Abstract
Angioimmunoblastic T-cell lymphoma (AITL) is a unique type of peripheral T-cell lymphoma. Patients with AITL may have occasional reactive plasma cells present in the peripheral circulation. Prominent peripheral blood polyclonal plasmacytosis mimicking plasma cell leukemia, however, is distinctly uncommon. Here we describe 3 such cases from two large tertiary medical centers and discuss the role of ancillary studies in the differential diagnosis of peripheral blood plasmacytosis.
Keywords: Angioimmunoblastic T-cell lymphoma, peripheral blood, plasmacytosis, flow cytometric immunopheno-typing
Introduction
Angioimmunoblastic T-cell lymphoma (AILT) is the second most common peripheral T-cell lymphoma with a constellation of clinical signs and symptoms including fever, weight loss, chills, skin rash, pruritus, lymphadenopathy, hepa-tosplenomegaly, anemia, thrombocytopenia and polyclonal hypergammaglobulinemia [1, 2]. It was first described by Frizzera et al in the early 1970s as “angio-immunoblastic lymphad-nopahy with dysproteinemia (AILD),” and was originally thought to be a benign, albeit prema-lignant, atypical T-cell proliferation [3]. AILD was reclassified in the Revised European and American classification of Lymphoid neoplasms, and in the 2008 World Health Organization classification of hematolymphoid neoplasms, as a distinct type of peripheral T-cell lymphoma.
In the lymph node, AITL is characterized by proliferation of high-endothelial venules surrounded by a polymorphous infiltrate of medium -sized lymphoid cells of follicular T-cell origin with moderate amounts of clear cytoplasm and follicular dendritic cell networks. Admixed are small reactive lymphocytes and occasional large immunoblasts of B-cell lineage, as well as eosi-nophils, histiocytes and plasma cells. In the bone marrow, the disease is characterized by a nodular or interstitial pattern of hypocellular fibrovascular proliferations, infiltrated by lymphoid cells, eosinophils and reactive plasma cells [4, 5].
In the peripheral blood, patients with AITL frequently present with anemia, thrombocytopenia and absolute lymphopenia. Occasional circulating plasma cells, plasmacytoid lymphocytes and immunoblasts have been reported in about one third of patients with AITL. Exuberant reactive plasmacytosis mimicking plasma cell leukemia, however, has only rarely been described [5-7]. Here we present 3 such cases from two large tertiary medical centers, and discuss the differential diagnosis of peripheral blood plasmacytosis in light of ancillary studies that are now routinely available.
Materials and methods
Case report
Case #1: A 76 year-old woman presented with hypercalcemia, fever and diffuse lymphade-nopathy. Serum protein electrophoresis demonstrated hypoalbuminemia and polyclonal hyper-gammaglobulinemia. Peripheral blood findings included an elevated total white blood cell count (16,000 cells/μL) with absolute lymphopenia (1,600 cells/μL) and normochromic normocytic anemia (hematocrit 28.6%). The peripheral blood smear demonstrated Rouleaux formation, and numerous circulating plasma cells, plas-macytoid lymphocytes and scattered immuno-blasts, accounting for about 30% of all white blood cells. Platelets were normal in number and appearance.
Case #2: A 43 year-old woman had a past medical history of AITL status six years post treatment. She was subsequently diagnosed with stage IIC ovarian cancer and treated with chemotherapy, which was stopped early due to development of idiopathic thrombocytopenic pur-pura. She presented with hemolytic anemia and thrombocytopenia refractory to plasmapheresis, intravenous pooled immunoglobulin, and steroid treatment. She underwent emergent sple-nectomy and was admitted to the Medical Intensive Care Unit.
Peripheral blood findings included an elevated white count (18,100 cells/μL) with marked anemia (hematocrit 12.7%) and thrombocytopenia (5,000 platelets/μL). The peripheral smear demonstrated moderate Rouleaux formation with increased numbers of circulating nucleated red blood cells, plasmacytoid lymphocytes and plasma cells. A manual differential count demonstrated 63% segmented neutrophils and 6% lymphocytes, with 13% plasmacytoid lymphocytes and plasma cells.
Case #3: A 60 year-old man presented with generalized lymphadenopathy and splenomegaly. Peripheral blood counts were significant for normochromic, normocytic anemia (hematocrit 27.6%), absolute lymphopenia (700 cells/μL), and thrombocytopenia (12,000 platelets/μL). Serum protein electrophoresis demonstrated polyclonal hypergammaglobulinemia. The peripheral smear demonstrated moderate Rouleaux formation with a marked increase in plasma cells and plasmacytoid lymphocytes (20%) showing large size, occasional multiple nuclei, small nucleoli, and frequent mitoses.
