution were made in PBS for a total well volume of 0.15ml. 10ml of sample (value utilized in equation 3: B) and 140ml of PBS was added to each well in triplicate. Samples were collected immediately after lung homogenization (containing salt soluble and acid soluble collagens) and after centrifugation (containing only salt soluble collagens).
The plate was dried overnight at 37°C and sub-sequently washed three times with distilled water (200ml/well). To each well, 150 ml of 0.1% Sirius red stain (Direct Red 80, Sigma) in saturated picric acid was added and the wells were incubated for 1 hour at room temperature on a rocker. The plate was washed four times with acidified water (200ml/well, 5% acetic acid) and incubated with 150ml 0.1 M NaOH for 30 minutes at room temperature on a rocker. The contents of each well were transferred to a new 96-well plate to avoid any background from dried samples and read on a spectrophotometer at 550 nm. The standards were fit to a 4-parameter curve, R2= 0.99. The spectrophotometer data out was mg collagen for each sample (see equation 3: A). 10 ml of each sample was assayed in the Sirius red assay. Conversion calculations were completed to adjust the value for the initial volume that both lungs were homogenized in, 3 ml.
Equation 3: To calculate the total collagen per total lung:
A = Sirius red plate raw data (mg collagen); B = Volume of lung homogenate assayed; C = mg collagen / ml; D = Initial volume used for lung homogenization (see the tissue processing method section); X = mg collagen / total lung.
Statistical analysis
Mean concentrations and all other quantitative data (mean ± SEM) were assessed for significance using a 2-way ANOVA followed by Bon-ferroni's post-test using Graphpad Prism software (Graphpad, San Diego, CA). Significance was achieved by a p-value < 0.05. Sample sizes (n) are indicated in the Figure legends.
Results
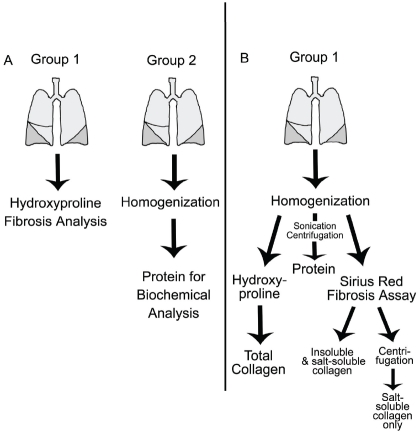
The determination of collagen burden in the lung, when using intratracheal injury models, is challenged by the necessity to assay both lungs in order to get an accurate assessment of fibrosis. This requires significantly more animals to be utilized so that fibrosis and biochemical analyses can be done (Figure 1A). The schematic of Figure 1B shows that modifications to the experimental design and tissue processing can maintain the integrity and accuracy of a study, while allowing for additional analysis so that the number of animals required is markedly reduced.
Figure 1.
Experimental Schematic A) This schematic represents the traditional experimental design to assay for fibrosis in the lungs. Group 1: Both lungs are utilized for hydroxyproline quantification. Group 2: A second set of animals is required to attain tissue homogenates for biochemical assays. B) This schematic represents the new method for fibrosis evaluation. Group 1: The lungs of one set of animals are homogenized in neutral salt buffer with protease inhibitors. One half of the volume is dried for hydroxyproline quantification and 100ml is reserved for the Sirius red assay for acid soluble and salt soluble collagens. The remaining volume is rotated and centrifuged to attain a protein fraction for biochemical assays. This fraction can also be applied to the Sirius red assay to determine salt-soluble collagen.
Accurate Determination of Fibrosis Burden with decreased animal numbers
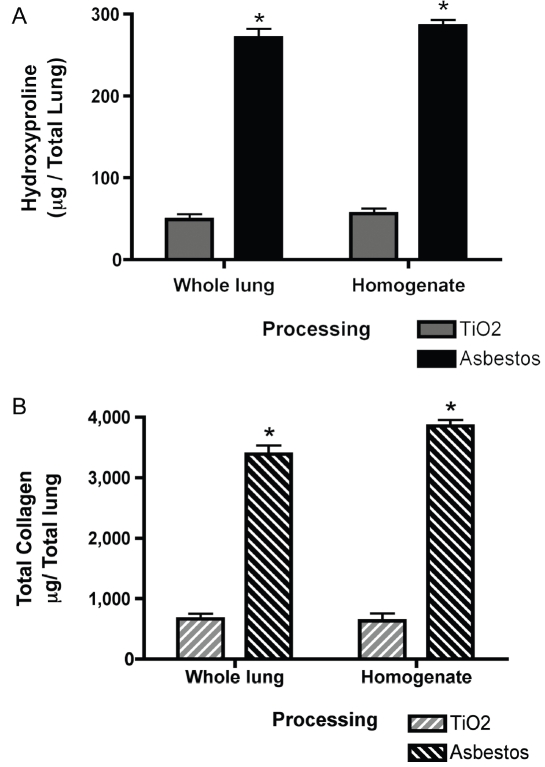
To determine if comparable hydroxyproline analysis can be completed on one half of the volume of total lung homogenates, we compared hydroxyproline data from mice treated with intratracheal asbestos or control titanium dioxide (TiO2). For group 1, both lungs were dried for hydroxyproline, while for group 2, both lungs were homogenized and one half the volume was dried for hydroxyproline analysis. Both processing methods showed that asbestos causes a greater than three-fold increase in hydroxyproline in the lungs (*p<0.05 compared to TiO2 controls, Figure 2A). Homogenization of both lungs prior to the assay did not compromise the accuracy of the analysis. In addition, no difference was seen between the tissue processing methods when data was converted to total collagen per total lung (Figure 2B).
