Abstract
AIMS
To assess the effect of the calcitonin gene-related peptide (CGRP) receptor antagonist, telcagepant, on the haemodynamic response to sublingual nitroglycerin (NTG).
METHODS
Twenty-two healthy male volunteers participated in a randomized, placebo-controlled, double-blind, two-period, crossover study. Subjects received 500 mg telcagepant or placebo followed, 1.5 h later, by 0.4 mg NTG. To assess the haemodynamic response the following vascular parameters were measured: blood pressure, aortic augmentation index (AIx) and brachial artery diameter (BAD). Data are presented as mean (95% confidence interval, CI).
RESULTS
The aortic AIx following NTG decreased by −18.50 (−21.02, −15.98) % after telcagepant vs. −17.28 (−19.80, −14.76) % after placebo. The BAD fold increase following NTG was 1.14 (1.12, 1.17) after telcagepant vs. 1.13 (1.10, 1.15) after placebo. For both AIx and BAD, the hypothesis that telcagepant does not significantly affect the changes induced by NTG is supported (P < 0.0001). In addition, no vasoconstrictor effect of telcagepant could be demonstrated.
CONCLUSIONS
Telcagepant did not affect NTG-induced haemodynamic changes. These data suggest that NTG-induced vasodilation is not CGRP dependent.
Keywords: augmentation index, calcitonin gene-related peptide, cardiovascular safety, echo tracking, migraine therapy
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Telcagepant (MK-0974, Merck & Co., Inc.), an oral calcitonin gene-related peptide (CGRP) antagonist, is effective in treating acute migraine headache. Although CGRP receptor antagonists seem devoid of direct vasoconstrictor activity, animal research suggests a role for CGRP in nitroglycerin (NTG) induced vasodilation. Pulse wave analysis and high resolution ultrasound are sensitive techniques to investigate cardiovascular responses to drugs in vivo in humans.
WHAT THIS STUDY ADDS
This study shows that telcagepant, at a supra-therapeutic dose for the treatment of acute migraine headache, has no clinically relevant effect on NTG-induced haemodynamic changes in healthy male volunteers. There is also no measurable vasoconstrictor effect of telcagepant as such in both the central and peripheral vascular bed. The results of the present study support the favourable cardiovascular safety profile of telcagepant and indicate that CGRP is not involved in NTG-induced vasodilation in humans.
Introduction
The introduction of the so-called ‘triptans’ (i.e. 5-HT1B/1D receptor agonists), with sumatriptan being the first compound of this class, has improved migraine therapy considerably over the past decade [1]. However, despite their well-established efficacy and the demonstrable need to reduce migraine-associated disability, triptans remain underused in clinical practice. Several factors, such as cost and availability, contribute to the relatively restricted use of triptans. In addition, concern among patients and doctors about their vasoconstrictor properties and the fact that triptans are contraindicated in patients with cardiovascular disease or at high cardiovascular risk, limits their more widespread use [2].
At the same time, the development rationale for acute anti-migraine drugs radically changed due to conceptual changes in the understanding of the pathophysiology of migraine [3]. Whereas triptans were developed as cranial vasoconstrictors, the focus later shifted towards compounds that counteract inflammation resulting from the release of neuropeptides from the pain-producing trigeminal neurons innervating the cranial circulation and dura mater [4]. In the following years, several compounds that were able to inhibit neurogenic inflammation in animal models failed to show efficacy in human migraine trials [5]. Numerous observations linked migraine headache with the release of one neuropeptide in particular, namely calcitonin gene-related peptide (CGRP) [6, 7]. Interestingly, experimental data also demonstrated an important role for CGRP receptors in nociceptive trigeminovascular processing in animal models [8]. The hypothesis that CGRP receptor antagonism would relieve pain in migraine was first confirmed by the intravenous administration of olcegepant (BIBN4096, Boehringer-Ingelheim) [9]. More recently, telcagepant (MK-0974, Merck & Co., Inc.), an oral CGRP antagonist, was also shown to be effective in treating moderate to severe migraine with an efficacy comparable with the triptans while a more favourable side effect profile was observed [10].
The exact site of action of CGRP receptor antagonists remains controversial as CGRP is present in both the central and peripheral nervous system, notably within the human trigeminal ganglion, as well as in blood vessels [5, 11, 12]. However, at this time, CGRP receptor antagonists seem devoid of direct vasoconstrictor activity and may thus present an alternative and safe treatment for migraineurs with cardiovascular risk/disease [13–15]. Nevertheless, it has been hypothesized that CGRP antagonism could result in inhibition of CGRP-dependent vasodilation and might thus have cardiovascular consequences [16]. Furthermore, some data suggest that administration of nitroglycerin (NTG), which is widely used in the treatment of cardiac ischaemic pain (i.e. angina pectoris), causes release of CGRP in the cardiovascular system resulting in coronary artery vasodilatation [17–19]. However, more recent data, including from our group, suggest that vasodilatation induced by exogenous NO is not CGRP dependent in men [13, 20–22]. Nonetheless, the degree to which the vasodilatory properties of NTG depend on the release of CGRP remains a matter of debate and the observations concerning the effect of CGRP receptor antagonism on the vasodilatory response to NTG lack clinical study [23].