Flow cytometric immunophenotyping
Flow cytometric immunophenotyping was performed as previously described. Intracellular staining for kappa and lambda immunoglobulin light chains was performed after membrane permeablization. Stained cells were acquired with FACSCanto™ II flow cytometer and the list-mode data was analyzed with Diva software (BD Biosciences).
Results
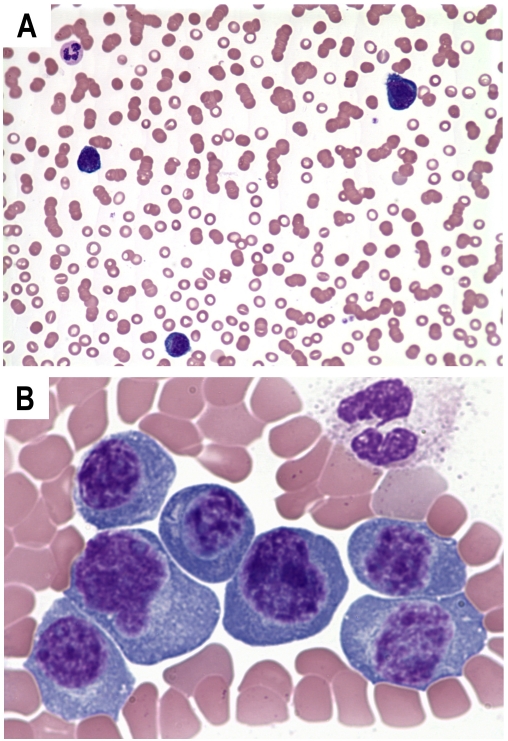
Review of the peripheral blood smear from all 3 patients revealed a prominent population of plasma cells, plasmacytoid lymphocytes and immunoblasts (more than 2 × 103 cells/μL or 20% of the nucleated cells on manual differential). Mitosis is occasionally observed. Rouleaux formation is prominent. Representative images are shown in Figure 1.
Figure 1.
Representative images of peripheral blood film (Wright and Giemsa stain) showing anemia with marked Rouleaux formation (A; 10×) and circulating plasma cells, plasmacytoid lymphocytes and immunoblasts (B; 100×).
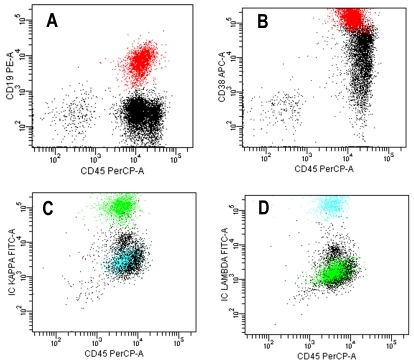
Flow cytometric immunophenotyping of the peripheral blood from all 3 cases showed that approximately 10-30% of the white blood cells expressed CD19, CD38 (high density) and CD45 (low density). Other B cells markers including CD20 were negative. CD56 expression was absent. Surface and intracytoplasmic light chains showed a mixed pattern of expression, confirming the polyclonal nature of the plasma cells and plasmacytoid lymphocytes. Representative scatter plots of the flow cytometric immunophe-notypic findings are shown in Figure 2. No aberrant T-cell populations were detected. Serum protein electrophoresis in 2 patients showed polyclonal hypergammaglobulinemia.
Figure 2.
Representative scatter plots of peripheral blood flow cytometric immunophenotyping. The plasma cells express CD19 (A) and high density CD38 (B), and intracytoplasmic polyclonal immunoglobulin light chain kappa (green) and lambda (cyan) proteins (C and D).
Molecular assay of the peripheral blood samples showed a polyclonal pattern for the immunoglobulin heavy chain gene and an oligoclonal pattern for the T-cell receptor gamma gene (data not shown). Lymph node and bone marrow biopsies were subsequently performed in all 3 patients and the morphologic, immunopheno-typic and molecular findings were diagnostic of de novo (cases #1 and #3) and recurrent (case #2) AITL (data not shown).
Discussion
We report here a prominent peripheral blood plasmacytosis in 3 patients prior to the diagnosis of de novo or recurrent AITL. All patients had generalized lymphadenopathy and/or splenomegaly. A mixture of plasma cells, plasmacytoid lymphocytes and immunoblasts meeting the diagnostic criterion of plasma cell leukemia was present in the peripheral blood, as determined by smear morphology and manual differential. These cells were polyclonal by flow cytometric immunophenotyping, serum protein electrophoresis, and/or molecular assay for immunoglobulin heavy chain gene rearrangement, consistent with a reactive process. No evidence of circulating lymphoma cells was detected in the blood samples.
