Abstract
Many drugs used for the treatment of HIV disease (including the associated opportunistic infections) can cause drug hypersensitivity reactions, which vary in severity, clinical manifestations and frequency. These reactions are not only seen with the older compounds, but also with the newer more recently introduced drugs. The pathogenesis is unclear in most cases, but there is increasing evidence to support that many of these are mediated through a combination of immunologic and genetic factors through the major histocompatibility complex (MHC). Genetic predisposition to the occurrence of these allergic reactions has been shown for some of the drugs, notably abacavir hypersensitivity which is strongly associated with the class I MHC allele, HLA-B*5701. Testing before the prescription of abacavir has been shown to be of clinical utility, has resulted in a change in the drug label, is now recommended in clinical guidelines and is practiced in most Western countries. For most other drugs, however, there are no good methods of prevention, and clinical monitoring with appropriate (usually supportive and symptomatic) treatment is required. There is a need to undertake further research in this area to increase our understanding of the mechanisms, which may lead to better preventive strategies through the development of predictive genetic biomarkers or through guiding the design of drugs less likely to cause these types of adverse drug reactions.
Keywords: abacavir, drug hypersensitivity, nevirapine, pharmacogenetics, SJS, TEN
Introduction
There are currently 22 antiretroviral drugs available in the UK which can be used in combinations of three or more drugs. Such combinations are known as highly active antiretroviral therapy (HAART) [1,2]. There are currently six groups of agents comprising nucleoside reverse transcriptase inhibitors (NRTIs), non nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs) and three new groups, namely entry inhibitors (fusion inhibitors and CCR5 inhibitors) and integrase inhibitors (Table 1). HAART is effective and has lead to decreases in mortality and morbidity from HIV [3]. However each of these drugs has a potential to cause serious adverse effects, including allergic reactions, as outlined in Table 2. The purpose of the article is to provide a succinct review of drug hypersensitivity associated with HAART, including the epidemiology, pathophysiology and management.
Table 1.
Drugs used for the treatment of HIV*
| NRTI | NNRTI | PI | Fusion inhibitors | CCR5 inhibitors | Integrase inhibitors |
|---|---|---|---|---|---|
| Zidovudine | Efavirenz | Lopinavir | Enfurvitide | Maraviroc | Raltegravir |
| Stavudine | Nevirapine | Atazanavir | |||
| Lamivudine | Etravirine | Saquinavir | |||
| Emtricitabine | Fosamprenavir | ||||
| Tenofovir | Tipranavir | ||||
| Didanosine | Darunavir | ||||
| Abacavir | Ritonavir | ||||
| Indinavir |
Drugs no longer used: Delavridine, Nelfinavir, Zalcitabine, Amprenavir.
Table 2.
HIV drugs associated with drug hypersensitivity
| Class | Drug | Reaction | Hepatotoxicity | Frequency |
|---|---|---|---|---|
| NRTI | Zidovudine | Exanthema | Not reported | Rare |
| Abacavir | Exanthema, HSR | Elevated LFTs hepatitis, liver failure | 2.3–9% [23] | |
| Emtricitabine | Rash | Elevated LFTs | 17% [134] | |
| NNRTIs | Efavirenz | SJS, TENExanthema, | Elevated LFTs | 0.1% [135] 4.6–20% [129] |
| Nevirapine | Exanthema, TEN, SJS, HSR, | Elevated LFTs immune mediated hepatitis, liver failure | 17–32% [49] 0.3–2% [49] 2–10% discontinuation [9] | |
| Etravirine | Rash, SJS, TEN | Elevated LFTs | 16% [136] 2% discontinuation [72] | |
| PIs | Tipranavir | Rash, dyslipidaemia | Elevated LFTs s, toxic hepatitis | 2–14% [85] 2–6.4% [87] |
| Atazanavir | Rash | Hyperbilirubinaemia | 6% [137] | |
| Fosamprenavir | Rash, HSR | Elevated LFTs | 1–19% [79] Discontinuation <1% | |
| Lopinavir | Rash | Elevated LFTs | 2–4% [77] | |
| Darunavir | Rash, HSR | Elevated LFTs | 6.7% [138] Rare | |
| Entry inhibitors | Enfuvirtide | Injection site reactions, HSR | Not reported | Rare |
| Maraviroc | Rash, cough, | Elevated LFTs | Rash [91,139] |
HSR, hypersensitivity reaction; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; LFTs, liver function tests.
General features of drug hypersensitivity in HIV
Skin reactions are the most common manifestation of drug hypersensitivity. These may present with exanthema without systemic symptoms or drug hypersensitivity syndromes typically manifesting as an erythematous, maculopapular confluent rash (Figure 1) with constitutional features (fever, rigors, myalgias, and arthralgias) in the presence or absence of internal organ involvement (hepatitis, pneumonitis, myocarditis, pericarditis and nephritis). The constitutional symptoms can either precede the rash or occur without it. Eosinophilia and mononucleosis are also more likely to occur than in the blistering reactions [4]. This syndrome has various names including DRESS (drug reaction with eosinophilia and systemic symptoms) and DIHS (drug-induced hypersensitivity syndrome).
Figure 1.
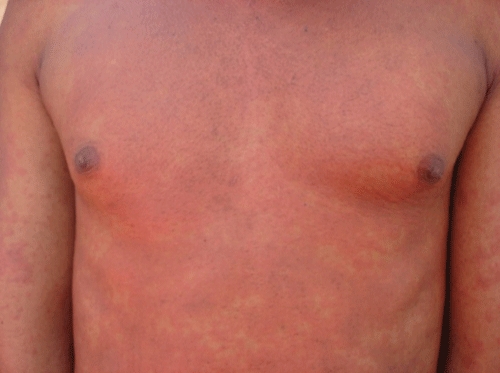
A typical maculopapular exanthema seen in hypersensitivity syndrome caused by antiretrovirals
Severe skin eruptions such as Stevens Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) develop in less than 0·5% of patients [5]. They are characterized by blistering affecting less than 10% (SJS), between 10 and 30% (overlap syndrome) and >30% (TEN) of body surface area, associated with mucosal membrane involvement. The most frequently affected mucosal membrane is the oropharynx (mouth ulcers), followed by the eyes (iritis/conjunctivitis) and genitourinary tract [5]. Extra-cutaneous involvement of variable severity is also seen in the blistering conditions [6]. In TEN, epidermal detachment may be extensive, and may affect the entire skin surface. Lesions may continue to erupt in crops for as long as 2 to 3 weeks [7].
The diagnosis of drug hypersensitivity in HIV patients is based on clinical criteria, but is complicated by the fact that many patients take multiple drugs and develop diseases such as opportunistic infections and immune restoration disease that can make determination of causality difficult. Diagnosis therefore does depend on carefully evaluating the temporal relationship, the effect of dechallenge and rechallenge, and exclusion of other causes. Usually the onset of an allergic reaction is delayed, between 1–6 weeks after commencing the drug. Rash or fever occurring more than 3 months after onset of therapy is almost always due to another agent. As in other conditions, rechallenge with the offending drug can lead to a serious and possibly fatal reaction [7], with the reaction occurring much sooner than on first exposure [8], and thus is rarely attempted. However, it is also important to note that (i) patients can sometimes be treated through the rash especially when it is mild to moderate and not accompanied by systemic symptoms such as fever or internal organ involvement [9] and (ii) desensitization techniques have been used when there is thought to be a clinical need for a particular agent. This was particularly the case with sulfamethoxazole [10], when it was more widely used for the treatment and/or prevention of opportunistic infections.
Mechanisms
The pathophysiology of drug hypersensitivity in HIV is multifactorial and related to a number of metabolic, immunologic, host and viral factors. Laboratory data showing that drug hypersensitivity is indeed immune mediated with data on the involvement of T-cells are now beginning to appear [11], and immunohistological analysis of skin lesions and analysis of the phenotype and functionality of drug-specific T-cell clones from hypersensitive patients [12] providing interesting insights.
The pathway by which drugs are presented in vivo is still unclear, with two prevailing hypotheses, the hapten-dependent and hapten-independent pathways. The former hypothesis states that most drugs are chemically inert, but become immunogenic through metabolism to reactive intermediates which are then able to bind covalently or haptenate with proteins [13,14], and are then presented via the HLA molecules to interact with T cells to form an immunological synapse [15]. The hapten-independent or pharmacological interaction (pI) hypothesis states that the parent drug itself interacts with T-cells through a pathway that is major histocompatibility complex (MHC)-restricted, but metabolism independent [13,16]. This implies that some drugs may actually activate T-cells directly by interacting with either the MHC-peptide or T-cell receptor. The ability of T-cells from allergic subjects to proliferate in vitro when exposed to the drug in the apparent absence of any metabolism is often used to support this hypothesis [17]. However, whether this is also occurring in vivo is unclear, and it is of course possible that both pathways may be important in different circumstances. In addition to the above hypotheses, a non-mutually exclusive mechanism known as the ‘danger hypothesis’ states that immune response to a drug-derived antigen requires the presence of co-stimulatory signals, incuding cytokines, to result in a hypersensitivity reaction [11,18].