Figure 2.
Comparison of hydroxyproline quantification of collagen in whole lung tissue and whole lung ho-mogenates. A) Hydroxyproline quantification (mg/ total lung), n=5 mice per group per treatment. *p<0.05. B) Total collagen (mg collagen /total lung) determined using a 13.5 conversion factor (see methods), n=5 mice per group per treatment. *p<0.05. All data was analyzed by 2-way ANOVA with Bonferroni post-test
Analysis of salt-soluble collagens by a Sirius Red Colorimetric plate assay
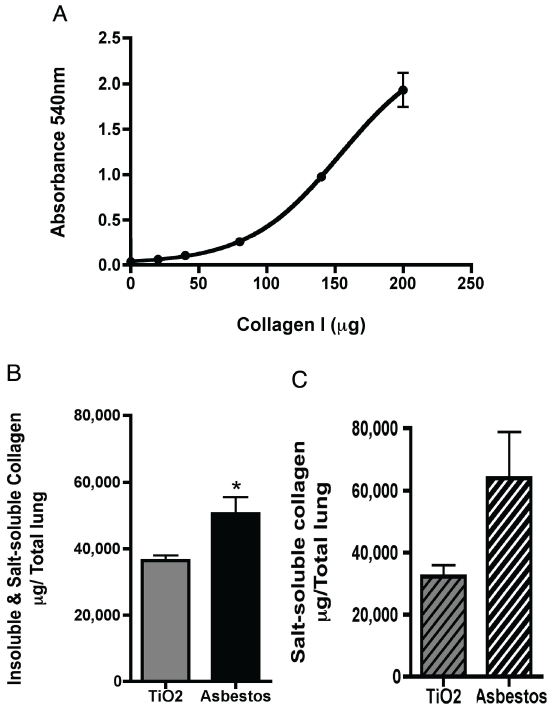
Lung homogenate samples were also analyzed for collagen using a modified Sirius red colorimetric 96-well plate assay with a 4-parameter fit collagen-I standard curve (Figure 3A). The Sirius red assay also showed a significant increase in collagen content in asbestos-treated lung samples containing both insoluble and soluble collagens (Figure 3B, *p<0.05). However, the magnitude of the increase in collagen between control and fibrotic lungs was markedly lower in this assay (25% increase) compared to the hydroxyproline assay (300% increase). After homogenization of both lungs, the homogenate samples were centrifuged with only salt-soluble collagens remaining. The Sirius red assay was able detect a trend towards increased soluble collagens however, with much variability in the results (Figure 3C). In addition, the absolute values of collagen per total lung are very similar between Figure 3B and 3C, when the values for insoluble and soluble collagen (Figure 3B) should be much greater.
Figure 3.
Sirius red plate assay for neutral-salt soluble collagens in whole lung homogenates. A) Sirius red collagen quantification in homogenate samples contain insoluble and salt-soluble collagens (prior to sample centrifugation). *p<0.05, Student's t-test. B) Collagen quantification of homogenate samples with only salt-soluble collagens. C) Collagen-I standard curve for the Sirius red colorimetric plate assay with a 4-parameter non-linear curve fit (Graphpad Prism). The coefficient for the curve is R2 = 0.99.
Discussion
The lung provides a unique challenge for fibrosis investigations, in that many stimuli for fibrosis are administered intratracheally and create a heterogeneic pattern of injury in the lung.
Evaluating only one lung for fibrosis or protein analysis likely introduces sampling error, as one lung may contain more fibrosis than the other. This study presents a simplified method of evaluating lung fibrosis that eliminates this error and provides additional information about insoluble collagen (mature fibers) and soluble collagen burdens (immature, newly synthesized fibers). Additionally, proteomic analysis can be performed on the remaining 1.5 ml of lung homogenate.
Homogenization of both lungs and then sampling only a portion of the homogenate, prior to the hydroxyproline assay results in comparable data to performing hydroxyproline analysis on whole lungs (Figure 2). The amounts of collagen determined for the TiO2-treated mice through this assay are very close to previous reports suggesting that the lung of a normal 20-g mouse contains approximately 1.2 mg of collagen (∼15-20% of the dry lung weight). This data suggests that this method provides equivalent results and efficiency allowing sampling from a single mouse. This homogenization method could be utilized to quantify fibrosis in other tissues where protein homogenates are also needed.