This study was aimed at evaluating the effect of a single 500 mg dose of the CGRP receptor antagonist telcagepant on the vasodilatory response to a therapeutic dose of 0.4 mg NTG. To that end, two techniques were used: pulse wave analysis and high resolution ultrasound. Pulse wave analysis allows for derivation of the aortic pressure waveform (PWF) from the peripheral PWF obtained by applanation tonometry at the radial artery using a validated transfer function [24]. From the aortic PWF, aortic diastolic and systolic blood pressure (ADBP, ASBP) values and augmentation index (aortic AIx) can be calculated. The aortic AIx is the proportion of central pulse pressure that results from arterial wave reflection and is a commonly used measure for arterial stiffness. This PWF information has become a powerful tool to investigate the cardiovascular response in vivo in humans to disease states and drugs. It was notably shown more reliable in detecting arterial triptan and nitrate-induced vascular effects than the classically used brachial artery diastolic (DBP) and systolic blood pressure (SBP) [25–28]. High resolution ultrasound allows the real time measurement of changes in the brachial artery diameter (BAD) induced by vasoactive drugs, notably triptans and nitrates [25]. In addition to exploring the effects of telcagepant on NTG response, the present study allowed us to investigate the vascular effects of a single 500 mg dose of telcagepant itself using the same highly sensitive techniques.
Methods
Subjects
After approval by the ethics committee of the University Hospital of Leuven, 43 healthy, non-smoking, male subjects were screened from the general population. Written informed consent was obtained during a screening visit from all subjects. Major exclusion criteria were: age < 20 and > 50 years; a history of major gastrointestinal, genitourinary, renal, hepatic, pulmonary, haematological, immunological, endocrine or metabolic, neurological or cerebrovascular abnormalities or disease and use of any kind of drugs. Subjects with a history of previous myocardial infarction or any clinically relevant cardiovascular disease were also excluded from participation in the study. On the basis of medical history, physical examination, laboratory tests and electrocardiography underlying disease was excluded. During the screening session, the subject's pressure waveform was assessed for reliability as per Sphygmocor system specifications. Only subjects with a positive aortic AIx during the screening visit were enrolled. The study was conducted in accordance with the guidelines on GCP and ethical standards for human experimentation established by the declaration of Helsinki [29].
Study design
All predefined criteria were fulfilled by 22 screened subjects. These 22 eligible subjects participated in a randomized, placebo-controlled, double-blind, two-way, crossover study in which the treatments were 500 mg telcagepant or placebo. In each period, each subject received a single oral dose of either 500 mg telcagepant or matching placebo followed 1.5 h later, by a dose of 0.4 mg sublingual NTG (flow chart in Figure 1). There was at least a 5 day washout interval between both study periods. For each individual subject, treatment was always administered at the same time of the day during each study period. Placebo and telcagepant doses were given as three capsules and taken under the supervision of a study nurse with 240 ml of water.
Figure 1.
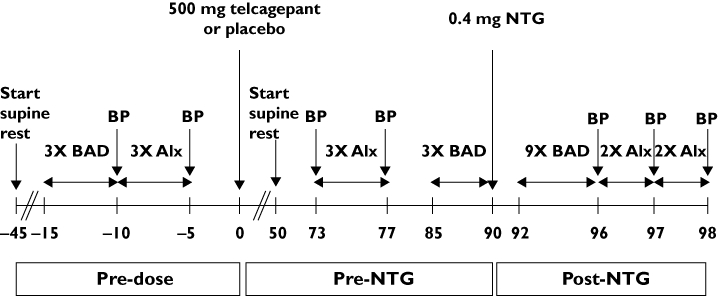
Study flowchart. BP, brachial blood pressure; BAD, brachial artery diameter; AIx, pressure waveform measurements with resulting aortic and radial augmentation index
Measurements
All measurements were performed in a quiet, temperature-controlled (24°C ± 1°C) room by two blinded observers. Subjects abstained from any drug for at least 3 days and from alcohol- and caffeine-containing beverages for at least 12 h before drug administration. Subjects fasted at least 8 h before drug administration and remained in a fasting state during all measurements. Before performing baseline measurements, subjects rested for 30 min in the supine position on a comfortable bed for acclimatization (flow chart in Figure 1). Fifteen minutes prior to drug administration, BAD followed by PWF measurements were performed in triplicate. Three PWF measurements were repeated 73 min after the administration of telcagepant or placebo. Thereafter and 5 min prior to NTG administration (i.e. 85 min post-dose), three BAD recordings were performed. The same image of the brachial artery was maintained by use of a probe-holder and arm supports throughout the sublingual administration of 0.4 mg NTG at exactly 90 min after drug intake. The time point of NTG administration was chosen at the expected time of maximal plasma concentration of telcagepant [30]. Between 2 and 6 min following NTG administration (i.e. between 92 and 96 min post-dose), a maximum of nine BAD measurements was performed, followed by four PWF recordings. All brachial oscillometric SBP and DBP values were obtained before and after PWF recordings and used for calibration of the PWFs. The study flow chart is given in Figure 1.
Brachial blood pressure and heart rate
Supine SBP, DBP and heart rate (HR) were recorded at the left upper arm by use of a validated, non-invasive semi-automated oscillometric device (OMRON 705IT; Omron Healtcare, Hamburg, Germany) [31].
Applanation tonometry
PWFs were recorded with a high-fidelity tonometer (SPT-301; Millar instruments). Each recording lasted 8 s, and the mean of at least two recordings at each time point was used for analysis. After calibration by use of brachial oscillometric SBP and DBP values, these PWFs were automatically transformed by computer software (Sphygmocor; Atcor Medical, West Ryde, New South Wales, Australia) into the corresponding aortic PWF by use of a generalized transfer function [32]. The following parameters were calculated by the software: ADBP, ASBP and both radial and aortic AIx. The Sphygmocor system has been validated and demonstrated adequate test-retest reproducibility in healthy volunteers [33].