In the acute phase of drug hypersensitivity syndrome, for instance with co-trimoxazole, T-cells have been shown to infiltrate the skin [17] and following drug stimulation, CD4+ T-cells secrete cytokines such as IL-5, granzyme and eotaxin which are involved in the recruitment, growth and differentiation of eosinophils [15]. CD4+ T-cells have also been implicated in the hypersensitivity syndrome associated with drugs such as carbamazepine [12–14]. The neutrophil attractant chemokine IL-8 which also kills target cells via both perforin and FAS-mediated pathways is involved in the condition known as acute generalized exanthematous pustulosis [19]. Drug-stimulated T-cells can also kill autologous target cells via the perforin pathway [20]. CD8+ T lymphocytes are primarily responsible for bullous reactions such as SJS and TEN, but have also been implicated in abacavir hypersensitivity [20–22]. An important aspect of the pathogenesis of hypersensitivity to HIV drugs is that of individual susceptibility, in particular the role of HLA alleles. This is covered in the individual sections below.
Nucleoside reverse transcriptase inhibitors (NRTI)
Abacavir (ABC) hypersensitivity reaction occurs in 2.3–9% of adults and children [23] with some differences by ethnicity [24]. The clinical diagnostic criteria for ABC hypersensitivity require at least two symptoms of fever, rash, nausea, vomiting, headache, lethargy, myalgia, arthralgia or gastrointestinal symptoms, occurring within 6 weeks after commencement and resolving within 72 h of withdrawal of the drug. Less common manifestations include respiratory symptoms, paraesthesia, oedema, renal or hepatic failure and anaphylaxis [21].
There is conclusive evidence on several levels that abacavir hypersensitivity has an immunological and genetic basis [25] Cellular studies have shown strong tumour necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) responses and CD8 proliferation after ex vivo exposure to ABC. ABC hypersensitivity seems to be a class I MHC disease mediated by CD8 lymphocytes [26]. The nature of the antigen is, however, unknown. Although proliferation has been witnessed after exposure to the parent drug [27], it is also known that ABC can be oxidized to an aldehyde intermediate mediated by class I alcohol dehydrogenase (ADH), which may be important in the pathogenesis of the hypersensitivity reactions [28].
Case reports of the familial occurrence of ABC hypersensitivity were early clues for a genetic basis for this syndrome [29]. Since that time, an enormous amount of progress has been made in this area with HLA-B*5701 genotyping now being used pre-prescription in most settings, and indeed this represents the best example of translational pharmacogenetics defined to date. Beginning with the first report of the association by Mallal et al. in 2002 [26], there has been rapid progress with replication of the genetic association [30–32], demonstration that genetic testing would be cost-effective [32–34], and the demonstration in a randomized controlled trial that pre-prescription genotyping was clinically effective [35]. Observational data from several clinics have shown that the use of the test reduces the incidence of hypersensitivity [36–38], and a change in the drug label with testing is now either mandatory or recommended in different countries.
A meta-analysis of 25 clinical studies involving 5248 participants showed that ethnic origin might influence ABC hypersensitivity, with a lower risk associated with the Black race [39,40]. It was initially thought that HLA-B*5701 did not have clinical utility in non-Caucasians, but this may largely have been due to the lower carriage rates of HLA-B*5701 [31] and most importantly due to the high rate of false positive clinical diagnosis of abacavir hypersensitivity. More recent data using patch testing has shown that HLA-B*5701 as a marker for ABC hypersensitivity has 100% sensitivity in both US White and Black patients suggesting that the test should be used irrespective of race [41].
Other NRTIs namely didanosine, tenofovir and zidovudine may cause allergic reactions such as rash, although these events are relatively rare despite the intensive use of these drugs over many years [42,43]. Emtricitabine (FTC) causes asymptomatic maculae on the palms or soles in 1.5% of patients, which are usually mild (grade 1 severity). FTC also causes increased alanine aminotransferase in 0.9% of patients and increased bilirubin in 0.6% of patients [44], but whether this is immune-mediated is unclear.
Non nucleoside reverse transcriptase inhibitors (NNRTI)
The non-nucleoside reverse transcriptase inhibitors (NNRTIs) delavirdine, efavirenz, nevirapine and etravirine all cause skin rash. The rash associated with NNRTIs is usually erythematous, maculopapular and widespread. Rash with NNRTIs as a class of drugs has been observed in 10–17% of patients [45].The incidence of moderate to severe rash is approximately 8–12% with rash-related discontinuation rates ranging from 2 to 10% [9,46–48].
Nevirapine can cause skin rash in 17% to 32% of patients although 13% of these are mild rashes [49]. Systemic symptoms may also be present. The DRESS syndrome (drug rash with eosinophilia and systemic symptoms), often accompanied by fever and hepatitis, is well documented with nevirapine [50]. Stevens-Johnson syndrome has been reported in 0.37% of nevirapine recipients [49]. There are some important ethnic differences; for example, nevirapine rash was 2·8 times higher in Thai adults than in White adults [47].
Hepatotoxicity associated with nevirapine has been described in at least two distinct patterns: an early form of liver enzyme elevation that occurs less than 6 weeks after the initiation of therapy and is associated with cutaneous hypersensitivity and a delayed variant that is usually devoid of extra hepatic findings and manifests after more than 2 to 3 months of exposure [51]. There is evidence that the former, but not the latter, type of hepatic injury is immune-mediated [52]. Hepatotoxicity occurs more frequently with nevirapine (1·4–17% of patients) than with efavirenz (1.1–8%) [53–55].
Consistent with the fact that nevirapine-induced skin reactions are immune-mediated is the fact that they occur within 3 months of treatment initiation [56], and are more rapid and severe with nevirapine rechallenge [57]. Furthermore, nevirapine hypersensitivity is associated with higher CD4+ counts while the reaction appears more frequently and is more severe amongst non-HIV-infected individuals receiving prophylactic nevirapine [54]. Furthermore, work by Uetrecht and co-workers in an animal model of nevirapine hypersensitivity has suggested the involvement of the immune system in the pathogenesis of the rash [58]. Taken together, the evidence is consistent with the involvement of a CD4+ dependent, MHC class II restricted immune response directed against NVP or its metabolites. Additionally, genetic studies in different populations have suggested associations with different HLA alleles (Table 3), although it is important to note that most of these studies have been small, the associations demonstrated have been relatively weak, apart from in the Thai population where a stronger association (OR = 49) was demonstrated with HLA-B*3505 [59]. Metabolic polymorphisms may also be important in predisposing to nevirapine hepatotoxicity. For example, associations have been demonstrated with CYP2B6 [60] and ABCB1 [61–63]. Interestingly, nevirapine is metabolized by CYP2B6, and the G516T polymorphism in this gene significantly influences nevirapine trough concentrations which have been linked with a higher risk of hepatotoxicity [64–66]. However, the relationship between high drug plasma concentrations and the risk of hepatotoxicity is controversial since the high concentrations may correlate with more severe liver disease rather than reflect a dose-related toxicity [56,67].
Table 3.
Genetic associations reported with nevirapine hypersensitivity
In patients presenting with delayed hepatotoxicity after starting nevirapine, other mechanisms may be important including direct antiretroviral toxicity, immune reconstitution in those with chronic viral hepatitis, and steatohepatitis caused by NRTIs such as stavudine and metabolic disease [68]. It is also known that alcohol abuse, hepatitis B or C co-infection and concomitant use of other hepatotoxic drugs increases the likelihood of NNRTI associated hepatotoxicity [69].
Efavirenz hypersensitivity is commonly manifested as a mild to moderate skin rash, with severe eruptions such as SJS, TEN and erythema multiforme being reported in 0.1% of patients, compared with 0.3–1% reported with nevirapine [70]. Hepatotoxicity occurs less often with efavirenz. Grade 2–3 events were seen in 4% of patients [51].
Etravirine hypersensitivity manifests as skin rash occurring most often during the second week of therapy and leads to drug discontinuation in 2% of patients [71], with women being at higher risk [72]. In September 2009, the marketing authorization holder issued a dear doctor letter warning about the risk of TEN and DRESS syndrome with this drug based on three cases of severe rash (SJS/TEN) or hypersensitivity [73]. Mild liver enzyme elevation (grade 1–2) may also occur [72]. The mechanism is unknown.
Rilpivirine, also known as TMC278, is undergoing phase III studies in treatment-naïve individuals. In phase II studies, rilpivirine was generally well tolerated. Skin rash was reported in 7.9% of subjects receiving rilpivirine compared with 19.1% of patients treated with efavirenz [74]. Mild liver enzyme elevation and hepatitis were also reported [74].
