Abstract
AIM
The aim of this study was to investigate the effects of orange juice and apple juice on the pharmacokinetics and pharmacodynamics of aliskiren.
METHODS
In a randomized crossover study, 12 healthy volunteers ingested 200 ml of orange juice, apple juice or water three times daily for 5 days. On day 3, they ingested a single 150-mg dose of aliskiren. Plasma aliskiren concentrations were measured up to 72 h, its excretion into urine up to 12 h and plasma renin activity up to 24 h.
RESULTS
Orange and apple juice reduced aliskiren peak plasma concentrations by 80% (95% CI 63%, 89%, P < 0.001) and 84% (95% CI 72%, 91%, P < 0.001), and the area under the plasma aliskiren concentration–time curve (AUC) by 62% (95% CI 47%, 72%, P < 0.001) and 63% (95% CI 46%, 74%, P < 0.001), respectively, but had no significant effect on its elimination half-life or renal clearance. The decreases in aliskiren AUC by orange and apple juice correlated with aliskiren AUC during the water phase (r = 0.98, P < 0.001). Plasma renin activity was 87% and 67% higher at 24 h after aliskiren during the orange juice and apple juice phases, respectively, than during the water phase (P < 0.05).
CONCLUSIONS
Orange juice and apple juice greatly reduce the plasma concentrations and renin-inhibiting effect of aliskiren, probably by inhibiting its OATP2B1-mediated influx in the small intestine. Concomitant intake of aliskiren with orange or apple juice is best avoided.
Keywords: aliskiren, apple juice, orange juice, organic anion transporting polypeptide 2B1, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Aliskiren is an antihypertensive drug with a low oral bioavailability.
Aliskiren is eliminated primarily unchanged into bile, and it is a substrate of the organic anion transporting polypeptide 2B1 (OATP2B1) influx transporter, the P-glycoprotein efflux transporter and cytochrome P450 3A4.
Flavonoids in orange and apple juice have been shown to inhibit OATP2B1 in vitro.
WHAT THIS STUDY ADDS
Orange juice and apple juice markedly reduce the plasma concentrations of aliskiren probably by inhibiting its OATP2B1-mediated intestinal absorption.
Concomitant intake of aliskiren with orange or apple juice is best avoided.
Introduction
Fruit juices are of interest as possible causes of food–drug interactions [1, 2]. Grapefruit juice is known to enhance the oral bioavailability of many cytochrome P-450 (CYP) 3A4 substrates by inactivating intestinal CYP3A4 [1, 3, 4]. Orange and apple juice, in contrast, have not been reported to affect CYP3A4 activity [3, 5, 6]. On the other hand, grapefruit, orange and/or apple juice have decreased the oral bioavailabilities of some drugs, such as fexofenadine, celiprolol, talinolol, atenolol and ciprofloxacin [2, 7–12]. These drugs are all substrates of either organic anion transporting polypeptide 1A2 (OATP1A2) or OATP2B1, or both [7, 13–19]. OATP1A2 and OATP2B1 are influx transporters expressed in the luminal membrane of small intestinal enterocytes [16, 20–22], potentially participating in the active absorption of drugs [19]. Certain constituents of grapefruit, orange and apple juice have been found to inhibit OATP1A2 and OATP2B1 in vitro[7, 23, 24].
Recently, grapefruit juice was found to reduce markedly the peak plasma concentration (Cmax) and the area under the plasma concentration–time curve (AUC) of the renin-inhibiting antihypertensive drug aliskiren [25]. Aliskiren is a substrate of OATP2B1, P-glycoprotein and CYP3A4 [26, 27], and the mechanism of this interaction may be inhibition of the OATP2B1-mediated influx of aliskiren in the small intestine by grapefruit juice. Aliskiren is a hydrophilic, poorly absorbed compound with a low oral bioavailability (2–3%) [26, 28, 29]. It is eliminated mainly by biliary and, to a smaller extent, by renal excretion, with only a small amount being metabolized by CYP3A4 [26]. These characteristics render it susceptible to transporter-mediated drug interactions and a good candidate for a probe substrate for transporters in vivo. In addition to the effects of grapefruit juice, aliskiren has also been found to be susceptible to induction and inhibition of the P-glycoprotein efflux transporter by rifampicin and itraconazole, respectively [30, 31].