Ultrasound
The end-diastolic BAD was measured at the antecubital crease of the right arm by use of an echo-tracking system (Wall track System; Pie Medical Imaging, Maastricht, the Netherlands). The Wall Track system consists of an ultrasound device (Esaote AU5; Esaote Biomedica, Genoa, Italy) equipped with a 7.5- to 10 MHz linear-array transducer connected to a data acquisition and processing unit [34]. This system has previously been validated and has demonstrated acceptable reproducibility in healthy subjects [35]. The brachial artery was scanned in longitudinal sections to obtain satisfactory ultrasonographic images. At each time point repeated measures were made to obtain at least 15 cardiac cycles for analysis.
Data analysis
Power calculation
Prior to the study, power calculations were performed for aortic AIx and BAD. For aortic AIx, in house data that were collected previously by our group were available. The power calculation assumed a within-subject variance and between subject variance of 17.79%2 and 101.14%2, respectively, for aortic AIx change from baseline. It also assumed a true effect of −12% when NTG is administered with placebo. With n = 21 subjects, α= 0.05 (one-sided) and n− 2 degrees of freedom for error, there is 99% power to conclude that the true aortic AIx ratio (effect of NTG with telcagepant/effect of NTG with placebo) is >0.5, if the true ratio is 1.0.
For BAD, the power calculation was based on data from the literature [36]. The power calculation assumed a true within-subject variance for ln-brachial artery diameter change from baseline of 0.0322 ln-µm2. With n = 21 subject, α= 0.05 (one-sided), and n− 2 degrees of freedom for error, there is >0.99 probability that the 90% confidence interval lower boundary for the true mean fold treatment difference (effect of NTG with telcagepant/effect of NTG with placebo) ≥0.50, if the true ratio is 1.0.
Primary analyses to evaluate the effect of telcagepant on NTG-induced vascular changes
The study was powered to detect changes in the maximal vasodilatory NTG response due to telcagepant for the parameters aortic AIx and BAD. Therefore, in the primary analyses, only aortic AIx and BAD were used. As NTG causes a decrease in aortic AIx and the baseline aortic AIx was positive (cf. inclusion criteria), the change in aortic AIx is a negative value. Therefore, the maximum effect on aortic AIx for a subject in a given treatment period was defined as the largest negative difference between the post- and pre-NTG measurements over the two predefined post-NTG time points. The maximum increase in BAD induced by NTG was defined as the maximum fold-change obtained over the four predefined post-NTG time points. Individual maximal effect values were analyzed in a mixed effects analysis of variance (anova) with fixed effects for treatment, period and treatment sequence and a random effect for subject nested within treatment sequence. For BAD, values were ln-transformed, whereas for AIx the data were analyzed on the original scale. Results for BAD were back-transformed to the original scale to obtain a geometric mean and corresponding 95% confidence interval (95% CI).
For aortic AIx, the hypothesis was tested that at least 50% of the maximum NTG effect was maintained following administration of telcagepant as compared with placebo, where 50% was predefined as the threshold for significance. The P value is for the test that the fold treatment difference (effect of NTG with telcagepant/effect of NTG with placebo) is less than 0.50 (null hypothesis) vs. at least 0.50 (alternative hypothesis). P < 0.05 signifies that at least 50% of the NTG effect is maintained when administered with telcagepant.
In addition, the hypothesis was tested that at least 50% of the maximum NTG effect on BAD is maintained following administration of telcagepant as compared with placebo. Therefore, a two-sided 90% CI (equivalent to a one-sided lower 95% CI) for the difference (effect of NTG with telcagepant – effect of NTG with placebo) in ln-BAD maximum effect was calculated using the mean square error from the anova and referencing a t-distribution. These confidence limits were exponentiated to obtain a 90% CI for the fold treatment difference (effect of NTG with telcagepant/effect of NTG with placebo). It was predefined that if the lower bound of the 90% CI was ≥0.50, the hypothesis that telcagepant has no effect on NTG-induced vascular changes would be supported.
Secondary analyses to evaluate the effect of telcagepant on NTG-induced vascular changes
In secondary analyses, the effect of telcagepant on the vasodilatory response to NTG was assessed for all vascular parameters. To this end, the change for each individual vascular parameter was calculated as the mean difference and corresponding 95% CIs between measurements recorded post-NTG minus those obtained pre-NTG. The mean NTG effect was then compared between both treatment periods using a mixed effects anova model with fixed effects for treatment, period, treatment sequence, time and treatment by time interaction and a random effect for nested within treatment sequence.
Secondary analysis to evaluate the effect of telcagepant
In secondary analyses, the mean and corresponding 95% CI for each vascular parameter at each individual time point (i.e. pre-dose, pre-NTG and post-NTG) weres calculated. Further, each vascular parameter was evaluated in the mixed effects anova model with fixed effects for treatment, period, treatment sequence, time and treatment by time interaction and a random effect for nested within treatment sequence. The treatment main effect was assessed by comparing each vascular parameter between the treatment and the placebo period over the three time-points and at each individual time point using the anova.
Results
All 22 subjects completed the study. One subject, with a positive aortic AIx at screening, had a negative aortic AIx at baseline during both study periods. Moreover, in this subject NTG had no effect on aortic AIx and thus the subject was considered to be a non-responder to NTG and his data were excluded from further analyses.
The remaining 21 subjects were aged 38 ± 10 years and had a body mass index of 24.4 ± 2.8 kg m−2 (mean ± SD).
Primary analyses to evaluate the effect of telcagepant on NTG-induced vascular changes
Aortic AIx
The maximum change in aortic AIx following sublingual NTG averaged −18.50 (−21.02, −15.98) % after telcagepant vs. −17.28 (−19.80, −14.76) % after placebo. The hypothesis that telcagepant does not significantly affect the mean change from baseline in aortic AIx induced by NTG is supported (P < 0.0001).