Protease inhibitors (PI)
Allergic reactions, such as skin rashes and abnormal liver function tests, have also been reported with all protease inhibitors. Rash has been reported in up to 6% of patients taking atazanavir, an azapeptide protease inhibitor [75]. Rash in this case often occurs in association with fever and hyperbilirubinaemia [76]. Lopinavir has been reported to cause rash in 2–4% of patients [77]. Fosamprenavir has been associated with skin rash of varying severity in 19% of patients in clinical studies. However, less than 1% of these were deemed severe or required drug discontinuation [78,79]. More recently, darunavir has been reported to cause rash in 6.7% of patients with severe rash occurring in less than 1% [48,80–82]. The sulphonamide-like structure of fosamprenavir and darunavir seems to influence if not determine the propensity for allergic reactions of these agents. Sulphonamide hypersensitivity is not an absolute contraindication in these patients, but fosamprenavir and darunavir should be used cautiously in such patients [78,83,84]. Tipranavir was associated with rash in 2–14% of subjects [85] and grade 3 alanine aminotransferase elevations in 6.3% of patients [86,87]. Again, very little mechanistic work has been undertaken with these compounds to ascertain whether these reactions are truly immune-mediated or not.
Entry inhibitors (EI)-fusion inhibitors and CCR5 inhibitors
The new classes of drugs have also been implicated in drug hypersensitivity. Enfuvirtide is a synthetic peptide which binds to HIV-1 gp41, a viral transmembrane protein, preventing the formation of an entry pore and thereby blocking HIV entry. The most common adverse event associated with this drug is a local reaction at the injection site although hypersensitivity reactions have been reported in less than 1% of patients [88].
An increase in liver enzymes was seen in patients receiving maraviroc, a CCR5 co-receptor antagonist in the MOTIVATE trials. However, there were no significant differences seen in grade 3 or 4 abnormalities [89–91], and whether this is an immune-mediated phenomenon is unknown. Hepatotoxicity, seen with the discontinued CCR5 co-receptor antagonist aplaviroc, does not appear to be a class effect [92].
Integrase inhibitors
Few cases hypersensitivity reactions have been reported with raltegravir [93] suggesting that this class of drugs may be safer from this perspective [94].
Drugs for opportunistic infections
Cotrimoxazole (TMP-SMX) used in the treatment of Pneumocystis jiroveci pneumonia (previously Pneumocystis carinii) in patients with AIDS is associated with allergic reactions. Such reactions are more common in HIV-positive patients being seen in up to 60% compared with 5% of HIV-negative patients [95–97].
The clinical manifestations vary considerably between different patients with urticaria, macular exanthemas, eczematous and fixed drug eruptions, erythema multiforme, and SJS and TEN being the cutaneous manifestations [98], with associated constitutional symptoms. Risk factors that have been identified include a history of syphilis and a higher total plasma protein concentration [97]. Low CD4 count [99] has also been associated with the development of hypersensitivity although this has been in the context of a higher CD4 : CD8 ratio [100]. It is thought to be related to a decline in T-cell sensitivity to cotrimoxazole with HIV disease progression [100] and possibly a slow acetylator phenotype (but not genotype) [98]. To date, no convincing genetic predisposing factor has been identified [95].
Sulfamethoxazole undergoes oxidation by cytochrome P450 to sulfamethoxazole hydroxylamine [17]. Sulfamethoxazole hydroxylamine is a reactive metabolite and may spontaneously form nitrosulfamethoxazole [101]. It has been shown that the nitroso metabolite binds covalently to host proteins, causing direct cellular toxicity, and that this necrotic cell death may provide a ‘danger signal’ to sensitized T-cells leading to the cascade of immune response and cytokine release manifesting as drug hypersensitivity [102]. Glutathione deficiency has also been proposed as another predisposing mechanism for TMP-SMX hypersensitivity by resulting in decreased inactivation of the toxic metabolites [103]. The overall pathogenesis seems to be highly complex with metabolic derangements interacting with immunoregulatory factors leading to the clinical manifestations in predisposed individuals [104].
Management
Screening tests
HLA-B*5701 testing prior to starting abacavir has been shown to decrease the incidence of hypersensitivity in several countries [36–38]. Screening prior to starting abacavir treatment is now recommended in international HIV treatment guidelines.
Hypersensitivity associated with nevirapine is more likely to occur at higher CD4 counts. Current guidelines thus recommend that nevirapine should be started only in antiretroviral naive men and women with CD4 counts of less than 400 and 250 cells µl–1, respectively [68]. Patients already receiving antiretrovirals who are virologically suppressed who switch to nevirapine above these CD4 thresholds do not necessarily have a greater risk of hypersensitivity [68].
Symptomatic and supportive treatment
The management of patients must be prompt; early recognition and early diagnosis are vital. For patients with mild symptoms, the best form of management is supportive care. Guidelines advise that patients with mild or moderate rash in the absence of constitutional symptoms can continue nevirapine therapy under close supervision [105,106]. About 50% of antiretroviral hypersensitivity cases, those with isolated mild to moderate skin rash, resolve spontaneously despite continuation of therapy [107]. The effectiveness of supportive measures such as antipyretics and antipruritics is unproven, but such agents are commonly used.
When to discontinue drugs
Therapy should be stopped if there is mucosal involvement, blistering, exfoliation, an elevation in ALT > five times the upper limit of normal or elevation in transaminases associated with symptoms such as jaundice and upper abdominal pain, fever greater than 39°C, or intolerable pruritus. It is also important to note that in abacavir hypersensitivity, rash may be a late or absent feature, and discontinuation should be based on progressive constitutional symptoms [108]. Reactions may worsen temporarily after cessation of drug therapy, particularly with drugs with longer half-lives such as nevirapine [45].
Specific treatment
Treatment of patients with corticosteroids within the first 24 h of TMP-SMX hypersensitivity has been shown to be of benefit [109]. By contrast, the prophylactic use of corticosteroids or antihistamines to prevent hypersensitivity reactions to nevirapine has not been shown to be of benefit, and could in fact increase the risk of developing the rash [110–112]. There have been case reports of successful treatment of allergic cases with intravenous immunoglobulins in TEN and DRESS [113,114]. Oral and intravenous N-acetylcysteine have also been used [115,116] but this cannot be recommended at present until better randomized data are available.
Diagnosis and clinical tests
Some degree of over-diagnosis may deprive the patient of a potentially valuable therapy but may be necessary to maintain the clinical safety of a drug (as per ABC). Over-diagnosis (or inaccurate clinical phenotyping) may clearly also contribute to the difficulty in undertaking pharmacogenetic/genomic studies and other studies examining the immunopathogenesis of hypersensitivity reactions. Patch testing (Figure 2), involving the application of 1% and 10% concentrations of ABC applied to the skin in petrolatum has been successfully used to identify correctly true immune mediated ABC hypersensitivity reactions, and may represent a useful adjunctive method for confirming suspected ABC hypersensitivity [25,117,118]. However, the use of patch testing is not widespread, and even with ABC hypersensitivity, the predictive value of testing has not been ascertained. Lymphocyte transformation tests have also been used with a number of drugs associated with hypersensitivity in HIV patients including SMX [119], ABC [25] and nevirapine [52]. However, this is very much a research tool, and not a clinically validated test.
Figure 2.

Patch testing has been used with some drugs such as abacavir to confirm the clinical diagnosis of hypersensitivity
Desensitization and rechallenge
The morbidity and mortality associated with ABC hypersensitivity occurs mainly with rechallenge and therefore a history of hypersensitivity to ABC is an absolute contraindication to subsequent treatment with any ABC-containing formulation [21,120]. Even a negative patch and HLA-B*5701 test should not be used as ground for rechallenge in a patient who has experienced a clinical syndrome in keeping with ABC hypersensitivity [121–123]. Desensitization is unstudied and, although useful for sulphonamide hypersensitivity, may be inappropriate for antiretroviral hypersensitivity, since it would necessitate a period of subtherapeutic drug concentrations leading to the development of drug resistance. That said, desensitization has been used with some success to re-initiate the drug in patients who have experienced an allergic reaction to zidovudine [43] and enfurvitide [88]. Since its safety is not established, NNRTI rechallenge should be medically observed, preferably in hospital, and is contraindicated when there is internal organ involvement. Desensitization protocols exist for hypersensitivity reactions to tipranavir [124], amprenavir [125], darunavir [126], efavirenz [127] and have been tried with nevirapine [128].
Cross reactivity
The rate of NNRTI cross-sensitivity is not known, and so new NNRTI therapy in patients with prior severe hypersensitivity to another NNRTI should also be monitored. Switching from nevirapine to efavirenz and vice versa following cutaneous hypersensitivity was associated with a recurrence of severe rash although the evidence for this comes from small retrospective cases [129–131]. Cross-reactivity is reported to be higher between nevirapine and delavirdine which have a similar structure, but delavirdine is no longer used for the treatment of HIV disease due to its toxicity profile [132].