Orange and apple juice are widely consumed, but there are only a few studies investigating their possible effects on the pharmacokinetics of drugs in vivo in humans [2]. Because the constituents of these juices inhibit OATP2B1 in vitro, we thought it important to study the effects of these juices on aliskiren. Accordingly, we have investigated the effects of orange juice and apple juice on the pharmacokinetics and pharmacodynamics of aliskiren in a randomized crossover study in healthy volunteers.
Methods
Subjects
Twelve healthy volunteers (five women, seven men) participated in the study after having given written informed consent. Their mean ± SD age was 22 ± 2 years (range 20–28 years), mean height 173 ± 7 cm (161–183 cm) and mean weight 69 ± 10 kg (55–85 kg). Each participant's health was ascertained by medical history, clinical examination and laboratory tests. Subjects with a systolic blood pressure less than 100 mmHg were not included in the study. None was on any continuous medication, including hormonal contraceptives, and none was a tobacco smoker.
Study design
The study protocol was approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District, and the National Agency for Medicines in Finland. In a randomized crossover study with three phases and a washout period of 2 weeks, the participants ingested either 200 ml of made-from-concentrate normal-strength orange juice (Valio appelsiinitäysmehu; Valio, Helsinki, Finland), made-from-concentrate normal-strength apple juice (Valio omenatäysmehu; Valio, Helsinki, Finland) or water three times a day at 0800 h, 1200 h and 2000 h for 5 days. On day 3, following an overnight fast, they ingested a single 150-mg dose of aliskiren (Rasilez; Novartis, Horsham, UK) with 200 ml of orange juice, apple juice or water at 0800 h. A standardized warm meal was served 4 h after aliskiren and a standardized light meal after 7 h and 10 h. Systolic and diastolic blood pressures and heart rate were measured twice (mean value was used in the calculations) from the forearm with an automatic oscillometric blood pressure monitor (Omron M5-I, Omron Healthcare Europe BV, Hoofddorp, the Netherlands), with the participant in a sitting position, prior to and 2, 4, 7, 9, 12 and 24 h after aliskiren ingestion. The participants were under direct medical supervision for 12 h after the administration of aliskiren. Use of fruit juice and orange, apple and grapefruit products was prohibited during the study, starting from 1 week before the first day of administration of aliskiren. Use of all drugs was prohibited for 1 week, and use of alcohol for 2 days before aliskiren administration and 3 days thereafter.
Timed ethylenediaminetetraacetic acid (EDTA) blood samples (5–10 ml each) for aliskiren concentration measurements were drawn prior to and 0.5, 1, 2, 3, 4, 5, 7, 9, 12, 24, 34, 48 and 72 h after aliskiren administration. Blood samples for the determination of plasma renin activity (5 ml each) were drawn before aliskiren administration and 4 h and 24 h thereafter into chilled EDTA tubes, which were placed on ice immediately after sampling. Plasma was separated within 30 min. Urine was collected up to 12 h after aliskiren administration. The samples were stored at −70°C until analysis.
Determination of aliskiren concentrations and renin activity
Aliskiren plasma and urine concentrations were quantified using an Applied Biosystems SCIEX Q Trap LC/MS/MS system (Sciex Division of MDS, Toronto, Ontario, Canada) [31]. Acebutolol served as an internal standard. The lower limit of quantification of plasma aliskiren was 0.24 ng ml−1, and the between-day coefficient of variation was 6.1% at 1.8 ng ml−1, 4.6% at 18 ng ml−1 and 7.0% at 180 ng ml−1 (n = 10). The lower limit of quantification of urine aliskiren was 21 ng ml−1, and the intra-day coefficient of variation was 4.0% at 21 ng ml−1, 2.3% at 54 ng ml−1, 4.2% at 450 ng ml−1 and 3.7% at 1350 ng ml−1 (n = 6). Plasma renin activity was determined by radioimmunoassay of generated angiotensin I with a commercially available method (RENCTK; DiaSorin, Saluggia, Italy) at Medix Laboratories (Espoo, Finland).