Sirius red staining of collagen has been used for many years. The present colorimetric plate assay allows for rapid assessment of collagen content. One major problem with the Sirius red assay is that it appears to over-estimate the amount of collagen in a sample. Only 1-5% of collagen in the adult lung is believed to be salt soluble [1]. The assay utilizes only a small fraction of the original sample (10ml of a an original samples volume of 3 ml) and small variations inherent to the staining procedure are amplified with subsequent calculations to determine the total collagen content. This data suggests that the Sirius red plate assay may be an overestimate of the true collagen burden of the lung. However, the assay does provide information about the relative changes of immature precursor collagen during fibrosis development. In addition, this assay under-estimates the relative increase in fibrosis between control and injured animals, further suggesting that there is nonspecific binding of the Sirius red dye to non-collagen proteins. Thus, the use of the Sirius red assay may not reflect significant changes in fibrosis that would be seen with the hydroxyproline assay.
There are several important advantages to this new method: 1) Only one mouse is required to attain fibrosis and protein/biochemical data compared to twice the number of animals with the previous method. 2) The homogenized tissue samples require less drying time during the hydroxyproline assay, which can shorten the procedure by 24 hours. 3) The assay provides an accurate quantification of total lung hydroxyproline and collagen through analysis of both lungs, not a single lung or lobe. 4) The Sirius red colorimetric plate assay can be utilized as a complementary assay to evaluate the relative changes in salt-soluble collagens, which are the precursors to mature collagen. One disadvantage to the new procedure is that is requires careful homogenization of tissues in identical volumes to ensure that error is not introduced through this step.
In summary, we provide an improved method for the assessment of collagen and biochemical analysis from a single mouse. This allows for a significant reduction in the number of animals used while producing accurate data on the fibrotic environment of the lung. Additionally, this homogenization technique would likely work for other pulmonary disease models, in which injury is induced by intratracheal instillation of a stimuli and which require multiple methods of lung tissue analysis.
Acknowledgments
This work was supported by the National Institutes of Health under National Research Service Awards (F30ES016483-01 to CRK and F30ES016973 to JME) and a Research Project Grant (R01HL063700-09 to TDO).
Glossary
Abbreviations
- IPF
Idiopathic Pulmonary Fibrosis
- TiO2
Titanium dioxide
References
- 1.Last JA, Reiser KM. Collagen biosynthesis. Environ Health Perspect. 1984;55:169–177. doi: 10.1289/ehp.8455169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 3.Lazo JS, Hoyt DG, Sebti SM, Pitt BR. Bleomyca pharmacologic tool in the study of the pathogenesis of interstitial pulmonary fibrosis. Pharmacol Ther. 1990;47:347–358. doi: 10.1016/0163-7258(90)90061-6. [DOI] [PubMed] [Google Scholar]
- 4.Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2003;35:763–771. doi: 10.1016/s0891-5849(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 5.Fattman C CC, Oury TD. Experimental Models of Asbestos-Related Diseases. In: Roggli V, Oury TD, Sporn TA, editors. Pathology of Asbestos-Associated Diseases. New York: Springer-Verlag; 2005. pp. 256–308. [Google Scholar]
- 6.Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, Jain A, Strawderman RL, Flint A, Lynch JP, Martinez FJ. Histopa-thologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164:1722–1727. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 7.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 8.Jackson DS, Bentley JP. On the significance of the extractable collagens. J Biophys Biochem Cytol. 1960;7:37–42. doi: 10.1083/jcb.7.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184:299–306. [PubMed] [Google Scholar]
- 10.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 11.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 12.Tullberg-Reinert H, Jundt G. In situ measurement of collagen synthesis by human bone cells with a sirius red-based colorimetric mi-croassay: effects of transforming growth factor beta2 and ascorbic acid 2-phosphate. Histo-chem Cell Biol. 1999;112:271–276. doi: 10.1007/s004180050447. [DOI] [PubMed] [Google Scholar]
- 13.Walsh BJ, Thornton SC, Penny R, Breit SN. Microplate reader-based quantitation of collagens. Anal Biochem. 1992;203:187–190. doi: 10.1016/0003-2697(92)90301-m. [DOI] [PubMed] [Google Scholar]
- 14.James J, Bosch KS, Aronson DC, Houtkooper JM. Sirius red histophotometry and spectrophotometry of sections in the assessment of the collagen content of liver tissue and its application in growing rat liver. Liver. 1990;10:1–5. doi: 10.1111/j.1600-0676.1990.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 15.Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol. 2004;97:2006–2013. doi: 10.1152/japplphysiol.00480.2004. [DOI] [PubMed] [Google Scholar]
- 16.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, Ramsgaard L, Richards TJ, Loutaev I, Nawroth PP, Kasper M, Bierhaus A, Oury TD. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol. 2008;172:583–591. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas IO, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2008 doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomy-cin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 19.Myllarniemi M, Lindholm P, Ryynanen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:321–329. doi: 10.1164/rccm.200706-945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]