Conclusions
Drug hypersensitivity is common in those living with HIV and its pathophysiology is complex and multifactorial. Early recognition and withdrawal of the drug is essential particularly in those with the more severe reactions. Further research is also needed to identify predisposing factors including the development of predictive biomarkers, as shown so beautifully with ABC hypersensitivity in the PREDICT trial [133], which will allow for better stratification of anti-HIV therapy. More studies are also needed to understand the mechanisms of antiretroviral hypersensitivity so that better strategies for prevention and treatment can be defined. The importance of this is emphasized by the fact that allergic reactions with anti-HIV drugs are not restricted to the older compounds, and will thus continue to be a clinical problem.
Acknowledgments
MC is funded by a Wellcome Trust Clinical Training Fellowship in Tropical Medicine. The authors would also like to acknowledge funding from the Department of Health (NHS Chair of Pharmacogenetics), the MRC (MRC Centre for Drug Safety Science) and the Wolfson Foundation (Wolfson Centre for Personalised Medicine).
Competing interests
There are no competing interests to declare.
REFERENCES
- 1.Breckenridge A. Pharmacology of drugs for HIV. Medicine. 2009;37:374–7. [Google Scholar]
- 2.Hughes CA, Robinson L, Tseng A, MacArthur RD. New antiretroviral drugs: a review of the efficacy, safety, pharmacokinetics, and resistance profile of tipranavir, darunavir, etravirine, rilpivirine, maraviroc, and raltegravir. Expert Opin Pharmacother. 2009;10:2445–66. doi: 10.1517/14656560903176446. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Seth D, Kamat D, Montejo J. DRESS syndrome: a practical approach for primary care practitioners. Clin Pediatr (Phila) 2008;47:947–52. doi: 10.1177/0009922808320703. [DOI] [PubMed] [Google Scholar]
- 5.Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171–81. doi: 10.1016/j.clindermatol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Williams PM, Conklin RJ. Erythema multiforme: a review and contrast from Stevens-Johnson syndrome/toxic epidermal necrolysis. Dent Clin North Am. 2005;49:67–76. doi: 10.1016/j.cden.2004.08.003. viii. [DOI] [PubMed] [Google Scholar]
- 7.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 8.Temesgen Z, Beri G. HIV and drug allergy. Immunol Allergy Clin North Am. 2004;24:521–31. doi: 10.1016/j.iac.2004.03.006. viii. [DOI] [PubMed] [Google Scholar]
- 9.Wit FW, Kesselring AM, Gras L, Richter C, van der Ende ME, Brinkman K, Lange JM, de Wolf F, Reiss P. Discontinuation of nevirapine because of hypersensitivity reactions in patients with prior treatment experience, compared with treatment-naive patients: the ATHENA cohort study. Clin Infect Dis. 2008;46:933–40. doi: 10.1086/528861. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa S, Yasuoka A, Kikuchi Y, Honda M, Gatanaga H, Tachikawa N, Hirabayashi Y, Oka S. A 5-day course of oral desensitization to trimethoprim/sulfamethoxazole (T/S) in patients with human immunodeficiency virus type-1 infection who were previously intolerant to T/S. Ann Allergy Asthma Immunol. 2000;85:241–4. doi: 10.1016/S1081-1206(10)62474-X. [DOI] [PubMed] [Google Scholar]
- 11.Pirmohamed M, Park BK. HIV and drug allergy. Curr Opin Allergy Clin Immunol. 2001;1:311–6. doi: 10.1097/01.all.0000011032.69243.10. [DOI] [PubMed] [Google Scholar]
- 12.Pichler WJ, Yawalkar N, Britschgi M, Depta J, Strasser I, Schmid S, Kuechler P, Naisbitt D. Cellular and molecular pathophysiology of cutaneous drug reactions. Am J Clin Dermatol. 2002;3:229–38. doi: 10.2165/00128071-200203040-00001. [DOI] [PubMed] [Google Scholar]
- 13.Park BK, Naisbitt DJ, Gordon SF, Kitteringham NR, Pirmohamed M. Metabolic activation in drug allergies. Toxicology. 2001;158:11–23. doi: 10.1016/s0300-483x(00)00397-8. [DOI] [PubMed] [Google Scholar]
- 14.Pichler WJ, Yawalkar N. Allergic reactions to drugs: involvement of T cells. Thorax. 2000;55(Suppl 2):S61–5. doi: 10.1136/thorax.55.suppl_2.S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnyder B, Mauri-Hellweg D, Zanni M, Bettens F, Pichler WJ. Direct, MHC-dependent presentation of the drug sulfamethoxazole to human alphabeta T cell clones. J Clin Invest. 1997;100:136–41. doi: 10.1172/JCI119505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanni MP, von Greyerz S, Schnyder B, Brander KA, Frutig K, Hari Y, Valitutti S, Pichler WJ. HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human alpha beta T lymphocytes. J Clin Invest. 1998;102:1591–8. doi: 10.1172/JCI3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naisbitt DJ. Drug hypersensitivity reactions in skin: understanding mechanisms and the development of diagnostic and predictive tests. Toxicology. 2004;194:179–96. doi: 10.1016/j.tox.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Naisbitt DJ, Pirmohamed M, Park BK. Immunopharmacology of hypersensitivity reactions to drugs. Curr Allergy Asthma Rep. 2003;3:22–9. doi: 10.1007/s11882-003-0006-9. [DOI] [PubMed] [Google Scholar]
- 19.Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, Burkhart C, Yawalkar N, Pichler WJ. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001;107:1433–41. doi: 10.1172/JCI12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassif A, Bensussan A, Dorothee G, Mami-Chouaib F, Bachot N, Bagot M, Boumsell L, Roujeau JC. Drug specific cytotoxic T-cells in the skin lesions of a patient with toxic epidermal necrolysis. J Invest Dermatol. 2002;118:728–33. doi: 10.1046/j.1523-1747.2002.01622.x. [DOI] [PubMed] [Google Scholar]
- 21.Hetherington S, McGuirk S, Powell G, Cutrell A, Naderer O, Spreen B, Lafon S, Pearce G, Steel H. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther. 2001;23:1603–14. doi: 10.1016/s0149-2918(01)80132-6. [DOI] [PubMed] [Google Scholar]
- 22.Naisbitt DJ, Britschgi M, Wong G, Farrell J, Depta JP, Chadwick DW, Pichler WJ, Pirmohamed M, Park BK. Hypersensitivity reactions to carbamazepine: characterization of the specificity, phenotype, and cytokine profile of drug-specific T cell clones. Mol Pharmacol. 2003;63:732–41. doi: 10.1124/mol.63.3.732. [DOI] [PubMed] [Google Scholar]
- 23.Clay PG. The abacavir hypersensitivity reaction: a review. Clin Ther. 2002;24:1502–14. doi: 10.1016/s0149-2918(02)80057-1. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Novoa S, Soriano V. Current trends in screening across ethnicities for hypersensitivity to abacavir. Pharmacogenomics. 2008;9:1531–41. doi: 10.2217/14622416.9.10.1531. [DOI] [PubMed] [Google Scholar]
- 25.Phillips EJ, Wong GA, Kaul R, Shahabi K, Nolan DA, Knowles SR, Martin AM, Mallal SA, Shear NH. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS. 2005;19:979–81. doi: 10.1097/01.aids.0000171414.99409.fb. [DOI] [PubMed] [Google Scholar]
- 26.Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, Sayer D, Castley A, Mamotte C, Maxwell D, James I, Christiansen FT. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–32. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 27.Martin AM, Nolan D, James I, Cameron P, Keller J, Moore C, Phillips E, Christiansen FT, Mallal S. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97–9. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- 28.Walsh JS, Reese MJ, Thurmond LM. The metabolic activation of abacavir by human liver cytosol and expressed human alcohol dehydrogenase isozymes. Chem Biol Interact. 2002;142:135–54. doi: 10.1016/s0009-2797(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 29.Peyrieere H, Nicolas J, Siffert M, Demoly P, Hillaire-Buys D, Reynes J. Hypersensitivity related to abacavir in two members of a family. Ann Pharmacother. 2001;35:1291–2. doi: 10.1345/aph.1A022. [DOI] [PubMed] [Google Scholar]