Pharmacokinetics and pharmacodynamics
Aliskiren pharmacokinetics were characterized by Cmax, time to Cmax (tmax), elimination half-life (t1/2), AUC(0–72 h), AUC(0–∞), amount of unchanged aliskiren excreted into urine from 0 to 12 h (Ae) and renal clearance (CLR). Pharmacokinetic parameters were calculated with conventional non-compartmental methods using MK-Model, version 5.0 (Biosoft, Cambridge, UK). The terminal log-linear part of each concentration–time curve was identified visually. The elimination rate constant (λz) was determined by linear regression analysis of the log-linear part of the plasma drug concentration–time curve. The t1/2 was calculated by the equation t1/2= ln2/λz. The AUC values were calculated by a combination of the linear and log-linear trapezoidal rules with extrapolation to infinity, when appropriate, by division of the last measured concentration by λz. The CLR was calculated by the equation CLR=Ae/AUC(0–12 h). Aliskiren pharmacodynamics were characterized by plasma renin activity at 4 h and 24 h after aliskiren administration, as well as average systolic and diastolic blood pressures and heart rate. Average values of systolic and diastolic blood pressures and heart rate were calculated by dividing the area under the effect–time curve from 0 to 24 h by 24 h.
DNA preparation and genotyping
A 10-ml EDTA blood sample was obtained from each participant and stored at −20°C until deoxyribonucleic acid (DNA) extraction. Genomic DNA was extracted using standard methods (QIAamp DNA Blood Mini Kit; Qiagen, Hilden, Germany). The participants were genotyped for SLCO2B1 g.-282G > A (rs2712807), c.601G > A (p.Val201Met, rs35199625), c.935G > A (p.Arg312Gln, rs12422149) and c.1457C > T (p.Ser486Phe, rs2306168) alleles by allelic discrimination with Taqman® Genotyping Assays [32].
Statistical analysis
The data were analysed using spss 17.0 (SPSS Inc., Chicago, IL, USA). The results are expressed as geometric means and geometric mean ratios with 95% confidence intervals, or median with range (tmax). All data except tmax were logarithmically transformed before statistical analysis. Statistical comparisons between the orange juice, apple juice and water phases were made using repeated measures analysis of variance (anova) with a priori pairwise comparisons with the Fisher's least significant difference method. The tmax values were compared with Friedman's anova with a priori pairwise comparisons with the Wilcoxon signed-rank test. Possible correlations among the AUC(0–∞) of aliskiren during the water phase and the changes in aliskiren AUC(0–∞) by orange juice and apple juice, between the plasma aliskiren concentration at 24 h and the plasma renin activity at 24 h, and between the relative change in plasma aliskiren concentration at 24 h by orange juice or apple juice and the corresponding relative change in plasma renin activity at 24 h were investigated with the Pearson correlation coefficient. Differences were considered statistically significant when P < 0.05.
Results
The consumption of either normal-strength orange juice or normal-strength apple juice three times daily greatly reduced the plasma concentrations of aliskiren (Figure 1, Table 1). Orange juice reduced aliskiren Cmax by 80% (the effect on Cmax ranged from a 95% decrease to a 24% increase, P < 0.001), AUC(0–∞) by 62% (range 9–87%, P < 0.001) and Ae by 67% (range 32–87%, P < 0.001; Figure 2). Apple juice reduced aliskiren Cmax by 84% (range, 2–96%, P < 0.001), AUC(0–∞) by 63% (range, 13–87%, P < 0.001) and Ae by 69% (range 14–87%, P < 0.001; Figure 2). Neither orange juice nor apple juice affected the tmax, t1/2 or CLR of aliskiren.
Figure 1.
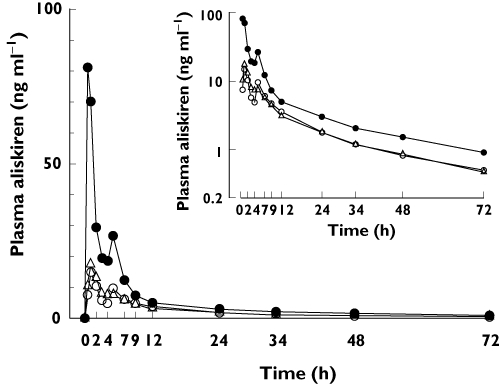
Geometric mean plasma concentrations of aliskiren in 12 healthy volunteers during orange juice (○), apple juice (▵) and water (•) phases. The volunteers ingested 200 ml of orange juice, apple juice or water three times a day for 5 days, and a single 150-mg dose of aliskiren on day 3, together with the first orange juice, apple juice or water dose of the day. Inset depicts the same data on a semi-logarithmic scale. Error bars have been omitted for clarity
Table 1.