Plasma cell leukemia is defined by a neoplastic proliferation of clonal plasma cells in excess of 2 × 103 cells/uL or 20% of the white blood cells in the peripheral blood. The clonality of these cells can be demonstrated by serum protein electrophoresis and/or flow cytometric immunophenotyping. In rare cases of nonsecretory plasma cells, molecular studies for immunoglobulin heavy chain gene rearrangement might be needed. Patients with plasma cell leukemia have a dismal prognosis with a median survival less than 1 year in most cases.
Prominent peripheral blood plasmacytosis mimicking plasma cell leukemia in patients with AITL was first reported by Pangalis et al in 1978.[8] Among the 38 AITL cases reported, about a third had circulating “immunocytes", which were defined as plasma cells, plasmacytoid lymphocytes, and immunoblasts. One patient demonstrated a prominent plasmacytosis with as many as 32,000 cells/μL. It has been reported that a variable number of circulating lymphoma cells are present in the peripheral blood in approximately 30% of patients with AITL [9]. It is unclear whether some of these plasmacytoid or immuoblastic cells reported by Pangalis et al may in fact represent malignant lymphoma cells since immunophenotyping and/or molecular studies were not performed.
More recently, Sakai et al and Yamane et al each reported a single case of a patient with exuberant peripheral blood plasmacytosis and generalized lymphadenopathy prior to the diagnosis of AITL [6, 7]. In both cases, the circulating plasma cells and plasmacytoid lymphocytes were assayed by flow cytometric immunophenotyping, and were found to express CD19 and CD38 (high density). CD10, CD20 and CD56 expression were absent. Expression of surface heavy chains with an IgM predominance was studied and observed in one case [7]. The majority of these cells were reported to lack cyto-plasmic immunoglobulin. In the other case, the cells express polytypic surface immunoglobulin light chains [6]. Similar to cases described here, this phenotype is consistent with a mixture of reactive plasma cells and plasmacytoid lymphocytes, and the finding of a polyclonal increase in immunoglobulins by serum protein electropho-resis.
The presence of plasma cells, plasmacytoid lymphocytes and immunoblasts in the peripheral blood smear is usually suggestive of a reactive process. These include bacterial or viral infections (such as parvovirus B19, hepatitis, dengue fever or Epstein-Barr virus), autoimmune phenomenon (such as rheumatoid arthritis, systemic lupus erythematosus, or Sjögren's Syndrome), and serum sickness [10-18]. It is more rarely associated with a neoplastic process (such as AITL). These cells are polyclonal in nature, and should be distinguished from the clonal proliferation of plasma cells, plasmacytoid cells or immunoblasts in plasma cell leukemia and nonHodgkin lymphomas with circulating plasmacytoid cells or immunoblasts. Serum protein electrophoresis may be useful to demonstrate clonality if a paraprotein is present. Flow cytometric immunophenotyping on a peripheral blood sample can rapidly differentiate reactive from clonal plasma cells, plasmacytoid lymphocytes or immunoblasts.
The mechanism of polyclonal proliferation of plasma cells in the peripheral blood of patients with AITL is probably multifactorial. The known association of Epstein-Barr virus (EBV) infection of the B cells in AITL may contribute to the reactive plasma cell proliferation. Increased release of cytokines, such IL-6 or IL-10 that are known to stimulate plasma cell generation, may also contribute to this process.
In these three cases reported here, only the subsequent examination of additional tissues demonstrated the presence of AITL, similar to each one of the cases reported by Sakai et al and Yamane et al. [6, 7] Thus, it must be stressed that polyclonal plasmacytosis in the peripheral blood should be investigated for AITL, especially in the presence of cardinal signs of lymphoma such as B symptoms, lymphadenopathy and/or hepatosplenomegaly.