- 30.Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W, Lai E, Davies K, Handley A, Dow DJ, Fling ME, Stocum M, Bowman C, Thurmond LM, Roses AD. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–2. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 31.Hughes AR, Mosteller M, Bansal AT, Davies K, Haneline SA, Lai EH, Nangle K, Scott T, Spreen WR, Warren LL, Roses AD. Association of genetic variations in HLA-B region with hypersensitivity to abacavir in some, but not all, populations. Pharmacogenomics. 2004;5:203–11. doi: 10.1517/phgs.5.2.203.27481. [DOI] [PubMed] [Google Scholar]
- 32.Hughes DA, Vilar FJ, Ward CC, Alfirevic A, Park BK, Pirmohamed M. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics. 2004;14:335–42. doi: 10.1097/00008571-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Schackman BR, Scott CA, Walensky RP, Losina E, Freedberg KA, Sax PE. The cost-effectiveness of HLA-B*5701 genetic screening to guide initial antiretroviral therapy for HIV. AIDS. 2008;22:2025–33. doi: 10.1097/QAD.0b013e3283103ce6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dervieux T, Bala MV. Overview of the pharmacoeconomics of pharmacogenetics. Pharmacogenomics. 2006;7:1175–84. doi: 10.2217/14622416.7.8.1175. [DOI] [PubMed] [Google Scholar]
- 35.Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jagel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 36.Rauch A, Nolan D, Martin A, McKinnon E, Almeida C, Mallal S. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study. Clin Infect Dis. 2006;43:99–102. doi: 10.1086/504874. [DOI] [PubMed] [Google Scholar]
- 37.Waters LJ, Mandalia S, Gazzard B, Nelson M. Prospective HLA-B*5701 screening and abacavir hypersensitivity: a single centre experience. AIDS. 2007;21:2533–4. doi: 10.1097/QAD.0b013e328273bc07. [DOI] [PubMed] [Google Scholar]
- 38.Zucman D, Truchis P, Majerholc C, Stegman S, Caillat-Zucman S. Prospective screening for human leukocyte antigen-B*5701 avoids abacavir hypersensitivity reaction in the ethnically mixed French HIV population. J Acquir Immune Defic Syndr. 2007;45:1–3. doi: 10.1097/QAI.0b013e318046ea31. [DOI] [PubMed] [Google Scholar]
- 39.Park WB, Choe PG, Song KH, Lee S, Jang HC, Jeon JH, Park SW, Park MH, Oh MD, Choe W. Should HLA-B*5701 screening be performed in every ethnic group before starting abacavir? Clin Infect Dis. 2009;48:365–7. doi: 10.1086/595890. [DOI] [PubMed] [Google Scholar]
- 40.Symonds W, Cutrell A, Edwards M, Steel H, Spreen B, Powell G, McGuirk S, Hetherington S. Risk factor analysis of hypersensitivity reactions to abacavir. Clin Ther. 2002;24:565–73. doi: 10.1016/s0149-2918(02)85132-3. [DOI] [PubMed] [Google Scholar]
- 41.Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, Stancil B, Mosteller M, Brothers C, Wannamaker P, Hughes A, Sutherland-Phillips D, Mallal S, Shaefer M. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008;46:1111–8. doi: 10.1086/529382. [DOI] [PubMed] [Google Scholar]
- 42.Ripamonti D, Maggiolo F, Suter F. Possible allergic cross-reaction to didanosine and tenofovir in an HIV-1-infected woman. AIDS. 2007;21:1059–60. doi: 10.1097/QAD.0b013e3281053a42. [DOI] [PubMed] [Google Scholar]
- 43.Carr A, Penny R, Cooper DA. Allergy and desensitization to zidovudine in patients with acquired immunodeficiency syndrome (AIDS) J Allergy Clin Immunol. 1993;91:683–5. doi: 10.1016/0091-6749(93)90276-l. [DOI] [PubMed] [Google Scholar]
- 44.Saez-Llorens X, Violari A, Ndiweni D, Yogev R, Cashat M, Wiznia A, Chittick G, Harris J, Hinkle J, Blum R, Adda N, Rousseau F. Long-term safety and efficacy results of once-daily emtricitabine-based highly active antiretroviral therapy regimens in human immunodeficiency virus-infected pediatric subjects. Pediatrics. 2008;121:E827–35. doi: 10.1542/peds.2006-3078. [DOI] [PubMed] [Google Scholar]
- 45.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–30. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 46.Sivadasan A, Abraham OC, Rupali P, Pulimood SA, Rajan J, Rajkumar S, Zachariah A, Kannangai R, Kandathip AJ, Sridharan G, Mathai D. High rates of regimen change due to drug toxicity among a cohort of South Indian adults with HIV infection initiated on generic, first-line antiretroviral treatment. J Assoc Physicians India. 2009;57:384–8. [PubMed] [Google Scholar]
- 47.Ananworanich J, Moor Z, Siangphoe U, Chan J, Cardiello P, Duncombe C, Phanuphak P, Ruxrungtham K, Lange J, Cooper DA. Incidence and risk factors for rash in Thai patients randomized to regimens with nevirapine, efavirenz or both drugs. AIDS. 2005;19:185–92. doi: 10.1097/00002030-200501280-00011. [DOI] [PubMed] [Google Scholar]
- 48.Clotet B, Bellos N, Molina JM, Cooper D, Goffard JC, Lazzarin A, Wohrmann A, Katlama C, Wilkin T, Haubrich R, Cohen C, Farthing C, Jayaweera D, Markowitz M, Ruane P, Spinosa-Guzman S, Lefebvre E. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–78. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 49.Warren KJ, Boxwell DE, Kim NY, Drolet BA. Nevirapine-associated Stevens-Johnson syndrome. Lancet. 1998;351:567. doi: 10.1016/S0140-6736(98)24008-6. [DOI] [PubMed] [Google Scholar]
- 50.Claudio GA, Martin AF, de Dios Perrino S, Velasco AA. DRESS syndrome associated with nevirapine therapy. Arch Intern Med. 2001;161:2501–2. doi: 10.1001/archinte.161.20.2501. [DOI] [PubMed] [Google Scholar]
- 51.Martin-Carbonero L, Nunez M, Gonzalez-Lahoz J, Soriano V. Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine. HIV Clin Trials. 2003;4:115–20. doi: 10.1310/N4VT-3E9U-4BKN-CRPW. [DOI] [PubMed] [Google Scholar]
- 52.Drummond NS, Vilar FJ, Naisbitt DJ, Hanson A, Woods A, Park BK, Pirmohamed M. Drug-specific T cells in an HIV-positive patient with nevirapine-induced hepatitis. Antivir Ther. 2006;11:393–5. [PubMed] [Google Scholar]
- 53.Pulido F, Torralba M. NNRTI hepatotoxicity: efavirenz versus nevirapine. J HIV Ther. 2002;7(Suppl 2):S3–16. [PubMed] [Google Scholar]
- 54.Bruck S, Witte S, Brust J, Schuster D, Mosthaf F, Procaccianti M, Rump JA, Klinker H, Petzold D, Hartmann M. Hepatotoxicity in patients prescribed efavirenz or nevirapine. Eur J Med Res. 2008;13:343–8. [PubMed] [Google Scholar]
- 55.Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, Wakeford C, Shaw A, Quinn J, Gish RG, Rousseau F. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191:825–9. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 56.Dieterich DT, Robinson PA, Love J, Stern JO. Drug-induced liver injury associated with the use of nonnucleoside reverse-transcriptase inhibitors. Clin Infect Dis. 2004;38(Suppl 2):S80–9. doi: 10.1086/381450. [DOI] [PubMed] [Google Scholar]
- 57.Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S21–33. doi: 10.1097/00126334-200309011-00005. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Tharmanathan T, Mannargudi B, Gou H, Uetrecht JP. A study of the specificity of lymphocytes in nevirapine-induced skin rash. J Pharmacol Exp Ther. 2009;331:836–41. doi: 10.1124/jpet.109.157362. [DOI] [PubMed] [Google Scholar]
- 59.Chantarangsu S, Mushiroda T, Mahasirimongkol S, Kiertiburanakul S, Sungkanuparph S, Manosuthi W, Tantisiriwat W, Charoenyingwattana A, Sura T, Chantratita W, Nakamura Y. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19:139–46. doi: 10.1097/FPC.0b013e32831d0faf. [DOI] [PubMed] [Google Scholar]