Brachial artery diameter
The maximum fold change in BAD following sublingual NTG averaged 1.14 (1.12, 1.17) after telcagepant vs. 1.13 (1.10, 1.15) after placebo. The geometric mean ratio (effect of NTG with telcagepant/effect of NTG with placebo) was 1.02 with a corresponding 90% CI of 1.00, 1.03. Since the confidence interval lies completely above 0.50, the hypothesis that telcagepant does not significantly affect the mean fold change from baseline in BAD induced by NTG is supported.
Secondary analyses to evaluate the effect of telcagepant on NTG-induced vascular changes
Using the previously described anova, no difference could be detected for any of the vascular parameters in mean response to NTG following telcagepant compared with placebo (Table 1, Figures 2 and 3).
Table 1.
Vascular parameters
| Period | Pre-dose | Pre-NTG | Post-NTG | NTG-induced change | |
|---|---|---|---|---|---|
| HR (beats min−1) | Placebo | 53 (50, 57) | 53 (49, 56) | 56 (52, 61) | 4 (2, 6) |
| Telcagepant | 54 (50, 57) | 53 (49,57) | 58 (53, 62) | 5 (3, 6) | |
| SBP (mmHg) | Placebo | 119 (115, 123) | 119 (116, 123) | 118 (114, 121) | −2 (−4, 0) |
| Telcagepant | 119 (115, 122) | 121 (117,124) | 121 (117,125) | 0 (−2, 2) | |
| DBP (mmHg) | Placebo | 72 (69, 76) | 75 (71, 78) | 67 (64, 70) | −8 (−9, −6) |
| Telcagepant | 73 (70, 76) | 75 (71, 78) | 69 (65, 72) | −6 (−7, −5) | |
| ASBP (mmHg) | Placebo | 105 (101, 110) | 107 (103, 111) | 99 (95, 102) | −8 (−10, −7) |
| Telcagepant | 104 (101, 108) | 107 (104, 111) | 101 (97, 104) | −7 (−8, −5) | |
| ADBP (mmHg) | Placebo | 73 (70, 76) | 75 (72, 79) | 67 (64, 71) | −8 (−10, −6) |
| Telcagepant | 74 (71, 76) | 75 (72, 79) | 69 (66, 73) | −6 (−8, −5) | |
| Radial AIx (%) | Placebo | 65 (59, 71) | 67 (61, 70) | 44 (39, 48) | −23 (−26, −20) |
| Telcagepant | 62 (56, 67) | 66 (61, 70) | 42 (38, 47) | −23 (−26, −20) | |
| Aortic AIx (%) | Placebo | 13 (9, 18) | 15 (11, 19) | −2 (−5, 2) | −16 (−19, −13) |
| Telcagepant | 11 (7, 15) | 15 (11, 18) | −3 (−6, 0) | −17 (−19, −15) | |
| BAD (µm) | Placebo | 6430 (6089, 6770) | 6445 (6134, 6756) | 7048 (6705, 7391) | 603 (464, 741) |
| Telcagepant | 6441 (6112, 6770) | 6410 (6048, 6771) | 7069 (6676, 7461) | 659 (533, 785) |
Data (mean ± 95% CI); n = 21; HR, heart rate, SBP brachial systolic blood pressure, DBP brachial diastolic blood pressure, ASBP aortic systolic blood pressure, ADBP aortic diastolic blood pressure, radial AIx augmentation index from radial pressure waveform, aortic AIx, augmentation index from aortic pressure waveform, BAD brachial artery diameter.
Figure 2.
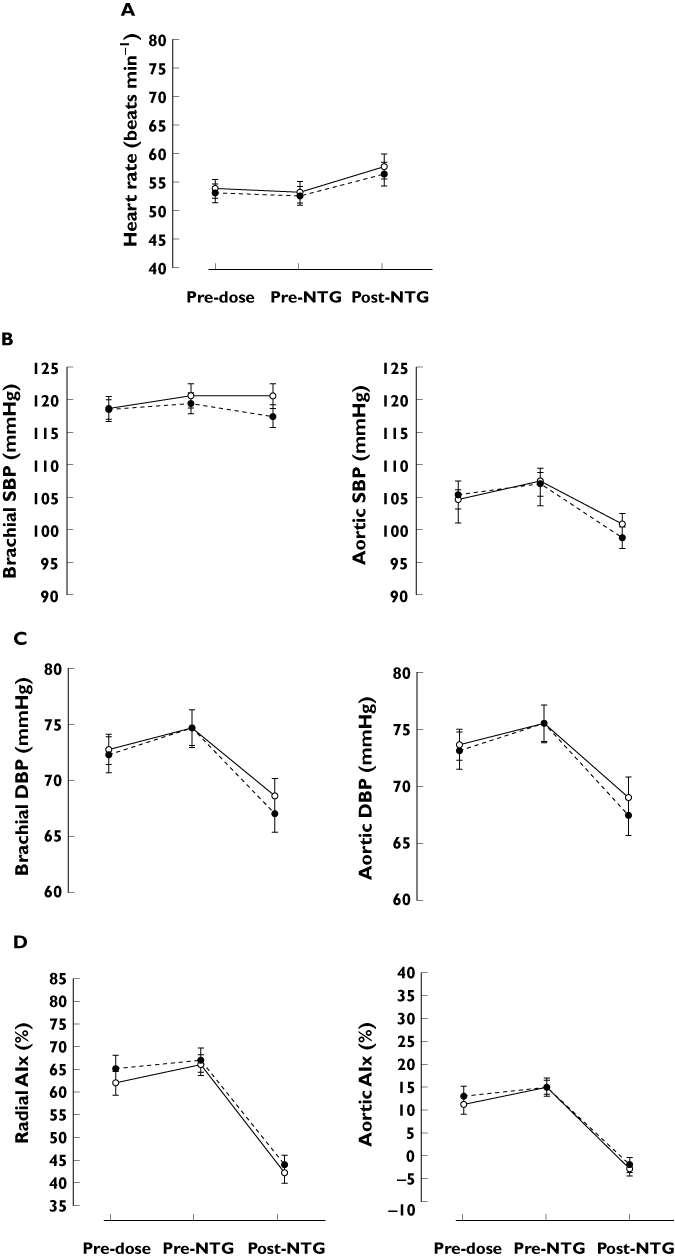
Heart rate (A), brachial and aortic systolic blood pressure (B), diastolic blood pressure (C) and radial and aortic augmentation index (D) after oral administration of 500 mg telcagepant (solid line, open circle) or placebo (dotted line, full circle) in combination with 0.4 mg of sublingual NTG. Number of subjects = 21. Data presented as mean ± SEM. SBP systolic blood pressure, DBP diastolic blood pressure, AIx augmentation Index
Figure 3.