Pharmacokinetic variables of a single 150-mg oral dose of aliskiren in 12 healthy volunteers during orange juice, apple juice and water phases
| Variable | Water phase (control) | Orange juice phase | Geometric mean ratio (orange juice/water; 95% CI) | Apple juice phase | Geometric mean ratio (apple juice/water; 95% CI) |
|---|---|---|---|---|---|
| Aliskiren | |||||
| Cmax (ng ml−1) | 110 (67, 182) | 22 (12, 40)* | 0.20 (0.11, 0.37) | 18 (11, 28)* | 0.16 (0.09, 0.28) |
| tmax (h) | 0.5 (0.5–5) | 1 (0.5–5) | 1 (0.5–2) | ||
| t1/2 (h) | 28 (27, 31) | 28 (25, 30) | 0.97 (0.86, 1.09) | 26 (24, 29) | 0.92 (0.83, 1.02) |
| AUC(0–72 h; ng ml−1 h−1) | 441 (276, 704) | 162 (122, 216)* | 0.37 (0.27, 0.51) | 161 (126, 207)* | 0.37 (0.25, 0.53) |
| AUC(0–∞; ng ml−1 h−1) | 481 (302, 767) | 183 (138, 243)* | 0.38 (0.28, 0.53) | 180 (142, 229)* | 0.37 (0.26, 0.54) |
| Ae (mg) | 0.405 (0.281, 0.582) | 0.132 (0.110, 0.158)* | 0.33 (0.24, 0.44) | 0.127 (0.102, 0.157)* | 0.31 (0.22, 0.45) |
| CLR (l h−1) | 1.34 (1.07, 1.66) | 1.54 (1.32, 1.81) | 1.16 (0.97, 1.38) | 1.47 (1.24, 1.75) | 1.10 (0.89, 1.37) |
The volunteers ingested 200 ml of orange juice, apple juice or water three times a day for 5 days and a single 150-mg dose of aliskiren on day 3, together with the first orange juice, apple juice or water dose of the day. Data are given as geometric mean (95% CI) and geometric mean ratio (95% CI), except for tmax, which is given as median (range). Ae, amount excreted into urine within 12 h; AUC(0–72 h), area under the plasma concentration–time curve from 0 to 72 h; AUC(0–∞), area under the plasma concentration–time curve from time 0 to infinity; CI, confidence interval; CLR, renal clearance; Cmax, peak plasma concentration; t1/2, elimination half-life; tmax, time to Cmax.
P < 0.001 compared with water phase.
Figure 2.
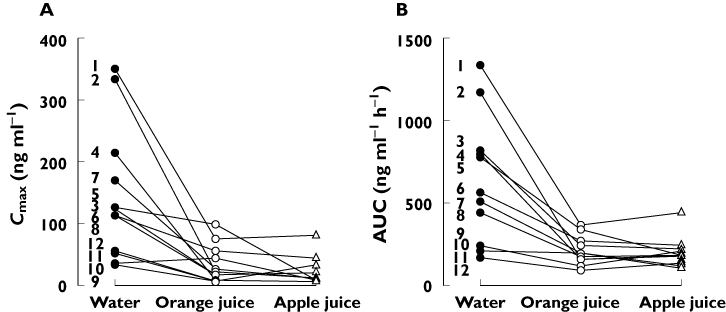
Individual Cmax (A) and AUC (B) values of aliskiren in 12 healthy volunteers during orange juice, apple juice and water phases. The numbers refer to the participant numbers in Table 2. The volunteers ingested 200 ml of orange juice, apple juice or water three times a day for 5 days, and a single 150-mg dose of aliskiren on day 3, together with the first orange juice, apple juice or water dose of the day. AUC, area under the plasma concentration–time curve; Cmax, peak plasma concentration
Aliskiren AUC(0–∞) values varied 7.9-fold, 4.0-fold and 4.2-fold between individual participants during the water, orange juice and apple juice phases, respectively. The changes in aliskiren AUC(0–∞) by orange juice and apple juice correlated with each other and with the AUC(0–∞) of aliskiren during the water phase (r = 0.98, P < 0.001 for all pairs; Figure 3). The SLCO2B1 genotypes of the participants are shown in Table 2. No obvious differences were seen in the pharmacokinetics of aliskiren between participants with different SLCO2B1 genotypes, but the number of individuals with different genotypes was too small to draw any conclusions (Figure 2).