- 60.Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, Keiser O, Biollaz J, Decosterd L, Telenti A. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Ciccacci C, Borgiani P, Ceffa S, Sirianni E, Marazzi MC, Altan AM, Paturzo G, Bramanti P, Novelli G, Palombi L. Nevirapine-induced hepatotoxicity and pharmacogenetics: a retrospective study in a population from Mozambique. Pharmacogenomics. 2010;11:23–31. doi: 10.2217/pgs.09.142. [DOI] [PubMed] [Google Scholar]
- 62.Haas DW, Bartlett JA, Andersen JW, Sanne I, Wilkinson GR, Hinkle J, Rousseau F, Ingram CD, Shaw A, Lederman MM, Kim RB. Pharmacogenetics of nevirapine-associated hepatotoxicity: an Adult AIDS Clinical Trials Group collaboration. Clin Infect Dis. 2006;43:783–6. doi: 10.1086/507097. [DOI] [PubMed] [Google Scholar]
- 63.Ritchie MD, Haas DW, Motsinger AA, Donahue JP, Erdem H, Raffanti S, Rebeiro P, George AL, Kim RB, Haines JL, Sterling TR. Drug transporter and metabolizing enzyme gene variants and nonnucleoside reverse-transcriptase inhibitor hepatotoxicity. Clin Infect Dis. 2006;43:779–82. doi: 10.1086/507101. [DOI] [PubMed] [Google Scholar]
- 64.Penzak SR, Kabuye G, Mugyenyi P, Mbamanya F, Natarajan V, Alfaro RM, Kityo C, Formentini E, Masur H. Cytochrome P450 2B6 (CYP2B6) G516T influences nevirapine plasma concentrations in HIV-infected patients in Uganda. HIV Med. 2007;8:86–91. doi: 10.1111/j.1468-1293.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 65.Kappelhoff BS, van Leth F, Robinson PA, MacGregor TR, Baraldi E, Montella F, Uip DE, Thompson MA, Russell DB, Lange JM, Beijnen JH, Huitema AD. Are adverse events of nevirapine and efavirenz related to plasma concentrations? Antivir Ther. 2005;10:489–98. [PubMed] [Google Scholar]
- 66.Mahungu T, Smith C, Turner F, Egan D, Youle M, Johnson M, Khoo S, Back D, Owen A. Cytochrome P450 2B6 516G – >T is associated with plasma concentrations of nevirapine at both 200 mg twice daily and 400 mg once daily in an ethnically diverse population. HIV Med. 2009;10:310–7. doi: 10.1111/j.1468-1293.2008.00689.x. [DOI] [PubMed] [Google Scholar]
- 67.Meynard JL, Lacombe K, Poirier JM, Legrand J, Morand-Joubert L, Girard PM. Influence of liver fibrosis stage on plasma levels of efavirenz in HIV-infected patients with chronic hepatitis B or C. J Antimicrob Chemother. 2009;63:579–84. doi: 10.1093/jac/dkn531. [DOI] [PubMed] [Google Scholar]
- 68.Calmy A, Hirschel B, Cooper DA, Carr A. Clinical update: adverse effects of antiretroviral therapy. Lancet. 2007;370:12–4. doi: 10.1016/S0140-6736(07)61027-7. [DOI] [PubMed] [Google Scholar]
- 69.Nunez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44(1) Suppl:S132–9. doi: 10.1016/j.jhep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 70.Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227–8. doi: 10.1086/313629. [DOI] [PubMed] [Google Scholar]
- 71.Schiller DS, Youssef-Bessler M. Etravirine: a second-generation nonnucleoside reverse transcriptase inhibitor (NNRTI) active against NNRTI-resistant strains of HIV. Clin Ther. 2009;31:692–704. doi: 10.1016/j.clinthera.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 72.Katlama C, Haubrich R, Lalezari J, Lazzarin A, Madruga JV, Molina JM, Schechter M, Peeters M, Picchio G, Vingerhoets J, Woodfall B, De Smedt G. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS. 2009;23:2289–300. doi: 10.1097/QAD.0b013e3283316a5e. [DOI] [PubMed] [Google Scholar]
- 73.Carter M. Warning issued about rare etravirine allergic reactions AIDSMAP. October 21, 2009.
- 74.Pozniak AL, Morales-Ramirez J, Katabira E, Steyn D, Lupo SH, Santoscoy M, Grinsztejn B, Ruxrungtham K, Rimsky LT, Vanveggel S, Boven K. Efficacy and safety of TMC278 in antiretroviral-naive HIV-1 patients: week 96 results of a phase IIb randomized trial. AIDS. 2010;24:55–65. doi: 10.1097/QAD.0b013e32833032ed. [DOI] [PubMed] [Google Scholar]
- 75.Ouagari Z, Tubiana R, Mohand HA, Dominguez S, Duvivier C, Bricaire F, Katlama C, Caumes E. Skin rash associated with atazanavir: report of three cases. AIDS. 2006;20:1207–8. doi: 10.1097/01.aids.0000226965.17966.3c. [DOI] [PubMed] [Google Scholar]
- 76.Herzmann C, Kinloch S, Johnson M. Rash in an HIV-positive patient. HIV Med. 2005;6:379. doi: 10.1111/j.1468-1293.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 77.Corbett AH, Lim ML, Kashuba AD. Kaletra (lopinavir/ritonavir) Ann Pharmacother. 2002;36:1193–203. doi: 10.1345/aph.1A363. [DOI] [PubMed] [Google Scholar]
- 78.Chapman TM, Plosker GL, Perry CM. Fosamprenavir: a review of its use in the management of antiretroviral therapy-naive patients with HIV infection. Drugs. 2004;64:2101–24. doi: 10.2165/00003495-200464180-00014. [DOI] [PubMed] [Google Scholar]
- 79.Gathe JC, Jr, Ive P, Wood R, Schurmann D, Bellos NC, DeJesus E, Gladysz A, Garris C, Yeo J. SOLO: 48-week efficacy and safety comparison of once-daily fosamprenavir/ritonavir versus twice-daily nelfinavir in naive HIV-1-infected patients. AIDS. 2004;18:1529–37. doi: 10.1097/01.aids.0000131332.30548.92. [DOI] [PubMed] [Google Scholar]
- 80.Phillips EJ, Knowles SR. Comment: sulfonamide cross-reactivity: fact or fiction? Ann Pharmacother. 2005;39:1372–3. doi: 10.1345/aph.1E350a. author reply 3. [DOI] [PubMed] [Google Scholar]
- 81.Arasteh K, Yeni P, Pozniak A, Grinsztejn B, Jayaweera D, Roberts A, Hoy J, De Meyer S, Vangeneugden T, Tomaka F. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antivir Ther. 2009;14:859–64. doi: 10.3851/IMP1301. [DOI] [PubMed] [Google Scholar]
- 82.Marcos Bravo MC, Ocampo Hermida A, Martinez Vilela J, Perez Rodriguez MT, Gavilan Montenegro MJ, Arenas Villarroel LJ, Miralles Alvarez C, Rodriguez Dasilva A, Martinez Vazquez C. Hypersensitivity reaction to darunavir and desensitization protocol. J Investig Allergol Clin Immunol. 2009;19:250–1. [PubMed] [Google Scholar]
- 83.Soriano V, Puoti M, Garcia-Gasco P, Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. doi: 10.1097/QAD.0b013e3282f0e2fd. [DOI] [PubMed] [Google Scholar]
- 84.Molina JM, Cohen C, Katlama C, Grinsztejn B, Timerman A, Pedro Rde J, Vangeneugden T, Miralles D, Meyer SD, Parys W, Lefebvre E. Safety and efficacy of darunavir (TMC114) with low-dose ritonavir in treatment-experienced patients: 24-week results of POWER 3. J Acquir Immune Defic Syndr. 2007;46:24–31. doi: 10.1097/QAI.0b013e3181359cfb. [DOI] [PubMed] [Google Scholar]
- 85.Gathe J, Cooper DA, Farthing C, Jayaweera D, Norris D, Pierone G, Jr., Steinhart CR, Trottier B, Walmsley SL, Workman C, Mukwaya G, Kohlbrenner V, Dohnanyi C, McCallister S, Mayers D. Efficacy of the protease inhibitors tipranavir plus ritonavir in treatment-experienced patients: 24-week analysis from the RESIST-1 trial. Clin Infect Dis. 2006;43:1337–46. doi: 10.1086/508353. [DOI] [PubMed] [Google Scholar]
- 86.Macias J, Orihuela F, Rivero A, Viciana P, Marquez M, Portilla J, Rios MJ, Munoz L, Pasquau J, Castano MA, Abdel-Kader L, Pineda JA. Hepatic safety of tipranavir plus ritonavir (TPV/r)-based antiretroviral combinations: effect of hepatitis virus co-infection and pre-existing fibrosis. J Antimicrob Chemother. 2009;63:178–83. doi: 10.1093/jac/dkn429. [DOI] [PubMed] [Google Scholar]