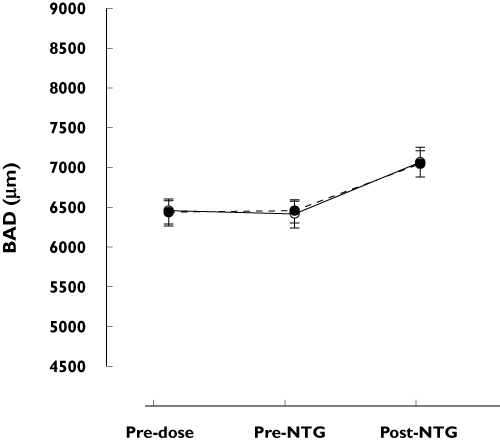
Brachial artery diameter (BAD) after oral administration of telcagepant (solid line, open circle) or placebo (dotted line, full circle) in combination with 0.4 mg of sublingual NTG. Number of subjects = 21. Data presented as mean ± SEM
Secondary analyses to evaluate the effect of telcagepant
Averaged over the three time points, vascular parameters did not differ between treatment periods except for the radial AIx, which was lower during the treatment period with telcagepant as compared with the placebo period (P = 0.040, previously described anova) (Table 1, Figure 2). This difference is largely explained by a lower value at the pre-dose time point during the telcagepant period (P = 0.027, previously described anova). Though small, the unexplained differences in the values obtained at baseline might contaminate the data.
At the pre-NTG time point, which falls at the time of the expected maximal plasma concentration of telcagepant, none of the vascular parameters differed between both treatment periods (previously described anova).
Discussion
We found that the orally administered CGRP antagonist, telcagepant, had no effect on the haemodynamic response of healthy volunteers to therapeutic doses of sublingual NTG. In addition, no vasoconstrictor effect was seen after a single 500 mg dose of telcagepant compared with placebo. These findings support a more favourable cardiovascular safety and tolerability profile of CGRP receptor antagonists over the triptans [10, 15].
Animal research, suggesting a role for CGRP in the vasodilatory effect of NTG, led us to investigate whether telcagepant attenuated NTG-induced vascular changes in healthy subjects [23]. NTG-induced vasodilation is associated with an endogenous CGRP release in both feline cerebral arterioles and rat aortas [17, 37]. These findings in animals suggest that CGRP antagonism might impede on the vasodilation induced by exogenous NO donors such as NTG. In humans, however, there is still much debate surrounding the interaction between NO and CGRP. In the resistance vessels of the human forearm, CGRP8-37, a CGRP receptor antagonist, has no effect on the vasodilation induced by the NO donor sodium nitroprusside [13]. The vasodilator mechanism of CGRP itself on the other hand seems to be partly mediated by the release of NO in the forearm vascular territory but not in the human skin [14, 38]. The interaction between NO and CGRP is thus different between vascular territories and between species. This clearly illustrates the need for sensitive techniques by which the drug-induced vascular effects of new compounds can be assessed at an early stage of clinical development.
In this respect, pulse wave analyses and brachial diameter measurements present very suitable techniques. They provide a non-invasive and more sensitive assessment of drug-induced haemodynamic changes than those obtained by more conventional oscillometric blood pressure measurements [25, 26]. In addition, the effect of NTG on these parameters is well known. Oliver et al. conducted a study in which they established the dose-relationship of sublingual NTG to changes in BAD and radial and aortic AIx in healthy men [28]. The change in heart rate, brachial diastolic blood pressure, aortic and radial AIx and BAD following 0.4 mg of NTG in our study are largely in agreement with their data.
Given the results, we conclude that telcagepant does not have a clinically significant effect on the decrease in aortic AIx following a single therapeutic dose of NTG in healthy men. Importantly, these data support the conclusion that NTG remains an effective treatment for ischaemia on the background of telcagepant therapy. This is crucial as one of the most important mechanisms of actions of NTG to relieve angina is to reduce the afterload of the heart, which is adequately reflected by the decrease in the aortic AIx [39]. More generally, this finding indicates that treatment with a potent CGRP-receptor antagonist does not interfere with the pharmacological effect of NTG, thus discarding a role for CGRP in NTG-induced vasodilation.
