Indication
Two new orphan medicines, rilonacept [1, 2] (Regeneron®) and canakinumab [3, 4] (Ilaris®), are indicated for the treatment of cryopyrin-associated periodic syndromes (CAPS). CAPS occur in a group of rare, inherited, inflammatory diseases where patients have a mutation in the NLRP3 (nucleotide-binding domain, leucine-rich family, pyrin domain-containing-3) gene encoding for cryopyrin, which is a regulator in the acute immune reaction. Both familial cold auto-inflammatory syndrome and Muckle–Wells syndrome belong to the CAPS group. Additionally, canakinumab may be used to treat other CAPS such as neonatal-onset multisystem inflammatory disease and familial cold urticaria. The indications for both drugs might be extended in future. Currently, these two drugs are being evaluated in more common diseases including rheumatoid arthritis and systemic-onset juvenile idiopathic arthritis [5, 6].
Mechanism
The pathophysiology underlying CAPS is characterized by overstimulation of the regulatory system of thepro-inflammatory cytokine interleukin-1β (Il-1β). The synthesis and release of Il-1β requires two distinct signals which are normally initiated by damage-associated molecular patterns, either pathogen-associated molecular patterns, e.g. bacterial RNA or lipopolysaccharides, or endogenous irritants, e.g. uric acid or heat shock proteins. One signal leads to synthesis of pro-Il-1β and components of the protein complex inflammasome NLRP3/cryopyrin. A second signal leads to the assembly of the inflammasome and activation of caspase-1. Active Il-1β is released from pro-Il-1β by caspase-1 (Figure 1).
Figure 1.
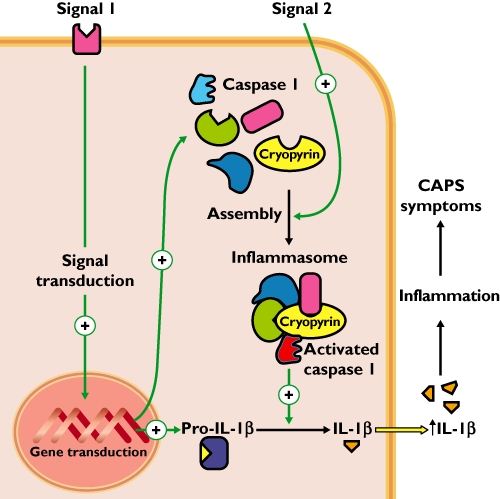
Pathophysiology of cryopyrin-associated periodic syndromes (CAPS). Because of a mutation in the NLRP3/cryopyrin gene, there is a constant activation of the regulatory system of Il-1β synthesis and secretion. A first signal activates expression of pro-Il-1β and components of the protein complex inflammasome NLRP3/cryopyrin. A second signal triggers the assembly of the inflammasome, and initiates caspase-1 activation required for the cleavage of pro-Il-1β into active Il-1β
In order to exert its pro-inflammatory actions, Il-1β must bind two receptors, the interleukin 1 receptor (Il-1-R1) and an accessory protein (Il-1-RAcP). These receptors form a complex at the membrane necessary for signal transduction.
Rilonacept and canakinumab each work by trapping Il-1β before it binds its receptor complex, preventing the pro-inflammatory effects that cause the symptoms of CAPS (Figure 2).
Figure 2.
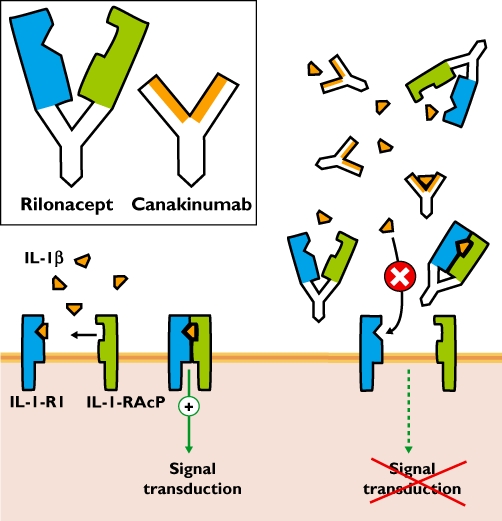
Mechanism of action of rilonacept and canakinumab. Inset: The antibody rilonacept is composed of a human IgG1 Fc domain (white) with the two arms specific for Il-1β binding: the extracellular domains of the Il-1 receptor 1 (Il-1-R1 in blue) and of the Il-1 receptor accessory protein (Il-1-RAcP in green). Canakinumab is a human IgG1 antibody designed against Il-1β. Main panel (left): Under (patho)physiological conditions Il-1β binds to both Il-1 receptor components on the cell surface mediating its pro-inflammatory effects. Main panel (right): In the presence of rilonacept or canakinumab, Il-1β is trapped before it can reach its membrane receptors and thereby preventing signal transduction leading to inflammation
Rilonacept consists of the extracellular domains of the Il-1-RAcP and the Il-1-R1 fused to the Fc portion of human IgG1. It binds Il-1β and Il-1α with high affinity and potently inhibits Il-1 activity. It is administered subcutaneously beginning with a loading dose followed by a weekly injection of half the loading dose.
Canakinumab is a specific human monoclonal IgG1 antibody targeted against Il-1β. This antibody has no cross-reactivity with Il-1α nor with the Il-1-R1 receptor. Canakinumab is also injected subcutaneously, but less often than rilonacept (once every 8 weeks).
Adverse effects
In general, the adverse effects of rilonacept and canakinumab are similar and have been mild. By blocking Il-1 actions, either of these drugs could interfere with the immune response. The most common adverse effects (>10% of treated patients) are reactions at the injection site, inflammation of the upper respiratory tract or sinuses and headache. CAPS patients suffering from severe, active infection should not be treated with either rilonacept or canakinumab.
Literature
- 1. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001047/human_med_000653.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124.
- 2.Hofman HM, Yasothan U, Kirkpatrick P. Fresh from the pipeline: rilonacept. Nat Rev Drug Discov. 2008;7:385–6. [Google Scholar]
- 3. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001109/human_med_000826.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124.
- 4.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, Gitton X, Widmer A, Patel N, Hawkins PH, for the Canakinumab in CAPS Study Group Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:2416–25. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 5.Stahl N, Radin A, Mellis S. Rilonacept – CAPS and beyond. Ann N Y Acad Sci. 2009;1182:124–34. doi: 10.1111/j.1749-6632.2009.05074.x. [DOI] [PubMed] [Google Scholar]
- 6.Geyer M, Müller-Ladner U. Actual status of anti-interleukin-1 therapies in rheumatic diseases. Curr Opin Rheumatol. 2010;22:246–51. doi: 10.1097/BOR.0b013e3283373fa0. [DOI] [PubMed] [Google Scholar]