- 87.Salazar JC, Cahn P, Yogev R, Negra MD, Castelli-Gattinara G, Fortuny C, Flynn PM, Giaquinto C, Ruan PK, Smith ME, Mikl J, Jelaska A. Efficacy, safety and tolerability of tipranavir coadministered with ritonavir in HIV-1-infected children and adolescents. AIDS. 2008;22:1789–98. doi: 10.1097/QAD.0b013e32830c481b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shahar E, Moar C, Pollack S. Successful desensitization of enfuvirtide-induced skin hypersensitivity reaction. AIDS. 2005;19:451–2. doi: 10.1097/01.aids.0000161779.23191.e5. [DOI] [PubMed] [Google Scholar]
- 89.Abel S, van der Ryst E, Rosario MC, Ridgway CE, Medhurst CG, Taylor-Worth RJ, Muirhead GJ. Assessment of the pharmacokinetics, safety and tolerability of maraviroc, a novel CCR5 antagonist, in healthy volunteers. Br J Clin Pharmacol. 2008;65(Suppl 1):5–18. doi: 10.1111/j.1365-2125.2008.03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abel S, Davis JD, Ridgway CE, Hamlin JC, Vourvahis M. Pharmacokinetics, safety and tolerability of a single oral dose of maraviroc in HIV-negative subjects with mild and moderate hepatic impairment. Antivir Ther. 2009;14:831–7. doi: 10.3851/IMP1297. [DOI] [PubMed] [Google Scholar]
- 91.Dubois EA, Cohen AF. Maraviroc and raltegravir. Br J Clin Pharmacol. 2009;68:651–2. doi: 10.1111/j.1365-2125.2009.03503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nichols WG, Steel HM, Bonny T, Adkison K, Curtis L, Millard J, Kabeya K, Clumeck N. Hepatotoxicity observed in clinical trials of aplaviroc (GW873140) Antimicrob Agents Chemother. 2008;52:858–65. doi: 10.1128/AAC.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borras-Blasco J, Navarro-Ruiz A, Borras C, Castera E. Adverse cutaneous reactions associated with the newest antiretroviral drugs in patients with human immunodeficiency virus infection. J Antimicrob Chemother. 2008;62:879–88. doi: 10.1093/jac/dkn292. [DOI] [PubMed] [Google Scholar]
- 94.Schafer JJ, Squires KE. Integrase inhibitors: a novel class of antiretroviral agents. Ann Pharmacother. 2010;44:145–56. doi: 10.1345/aph.1M309. [DOI] [PubMed] [Google Scholar]
- 95.Alfirevic A, Vilar FJ, Alsbou M, Jawaid A, Thomson W, Ollier WE, Bowman CE, Delrieu O, Park BK, Pirmohamed M. TNF, LTA, HSPA1L and HLA-DR gene polymorphisms in HIV-positive patients with hypersensitivity to cotrimoxazole. Pharmacogenomics. 2009;10:531–40. doi: 10.2217/pgs.09.6. [DOI] [PubMed] [Google Scholar]
- 96.Lin D, Li WK, Rieder MJ. Cotrimoxazole for prophylaxis or treatment of opportunistic infections of HIV/AIDS in patients with previous history of hypersensitivity to cotrimoxazole. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.CD005646.pub2. CD005646. [DOI] [PubMed] [Google Scholar]
- 97.Hennessy S, Strom BL, Berlin JA, Brennan PJ. Predicting cutaneous hypersensitivity reactions to cotrimoxazole in HIV-infected individuals receiving primary Pneumocystis carinii pneumonia prophylaxis. J Gen Intern Med. 1995;10:380–6. doi: 10.1007/BF02599836. [DOI] [PubMed] [Google Scholar]
- 98.Alfirevic A, Stalford AC, Vilar FJ, Wilkins EG, Park BK, Pirmohamed M. Slow acetylator phenotype and genotype in HIV-positive patients with sulphamethoxazole hypersensitivity. Br J Clin Pharmacol. 2003;55:158–65. doi: 10.1046/j.1365-2125.2003.01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rabaud C, Charreau I, Izard S, Raffi F, Meiffredy V, Leport C, Guillemin F, Yeni P, Aboulker JP, Delta trial group Adverse reactions to cotrimoxazole in HIV-infected patients: predictive factors and subsequent HIV disease progression. Scand J Infect Dis. 2001;33:759–64. doi: 10.1080/003655401317074581. [DOI] [PubMed] [Google Scholar]
- 100.Carr A, Swanson C, Penny R, Cooper DA. Clinical and laboratory markers of hypersensitivity to trimethoprim-sulfamethoxazole in patients with Pneumocystis carinii pneumonia and AIDS. J Infect Dis. 1993;167:180–5. doi: 10.1093/infdis/167.1.180. [DOI] [PubMed] [Google Scholar]
- 101.Reilly TP, Ju C. Mechanistic perspectives on sulfonamide-induced cutaneous drug reactions. Curr Opin Allergy Clin Immunol. 2002;2:307–15. doi: 10.1097/00130832-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 102.Naisbitt DJ, Farrell J, Gordon SF, Maggs JL, Burkhart C, Pichler WJ, Pirmohamed M, Park BK. Covalent binding of the nitroso metabolite of sulfamethoxazole leads to toxicity and major histocompatibility complex-restricted antigen presentation. Mol Pharmacol. 2002;62:628–37. doi: 10.1124/mol.62.3.628. [DOI] [PubMed] [Google Scholar]
- 103.Naisbitt DJ, Vilar FJ, Stalford AC, Wilkins EG, Pirmohamed M, Park BK. Plasma cysteine deficiency and decreased reduction of nitrososulfamethoxazole with HIV infection. AIDS Res Hum Retroviruses. 2000;16:1929–38. doi: 10.1089/088922200750054657. [DOI] [PubMed] [Google Scholar]
- 104.Sanderson JP, Naisbitt DJ, Farrell J, Ashby CA, Tucker MJ, Rieder MJ, Pirmohamed M, Clarke SE, Park BK. Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol. 2007;178:5533–42. doi: 10.4049/jimmunol.178.9.5533. [DOI] [PubMed] [Google Scholar]
- 105.Murphy RL. Defining the toxicity profile of nevirapine and other antiretroviral drugs. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S15–20. doi: 10.1097/00126334-200309011-00004. [DOI] [PubMed] [Google Scholar]
- 106.Zolopa AR. The evolution of HIV treatment guidelines: current state-of-the-art of ART. Antiviral Res. 2010;85:241–4. doi: 10.1016/j.antiviral.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 107.Carr A, Cooper DA. Pathogenesis and management of HIV-associated drug hypersensitivity. AIDS Clin Rev. 1995:65–97. [PubMed] [Google Scholar]
- 108.Davis CM, Shearer WT. Diagnosis and management of HIV drug hypersensitivity. J Allergy Clin Immunol. 2008;121:826–832. doi: 10.1016/j.jaci.2007.10.021. e825. [DOI] [PubMed] [Google Scholar]
- 109.Walmsley S, Levinton C, Brunton J, Muradali D, Rappaport D, Bast M, Spence D, Salit I. A multicenter randomized double-blind placebo-controlled trial of adjunctive corticosteroids in the treatment of Pneumocystis carinii pneumonia complicating the acquired immune deficiency syndrome. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:348–57. [PubMed] [Google Scholar]
- 110.Wit FW, Wood R, Horban A, Beniowski M, Schmidt RE, Gray G, Lazzarin A, Lafeuillade A, Paes D, Carlier H, van Weert L, de Vries C, van Leeuwen R, Lange JM. Prednisolone does not prevent hypersensitivity reactions in antiretroviral drug regimens containing abacavir with or without nevirapine. AIDS. 2001;15:2423–9. doi: 10.1097/00002030-200112070-00010. [DOI] [PubMed] [Google Scholar]
- 111.Montaner JS, Cahn P, Zala C, Casssetti LI, Losso M, Hall DB, Wruck J, McDonough M, Gigliotti M, Robinson PA. Randomized, controlled study of the effects of a short course of prednisone on the incidence of rash associated with nevirapine in patients infected with HIV-1. J Acquir Immune Defic Syndr. 2003;33:41–6. doi: 10.1097/00126334-200305010-00007. [DOI] [PubMed] [Google Scholar]
- 112.Barreiro P, Soriano V, Casas E, Estrada V, Tellez MJ, Hoetelmans R, de Requena DG, Jimenez-Nacher I, Gonzalez-Lahoz J. Prevention of nevirapine-associated exanthema using slow dose escalation and/or corticosteroids. AIDS. 2000;14:2153–7. doi: 10.1097/00002030-200009290-00012. [DOI] [PubMed] [Google Scholar]
- 113.Phan TG, Wong RC, Crotty K, Adelstein S. Toxic epidermal necrolysis in acquired immunodeficiency syndrome treated with intravenous gammaglobulin. Australas J Dermatol. 1999;40:153–7. doi: 10.1046/j.1440-0960.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 114.Fields KS, Petersen MJ, Chiao E, Tristani-Firouzi P. Case reports: treatment of nevirapine-associated dress syndrome with intravenous immune globulin (IVIG) J Drugs Dermatol. 2005;4:510–3. [PubMed] [Google Scholar]