Figure 3.
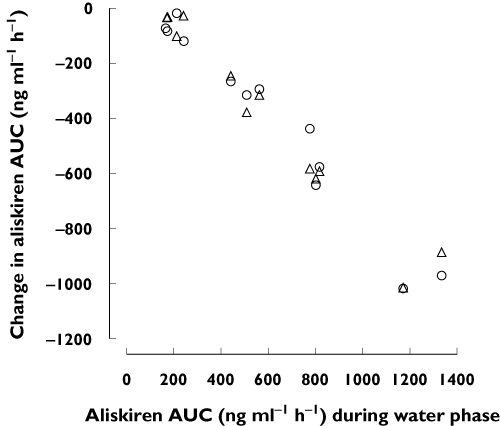
Change in aliskiren AUC by orange juice (○) and apple juice (▵) plotted against aliskiren AUC during the water phase. The volunteers ingested 200 ml of orange juice, apple juice or water three times a day for 5 days, and a single 150-mg dose of aliskiren on day 3, together with the first orange juice, apple juice or water dose of the day. AUC, area under the plasma concentration–time curve
Table 2.
SLCO2B1 genotypes of the healthy volunteers
| Participant | g.-282G > A | c.601G > A | c.935G > A | c.1457C > T |
|---|---|---|---|---|
| 1 | AA | GA | GA | CC |
| 2 | AA | GG | GG | CC |
| 3 | AA | GG | GG | CC |
| 4 | AA | GG | GG | CC |
| 5 | AA | GG | GG | CT |
| 6 | GA | GG | GG | CC |
| 7 | AA | GG | GG | CC |
| 8 | GA | GA | GA | CC |
| 9 | AA | GG | GG | CC |
| 10 | AA | GG | GA | CC |
| 11 | GA | GG | GG | CC |
| 12 | AA | GG | GA | CC |
Plasma renin activity at 4 and 24 h after aliskiren administration was 63% (P = 0.023) and 87% (P = 0.037) higher, respectively, during the orange juice phase than during the water phase (Figure 4, Table 3). During the apple juice phase, plasma renin activity was 67% higher at 24 h after aliskiren than during the water phase (P = 0.036). During the water phase, the plasma aliskiren concentration at 24 h correlated significantly with the plasma renin activity at 24 h (r=−0.615, P = 0.033). Moreover, the relative change in plasma aliskiren concentration at 24 h by orange juice or apple juice correlated with the corresponding relative change in plasma renin activity (orange juice r=−0.580, P = 0.048; apple juice r=−0.716, P = 0.009). No significant differences existed in the systolic or diastolic blood pressure or the heart rate between the phases.
Figure 4.
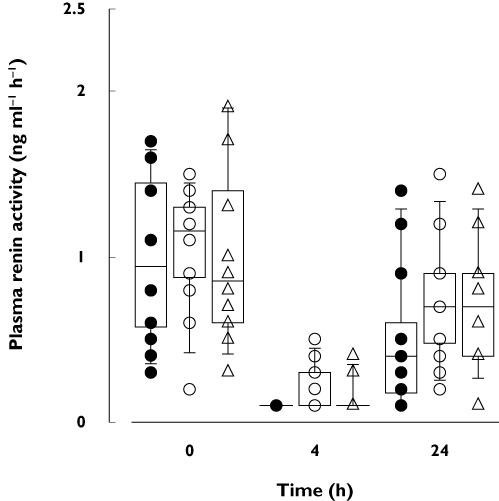
Box-and-whisker plots of plasma renin activity in 12 healthy volunteers 0, 4 and 24 h after a single 150-mg oral dose of aliskiren during orange juice, apple juice and water phases. The volunteers ingested 200 ml of orange juice, apple juice or water three times a day for 5 days, and a single 150-mg dose of aliskiren on day 3, together with the first orange juice, apple juice or water dose of the day. The horizontal lines inside the boxes represent the median, the box edges show the lower and upper quartiles, and the whiskers show the 5th and 95th percentiles. Individual data points are shown as • (water phase), ○ (orange juice phase) or ▵ (apple juice phase)
Table 3.