The analysis of the BAD permits an online assessment of the vasoactive effects of a drug on the brachial artery [25]. Whether the effect of a drug on the BAD reflects coronary artery behaviour has never been determined and would require simultaneous acquisition of both peripheral arterial ultrasound measurements and invasive coronary angiography. It is nevertheless of interest that previous studies have linked impairment of brachial artery response to NTG with coronary artery disease, suggesting similarities between both vascular territories [40]. The results obtained in the present study indicate that telcagepant has no significant effect on brachial vasodilation induced by a single dose of NTG in healthy men.
It was recently demonstrated, both in a dose finding phase II study and in a confirmatory phase III study, that a 300 mg dose of telcagepant has an efficacy comparable with that of the triptans for the treatment of acute migraine headache [10, 41]. The anticipated clinical dose of telcagepant (relative to the formulation used in this study) is a single 300 mg dose, with an optional second 300 mg dose 2 h later, if needed. A single 500 mg dose, as used in this study, achieves pharmacokinetic exposures similar to 2 × 300 mg doses, administered 2 h apart.
We are confident that the single 500 mg dose of telcagepant adequately blocks the peripheral CGRP receptor in healthy men based on a previous study conducted by our group in which we used topical capsaicin applications to elicit CGRP release in human skin and assessed the increase in dermal blood flow (DBF) using laser Doppler. Telcagepant inhibited the increase in DBF following capsaicin application and the subsequent analysis of the pharmacokinetic/pharmacodynamic relationship suggested that telcagepant engages the CGRP receptor with an EC90 of approximately 900 nm[42]. The concentration–response curve above 900 nM was relatively flat indicating that at or above this plasma concentration, telcagepant is maximally blocking the peripheral CGRP receptor in healthy men. In the capsaicin study, an early formulation of telcagepant was used that had lower bioavailability than the formulation used in this trial. The plasma concentrations achieved in this study are estimated to be approximately two- to four-fold higher than 900 nm and we are therefore confident that adequate blockade of the peripheral CGRP receptor was achieved [10, 43].
Despite the use of a high clinical dose in the present study (i.e. 500 mg), telcagepant alone (prior to NTG treatment) did not exert any unwanted cardiovascular effects either on the peripheral or on the central circulation of healthy men. The radial AIx was marginally lower when telcagepant was administered compared with placebo (P = 0.040), which results mostly from an unexplained difference in the pre-dose values and therefore merely reflects normal variability of the measurement. Our observations are in agreement with the findings of Petersen et al. who reported the absence of effect of olcegepant (a distinct CGRP receptor antagonist) on cerebral blood flow, temporal and radial artery diameter and oscillometric blood pressure measurements [15]. These data suggest that although CGRP circulates under measurable concentrations in the blood in basal conditions, it does not exert a homeostatic vasodilator activity in humans under resting conditions. As a consequence, a CGRP receptor antagonist, in contrast to the triptans, seems devoid of vasoconstrictor effects under resting conditions. In addition, in all recent studies telcagepant has been generally well tolerated and appears to have a profile consistent with safe use in patients with cardiovascular disease [10, 43–46].
A number of limitations of the present study need to be taken into account. First, the vascular effects of telcagepant were investigated in healthy men. Though CGRP has been demonstrated to play a role in ischaemic preconditioning in rats and is believed to mediate the cardioprotective effects of NTG-induced preconditioning in rabbits, there was no evidence of a major role of CGRP in regulating ischaemic blood flow in dogs [47–49]. Because of these differences between species and the lack of human studies, additional studies may be useful in patients with known cardiovascular disease [23]. In a first study in patients with stable cardiovascular disease, telcagepant did not appear to exacerbate spontaneous ischaemia [50]. Nevertheless, this study was performed under standardized resting conditions, whereas the effect of CGRP antagonism in cardiovascular patients might be rather different during normal daily activities or exercise. Second, central pressure effects were not measured invasively but were estimated by use of a generalized transfer function. This transfer function has received some criticism and has not been convincingly validated in young healthy subjects [39]. In addition calibrating the peripheral pressure wave form by use of brachial SBP and DBP might further flaw the prediction of aortic blood pressures [32]. However, in this study the use of radial AIx and aortic AIx yield the same conclusions, confirming that the untransformed radial pressure wave form provides similar information on pressure wave augmentation as the aortic PWF does. Third, the present study was not performed in migraine patients, the future patient population of telcagepant. This might be relevant as migraine patients are known to have alterations in arterial function when compared with healthy volunteers [51].
In summary, this study shows that telcagepant, at a high clinical dose for the treatment of migraine headache, had no clinically relevant effect on NTG-induced haemodynamic changes in healthy male volunteers. There was also no measurable vasoconstrictor effect of telcagepant as such in both the central and peripheral vascular bed. These results support the favourable cardiovascular safety profile of CGRP-receptor antagonists and indicate that CGRP is not involved in NTG-induced vasodilation in humans.
Acknowledgments
We acknowledge Jo Van Effen, Marc Oeyen, Karin Vaes and Lieve Janssens for assistance during the vascular measurements and Marissa Herbots for her help with the data analysis.
Competing Interests
BVdS, AVH and MD have no competing interests to declare. RB, GM, JP and IDL are employed by Merck & Co. Inc. In addition JP, RB, GM and IDL hold stock options in Merck & Co. Inc. JdH receives funds for research and research staff from the manufacture of telcagepant.