References
- 1.Iannitto E, Ferreri AJ, Minardi V, Tripodo C, Kreipe HH. Angioimmunoblastic T-cell lymphoma. Crit Rev Oncol Hematol. 2008;68:264–271. doi: 10.1016/j.critrevonc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Mourad N, Mounier N, Briere J, Raffoux E, Delmer A, Feller A, Meijer CJ, Emile JF, Bouabdallah R, Bosly A, Diebold J, Haioun C, Coiffier B, Gisselbrecht C, Gaulard P. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2008;111:4463–4470. doi: 10.1182/blood-2007-08-105759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frizzera G, Kaneko Y, Sakurai M. Angioimmunoblastic lymphadenopathy and related disorders: a retrospective look in search of definitions. Leukemia. 1989;3:1–5. [PubMed] [Google Scholar]
- 4.Khokhar FA, Payne WD, Talwalkar SS, Jorgensen JL, Bueso-Ramos CE, Medeiros U, Vega F. Angioimmunoblastic T-cell lymphoma in bone marrow: a morphologic and immunophenotypic study. Hum Pathol. 2009 doi: 10.1016/j.humpath.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Cho YU, Chi HS, Park CJ, Jang S, Seo EJ, Huh J. Distinct features of angioimmunoblastic T-cell lymphoma with bone marrow involvement. Am J Clin Pathol. 2009;131:640–646. doi: 10.1309/AJCPQXKCHQH4VAJ5. [DOI] [PubMed] [Google Scholar]
- 6.Yamane A, Awaya N, Shimizu T, Ikeda Y, Okamoto S. Angioimmunoblastic T-cell lymphoma with polyclonal proliferation of plasma cells in peripheral blood and marrow. Acta Haematol. 2007;117:74–77. doi: 10.1159/000096894. [DOI] [PubMed] [Google Scholar]
- 7.Sakai H, Tanaka H, Katsurada T, Yoshida Y, Okamoto E, Ohno H. Angioimmunoblastic T-cell lymphoma initially presenting with replacement of bone marrow and peripheral plasmacytosis. Intern Med. 2007;46:419–424. doi: 10.2169/internalmedicine.46.6121. [DOI] [PubMed] [Google Scholar]
- 8.Pangalis GA, Moran EM, Rappaport H. Blood and bone marrow findings in angioimmunoblastic lymphadenopathy. Blood. 1978;51:71–83. [PubMed] [Google Scholar]
- 9.Lachenal F, Berger F, Ghesquieres H, Biron P, Hot A, Callet-Bauchu E, Chassagne C, Coiffier B, Durieu I, Rousset H, Salles G. Angioim-munoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore) 2007;86:282–292. doi: 10.1097/MD.0b013e3181573059. [DOI] [PubMed] [Google Scholar]
- 10.Gawoski JM, Ooi WW. Dengue fever mimicking plasma cell leukemia. Arch Pathol Lab Med. 2003;127:1026–1027. doi: 10.5858/2003-127-1026-DFMPCL. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Hsu P, Patel K, Saffari Y, Ashley I, Brody J. Polyclonal plasma cell proliferation with marked hypergammaglobulinemia and multiple autoantibodies. Ann Clin Lab Sci. 2006;36:479–484. [PubMed] [Google Scholar]
- 12.Koduri PR, Naides SJ. Transient blood plasmacytosis in parvovirus B19 infection: a report of two cases. Ann Hematol. 1996;72:49–51. doi: 10.1007/BF00663017. [DOI] [PubMed] [Google Scholar]
- 13.Wada T, Maeba H, Ikawa Y, Hashida Y, Okumura A, Shibata F, Tone Y, Inoue M, Koizumi S, Takatori H, Sakai Y, Kaneko S, Yachie A. Reactive peripheral blood plasmacytosis in a patient with acute hepatitis A. Int J Hematol. 2007;85:191–194. doi: 10.1532/IJH97.06200. [DOI] [PubMed] [Google Scholar]
- 14.Shtalrid M, Shvidel L, Vorst E. Polyclonal reactive peripheral blood plasmacytosis mimicking plasma cell leukemia in a patient with Staphylococcal sepsis. Leuk Lymphoma. 2003;44:379–380. doi: 10.1080/1042819021000029713. [DOI] [PubMed] [Google Scholar]
- 15.Mori I, Parizot C, Dorgham K, Demeret S, Amoura Z, Bolgert F, Gorochov G. Prominent plasmacytosis following intravenous im-munoglobulin correlates with clinical improvement in Guillain-Barre syndrome. PLoS One. 2008;3:e2109. doi: 10.1371/journal.pone.0002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touzeau C, Pellat-Deceunynck C, Gastinne T, Accard F, Jego G, Avet-Loiseau H, Robillard N, Harousseau JL, Bataille R, Moreau P. Reactive plasmacytoses can mimick plasma cell leukemia: therapeutical implications. Leuk Lymphoma. 2007;48:207–208. doi: 10.1080/10428190601016159. [DOI] [PubMed] [Google Scholar]
- 17.Komiya I, Saito Y, Kuriya S. Peripheral blood plasmacytosis in a patient with infectious mononucleosis-like illness. Eur J Haematol. 1991;46:61–62. doi: 10.1111/j.1600-0609.1991.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 18.Thai KT, Wismeijer JA, Zumpolle C, de Jong MD, Kersten MJ, de Vries PJ. High incidence of peripheral blood plasmacytosis in patients with dengue virus infection. Clin Microbiol Infect. doi: 10.1111/j.1469-0691.2010.03434.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]