- 115.Walmsley SL, Khorasheh S, Singer J, Djurdjev O. A randomized trial of N-acetylcysteine for prevention of trimethoprim-sulfamethoxazole hypersensitivity reactions in Pneumocystis carinii pneumonia prophylaxis (CTN 057). Canadian HIV Trials Network 057 Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:498–505. doi: 10.1097/00042560-199812150-00009. [DOI] [PubMed] [Google Scholar]
- 116.Akerlund B, Tynell E, Bratt G, Bielenstein M, Lidman C. N-acetylcysteine treatment and the risk of toxic reactions to trimethoprim-sulphamethoxazole in primary Pneumocystis carinii prophylaxis in HIV-infected patients. J Infect. 1997;35:143–7. doi: 10.1016/s0163-4453(97)91578-4. [DOI] [PubMed] [Google Scholar]
- 117.Hughes S, Hughes A, Brothers C, Spreen W, Thorborn D. PREDICT-1 (CNA106030): the first powered, prospective trial of pharmacogenetic screening to reduce drug adverse events. Pharm Stat. 2008;7:121–9. doi: 10.1002/pst.286. [DOI] [PubMed] [Google Scholar]
- 118.Phillips EJ, Sullivan JR, Knowles SR, Shear NH. Utility of patch testing in patients with hypersensitivity syndromes associated with abacavir. AIDS. 2002;16:2223–5. doi: 10.1097/00002030-200211080-00017. [DOI] [PubMed] [Google Scholar]
- 119.Roujeau JC, Albengres E, Moritz S, Piacentino A, Cuny M, Revuz J, Touraine R. Lymphocyte transformation test in drug-induced toxic epidermal necrolysis. Int Arch Allergy Appl Immunol. 1985;78:22–4. doi: 10.1159/000233856. [DOI] [PubMed] [Google Scholar]
- 120.Escaut L, Liotier JY, Albengres E, Cheminot N, Vittecoq D. Abacavir rechallenge has to be avoided in case of hypersensitivity reaction. AIDS. 1999;13:1419–20. doi: 10.1097/00002030-199907300-00026. [DOI] [PubMed] [Google Scholar]
- 121.Shear NH, Milpied B, Bruynzeel DP, Phillips EJ. A review of drug patch testing and implications for HIV clinicians. AIDS. 2008;22:999–1007. doi: 10.1097/QAD.0b013e3282f7cb60. [DOI] [PubMed] [Google Scholar]
- 122.Calza L, Rosseti N, Biagetti C, Pocaterra D, Colangeli V, Manfredi R. Abacavir-induced reaction with fever and severe skin rash in a patient tested human leukocyte antigen-B*5701 negative. Int J STD AIDS. 2009;20:276–7. doi: 10.1258/ijsa.2008.008318. [DOI] [PubMed] [Google Scholar]
- 123.Bonta PI, Vermeulen LN, Speelman P, Prins JM. Severe abacavir hypersensitivity reaction in a patient tested HLA-B*5701 negative. AIDS. 2008;22:1522–3. doi: 10.1097/QAD.0b013e3283065ba1. [DOI] [PubMed] [Google Scholar]
- 124.Martinez Castro B, Ferrando Piqueres R, Martinez Garcia M, Soler Company E. [Desensitization to tipranavir caused by toxicodermia] Farm Hosp. 2009;33:340–2. doi: 10.1016/s1130-6343(09)72981-2. [DOI] [PubMed] [Google Scholar]
- 125.Kohli-Pamnani A, Huynh P, Lobo F. Amprenavir-induced maculopapular exanthem followed by desensitization in a patient with late-stage human immunodeficiency virus. Ann Allergy Asthma Immunol. 2006;96:620–3. doi: 10.1016/S1081-1206(10)63559-4. [DOI] [PubMed] [Google Scholar]
- 126.Bravo MCM, Hermida AO, Vilela JM, Rodriguez MTP, Montenegro MJG, Villarroel LJA, Alvarez CM, Dasilva AR, Vazquez CM. Hypersensitivity reaction to darunavir and desensitization protocol. J Invest Allerg Clin. 2009;19:250–1. [PubMed] [Google Scholar]
- 127.Phillips E, Gutierrez S, Jahnke N, Yip B, Lima VD, Hogg RS, Harrigan PR, Montaner JS. Determinants of nevirapine hypersensitivity and its effect on the association between hepatitis C status and mortality in antiretroviral drug-naive HIV-positive patients. AIDS. 2007;21:1561–8. doi: 10.1097/QAD.0b013e3282170a9d. [DOI] [PubMed] [Google Scholar]
- 128.Demoly P, Messaad D, Fabre J, Reynes J, Bousquet J. Nevirapine-induced cutaneous hypersensitivity reactions and successful tolerance induction. J Allergy Clin Immunol. 1999;104(2 Pt 1):504–5. doi: 10.1016/s0091-6749(99)70403-3. [DOI] [PubMed] [Google Scholar]
- 129.Mehta U, Maartens G. Is it safe to switch between efavirenz and nevirapine in the event of toxicity? Lancet Infect Dis. 2007;7:733–8. doi: 10.1016/S1473-3099(07)70262-1. [DOI] [PubMed] [Google Scholar]
- 130.Laureillard D, Prak N, Fernandez M, Ngeth C, Moeung S, Riel V, Chhneang V, Song S, Quillet C, Piketty C. Efavirenz replacement by immediate full-dose nevirapine is safe in HIV-1-infected patients in Cambodia. HIV Med. 2008;9:514–8. doi: 10.1111/j.1468-1293.2008.00597.x. [DOI] [PubMed] [Google Scholar]
- 131.Manosuthi W, Thongyen S, Chumpathat N, Muangchana K, Sungkanuparph S. Incidence and risk factors of rash associated with efavirenz in HIV-infected patients with preceding nevirapine-associated rash. HIV Med. 2006;7:378–82. doi: 10.1111/j.1468-1293.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- 132.Gangar M, Arias G, O'Brien JG, Kemper CA. Frequency of cutaneous reactions on rechallenge with nevirapine and delavirdine. Ann Pharmacother. 2000;34:839–42. doi: 10.1345/aph.19258. [DOI] [PubMed] [Google Scholar]
- 133.Nolan D. HLA-B*5701 screening prior to abacavir prescription: clinical and laboratory aspects. Crit Rev Clin Lab Sci. 2009;46:153–65. doi: 10.1080/10408360902937817. [DOI] [PubMed] [Google Scholar]
- 134.Saag MS, Cahn P, Raffi F, Wolff M, Pearce D, Molina JM, Powderly W, Shaw AL, Mondou E, Hinkle J, Borroto-Esoda K, Quinn JB, Barry DW, Rousseau F. Efficacy and safety of emtricitabine vs stavudine in combination therapy in antiretroviral-naive patients: a randomized trial. JAMA. 2004;292:180–9. doi: 10.1001/jama.292.2.180. [DOI] [PubMed] [Google Scholar]
- 135.Colebunders R, Vanwolleghem T, Meurrens P, Moerman F. Efavirenz-associated Stevens-Johnson syndrome. Infection. 2004;32:306–7. doi: 10.1007/s15010-004-4034-8. [DOI] [PubMed] [Google Scholar]
- 136.Lazzarin A, Campbell T, Clotet B, Johnson M, Katlama C, Moll A, Towner W, Trottier B, Peeters M, Vingerhoets J, de Smedt G, Baeten B, Beets G, Sinha R, Woodfall B. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:39–48. doi: 10.1016/S0140-6736(07)61048-4. [DOI] [PubMed] [Google Scholar]
- 137.Squires K, Lazzarin A, Gatell JM, Powderly WG, Pokrovskiy V, Delfraissy JF, Jemsek J, Rivero A, Rozenbaum W, Schrader S, Sension M, Vibhagool A, Thiry A, Giordano M. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004;36:1011–9. doi: 10.1097/00126334-200408150-00003. [DOI] [PubMed] [Google Scholar]
- 138.Madruga JV, Berger D, McMurchie M, Suter F, Banhegyi D, Ruxrungtham K, Norris D, Lefebvre E, de Bethune MP, Tomaka F, De Pauw M, Vangeneugden T, Spinosa-Guzman S. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 139.Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, Nadler J, Clotet B, Karlsson A, Wohlfeiler M, Montana JB, McHale M, Sullivan J, Ridgway C, Felstead S, Dunne MW, van der Ryst E, Mayer H. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gatanaga H, Yazaki H, Tanuma J, Honda M, Genka I, Teruya K, Tachikawa N, Kikuchi Y, Oka S. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS. 2007;21:264–5. doi: 10.1097/QAD.0b013e32801199d9. [DOI] [PubMed] [Google Scholar]
- 141.Littera R, Carcassi C, Masala A, Piano P, Serra P, Ortu F, Corso N, Casula B, La Nasa G, Contu L, Manconi PE. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. 2006;20:1621–6. doi: 10.1097/01.aids.0000238408.82947.09. [DOI] [PubMed] [Google Scholar]
- 142.Vitezica ZG, Milpied B, Lonjou C, Borot N, Ledger TN, Lefebvre A, Hovnanian A. HLA-DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540–1. doi: 10.1097/QAD.0b013e3282f37812. [DOI] [PubMed] [Google Scholar]