REFERENCES
- 1.Humphrey PPA, Feniuk W. Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol Sci. 1991;12:444–6. doi: 10.1016/0165-6147(91)90630-b. [DOI] [PubMed] [Google Scholar]
- 2.Dodick DW, Martin VT, Smith T, Silberstein S. Cardiovascular tolerability and safety of triptans: a review of clinical data. Headache. 2004;44(Suppl 1):S20–30. doi: 10.1111/j.1526-4610.2004.04105.x. [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Lipton RB, Ferrari MD. Migraine – current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 4.Buzzi MG, Bonamini M, Moskowitz MA. Neurogenic model of migraine. Cephalalgia. 1995;15:277–80. doi: 10.1046/j.1468-2982.1995.1504277.x. [DOI] [PubMed] [Google Scholar]
- 5.Edvinsson L. CGRP-receptor antagonism in migraine treatment. Lancet. 2008;372:2089–90. doi: 10.1016/S0140-6736(08)61710-9. [DOI] [PubMed] [Google Scholar]
- 6.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 7.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 8.Adwanikar H, Ji G, Li W, Doods H, Willis WD, Neugebauer V. Spinal CGRP1 receptors contribute to supraspinally organized pain behavior and pain-related sensitization of amygdala neurons. Pain. 2007;132:53–66. doi: 10.1016/j.pain.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 10.Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–12. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 11.Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–96. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet. 2010;376:645–55. doi: 10.1016/S0140-6736(10)60323-6. [DOI] [PubMed] [Google Scholar]
- 13.Vanmolkot FH, Van der Schueren BJ, de Hoon JN. Calcitonin gene-related peptide-induced vasodilation in the human forearm is antagonized by CGRP8-37: evaluation of a human in vivo pharmacodynamic model. Clin Pharmacol Ther. 2006;79:263–73. doi: 10.1016/j.clpt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Van der Schueren BJ, Rogiers A, Vanmolkot FH, Van Hecken A, Depre M, Kane SA, De Lepeleire I, Sinclair SR, de Hoon JN. Calcitonin gene-related peptide8-37 antagonizes capsaicin-induced vasodilation in the skin: evaluation of a human in vivo pharmacodynamic model. J Pharmacol Exp Ther. 2008;325:248–55. doi: 10.1124/jpet.107.133868. [DOI] [PubMed] [Google Scholar]
- 15.Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, Olesen J. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–47. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- 16.Verheggen R, Bumann K, Kaumann AJ. BIBN4096BS is a potent competitive antagonist of the relaxant effects of alpha-CGRP on human temporal artery: comparison with CGRP(8-37) Br J Pharmacol. 2002;136:120–6. doi: 10.1038/sj.bjp.0704682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei EP, Moskowitz MA, Boccalini P, Kontos HA. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ Res. 1992;70:1313–9. doi: 10.1161/01.res.70.6.1313. [DOI] [PubMed] [Google Scholar]
- 18.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–6. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 19.Ghatta S, O'Rourke ST. Nitroglycerin-induced release of calcitonin gene-related peptide from sensory nerves attenuates the development of nitrate tolerance. J Cardiovasc Pharmacol. 2006;47:175–81. doi: 10.1097/01.fjc.0000199681.35825.1d. [DOI] [PubMed] [Google Scholar]
- 20.Kruuse C, Iversen HK, Jansen-Olesen I, Edvinsson L, Olesen J. Calcitonin gene-related peptide (CGRP) levels during glyceryl trinitrate (GTN)-induced headache in healthy volunteers. Cephalalgia. 2010;30:467–74. doi: 10.1111/j.1468-2982.2009.01963.x. [DOI] [PubMed] [Google Scholar]
- 21.Chan KY, Edvinsson L, Eftekhari S, Kimblad PO, Kane SA, Lynch J, Hargreaves RJ, de Vries R, Garrelds IM, van den Bogaerdt AJ, Danser AH, Maassenvandenbrink A. Characterization of the calcitonin gene-related peptide receptor antagonist telcagepant (MK-0974) in human isolated coronary arteries. J Pharmacol Exp Ther. 2010;334:746–52. doi: 10.1124/jpet.110.165993. [DOI] [PubMed] [Google Scholar]
- 22.You JP, Wang Q, Zhang W, Jansen-Olesen I, Paulson OB, Lassen NA, Edvinsson L. Hypercapnic vasodilatation in isolated rat basilar arteries is exerted via low pH and does not involve nitric oxide synthase stimulation or cyclic GMP production. Acta Physiol Scand. 1994;152:391–7. doi: 10.1111/j.1748-1716.1994.tb09821.x. [DOI] [PubMed] [Google Scholar]
- 23.YJ Li, Du YH. CGRP-mediated cardiovascular effect of nitroglycerin. Med Hypotheses. 2003;60:693–8. doi: 10.1016/s0306-9877(03)00024-0. [DOI] [PubMed] [Google Scholar]
- 24.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–66. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- 25.de Hoon JN, Willigers JM, Troost J, Struijker-Boudier HA, Van Bortel LM. Vascular effects of 5-HT1B/1D-receptor agonists in patients with migraine headaches. Clin Pharmacol Ther. 2000;68:418–26. doi: 10.1067/mcp.2000.110502. [DOI] [PubMed] [Google Scholar]
- 26.Vanmolkot FH, de Hoon JN. Acute effects of sumatriptan on aortic blood pressure, stiffness, and pressure waveform. Clin Pharmacol Ther. 2006;80:85–94. doi: 10.1016/j.clpt.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Greig LD, Leslie SJ, Gibb FW, Tan S, Newby DE, Webb DJ. Comparative effects of glyceryl trinitrate and amyl nitrite on pulse wave reflection and augmentation index. Br J Clin Pharmacol. 2005;59:265–70. doi: 10.1111/j.1365-2125.2004.02334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliver JJ, Bowler A, Beudeker Q, Cate T, Webb DJ. Dose-response relationship of sublingual nitroglycerin with brachial artery dilatation and change in central and peripheral augmentation index. Clin Pharmacol Ther. 2005;77:337–8. doi: 10.1016/j.clpt.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 29.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–4. [PMC free article] [PubMed] [Google Scholar]
- 30.Salvatore CA, Hershey JC, Corcoran HA, Fay JF, Johnston VK, Moore EL, Mosser SD, Burgey CS, Paone DV, Shaw AW, Graham SL, Vacca JP, Williams TM, Koblan KS, Kane SA. Pharmacological characterization of MK-0974 [N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3- yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carbox amide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther. 2008;324:416–21. doi: 10.1124/jpet.107.130344. [DOI] [PubMed] [Google Scholar]
- 31.El Assaad MA, Topouchian JA, Asmar RG. Evaluation of two devices for self-measurement of blood pressure according to the international protocol: the Omron M5-I and the Omron 705IT. Blood Press Monit. 2003;8:127–33. doi: 10.1097/00126097-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher D, Adji A, O'Rourke MF. Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. Am J Hypertens. 2004;17:1059–67. doi: 10.1016/j.amjhyper.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–84. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 34.Hoeks APG, Brands PJ, Smeets FAM, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16:121–28. doi: 10.1016/0301-5629(90)90139-4. [DOI] [PubMed] [Google Scholar]
- 35.Kool MJF, van Merode T, Reneman RS, Hoeks APG, Struyker-Boudier HAJ, Van Bortel LMAB. Evaluation and reproducibility of a vessel wall movement detector system for assessment of large artery properties. Cardiovasc Res. 1994;28:610–4. doi: 10.1093/cvr/28.5.610. [DOI] [PubMed] [Google Scholar]
- 36.Dishy V, Sofowora G, Harris PA, Kandcer M, Zhan F, Wood AJ, Stein CM. The effect of sildenafil on nitric oxide-mediated vasodilation in healthy men. Clin Pharmacol Ther. 2001;70:270–9. doi: 10.1067/mcp.2001.117995. [DOI] [PubMed] [Google Scholar]
- 37.Booth BP, Tabrizi-Fard MA, Ho-Leung F. Calcitonin gene-related peptide-dependent vascular relaxation of rat aorta: an additional mechanism for nitroglycerin. Biochem Pharmacol. 2000;59:1603–09. doi: 10.1016/s0006-2952(00)00290-2. [DOI] [PubMed] [Google Scholar]
- 38.de Hoon JN, Pickkers P, Smits P, Struijker-Boudier HA, Van Bortel LM. Calcitonin gene-related peptide: exploring its vasodilating mechanism of action in humans. Clin Pharmacol Ther. 2003;73:312–21. doi: 10.1016/s0009-9236(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 39.Davies JI, Struthers AD. Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens. 2003;21:463–72. doi: 10.1097/00004872-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Aksoy Y, Acikgoz N, Sivri N, Bariskaner E, Akturk E, Turhan H, Yetkin E. Decreased nitrate-mediated dilatation in patients with coronary artery ectasia: an ultrasonographic evaluation of brachial artery. Coron Artery Dis. 2006;17:365–9. doi: 10.1097/00019501-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Koppen H, Winner P. Acute antimigraine efficacy and tolerability of the novel oral CGRP receptor antagonist MK-0974: a phase III clinical trial versus placebo and zolmitriptan. Headache. 2008;48:S7 (OR17). doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 42.Sinclair SR, Kane SA, Van der Schueren BJ, Xiao A, Willson KJ, Boyle J, de Lepeleire I, Xu Y, Hickey L, Denney WS, Li CC, Palcza J, Vanmolkot FH, Depre M, Van Hecken A, Murphy MG, Ho TW, de Hoon JN. Inhibition of capsaicin-induced increase in dermal blood flow by the oral CGRP receptor antagonist, telcagepant (MK-0974) Br J Clin Pharmacol. 2010;69:15–22. doi: 10.1111/j.1365-2125.2009.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–23. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 44.Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics. 2010;7:164–75. doi: 10.1016/j.nurt.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bigal ME, Ho TW. Is there an inherent limit to acute migraine treatment efficacy? J Headache Pain. 2009;10:393–4. doi: 10.1007/s10194-009-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, Fan X, Fei K, Assaid C, Lines C, Ho TW. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–7. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regan CP, Stump GL, Kane SA, Lynch JJ., Jr Calcitonin gene-related peptide receptor antagonism does not affect the severity of myocardial ischemia during atrial pacing in dogs with coronary artery stenosis. J Pharmacol Exp Ther. 2009;328:571–8. doi: 10.1124/jpet.108.144220. [DOI] [PubMed] [Google Scholar]
- 48.Luo D, Deng PY, Ye F, Peng WJ, Deng HW, Li YJ. Delayed preconditioning by cardiac ischemia involves endogenous calcitonin gene-related peptide via the nitric oxide pathway. Eur J Pharmacol. 2004;502:135–41. doi: 10.1016/j.ejphar.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 49.Li D, Li NS, Chen QQ, Guo R, Xu PS, Deng HW, Li YJ. Calcitonin gene-related peptide-mediated cardioprotection of postconditioning in isolated rat hearts. Regul Pept. 2008;147:4–8. doi: 10.1016/j.regpep.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Behm MO, Blanchard RL, Murphy MG, Chodakewitz JA, Palcza JS, Harris DE, Butterfield KL, Smith WB, Haynes EM, Sackner-Bernstein J, Preston RA, Krucoff MW. Assessment of the effect of MK-0974, an oral CGRP receptor antagonist, on spontaneous ischemia in patients with stable cardiovascular disease. Headache. 2008;48:S39 (S1). [Google Scholar]
- 51.Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology. 2007;68:1563–70. doi: 10.1212/01.wnl.0000260964.28393.ed. [DOI] [PubMed] [Google Scholar]