Pharmacodynamic variables of a single 150-mg oral dose of aliskiren in 12 healthy volunteers during orange juice, apple juice and water phases
| Variable | Water phase (control) | Orange juice phase | Geometric mean ratio (orange juice/water) (95% CI) | Apple juice phase | Geometric mean ratio (apple juice/water) (95% CI) |
|---|---|---|---|---|---|
| Plasma renin activity (ng ml−1 h−1) | |||||
| At baseline | 0.88 (0.60, 1.28) | 0.93 (0.66, 1.33) | 1.06 (0.61, 1.84) | 0.88 (0.61, 1.27) | 1.00 (0.66, 1.51) |
| At 4 h | 0.10 (0.10, 0.10) | 0.16 (0.11, 0.25)* | 1.63 (1.09, 2.45) | 0.12 (0.09, 0.17) | 1.23 (0.90, 1.68) |
| At 24 h | 0.35 (0.19, 0.62) | 0.65 (0.45, 0.94)† | 1.87 (1.05, 3.35) | 0.58 (0.37, 0.90)‡ | 1.67 (1.04, 2.67) |
| Systolic blood pressure (mmHg) | |||||
| Baseline | 127 (117, 137) | 122 (113, 132) | 0.96 (0.91, 1.02) | 123 (115, 132) | 0.97 (0.94, 1.00) |
| Average0–24 h | 125 (116, 135) | 125 (116, 134) | 1.00 (0.96, 1.03) | 123 (110, 137) | 0.98 (0.93, 1.03) |
| Average0–24 h/baseline | 0.99 (0.96, 1.01) | 1.02 (0.98, 1.07) | 1.04 (0.98, 1.09) | 1.00 (0.93, 1.07) | 1.01 (0.94, 1.09) |
| Diastolic blood pressure (mmHg) | |||||
| Baseline | 74 (71, 78) | 75 (69, 82) | 1.01 (0.95, 1.07) | 72 (67, 77) | 0.96 (0.91, 1.01) |
| Average0–24 h | 73 (68, 79) | 73 (68, 77) | 0.99 (0.95, 1.03) | 71 (64, 78) | 0.97 (0.92, 1.02) |
| Average0–24 h/baseline | 0.99 (0.95, 1.02) | 0.97 (0.93, 1.01) | 0.98 (0.93, 1.04) | 0.99 (0.91, 1.08) | 1.01 (0.94, 1.07) |
| Heart rate (beats min−1) | |||||
| Baseline | 63 (57, 70) | 60 (53, 69) | 0.96 (0.84, 1.11) | 62 (55, 70) | 0.99 (0.88, 1.10) |
| Average0–24 h | 66 (61, 72) | 66 (61, 71) | 1.00 (0.96, 1.04) | 65 (60, 69) | 0.98 (0.91, 1.04) |
| Average0–24 h/baseline | 1.06 (0.96, 1.16) | 1.09 (1.00, 1.20) | 1.04 (0.91, 1.18) | 1.04 (0.94, 1.16) | 0.99 (0.87, 1.12) |
The volunteers ingested 200 ml of orange juice, apple juice or water three times a day for 5 days and a single 150-mg dose of aliskiren on day 3, together with the first orange juice, apple juice or water dose of the day. Data are given as geometric mean (95% CI) and geometric mean ratio (95% CI). Baseline, before the administration of aliskiren; CI, confidence interval.
P = 0.023 compared with water phase.
P = 0.037 compared with water phase.
P = 0.036 compared with water phase.
Discussion
These data show that regular consumption of orange juice or apple juice can markedly reduce the plasma concentrations of the OATP2B1 substrate aliskiren. Because the Cmax, AUC and Ae of aliskiren were decreased, but the t1/2 and CLR remained unaffected, it is evident that orange juice and apple juice reduced the oral bioavailability of aliskiren. In line with the effects of orange and apple juice on aliskiren pharmacokinetics, the pharmacodynamic response to aliskiren was attenuated by these fruit juices.
The AUC of aliskiren showed about eightfold interindividual variation during the water phase (control), but only fourfold variability during the orange juice and apple juice phases. Moreover, the effects of orange and apple juices on aliskiren AUC varied greatly between individuals, with the largest effects seen in those individuals with the highest aliskiren exposures during the water phase. Interestingly, at the individual level, orange juice and apple juice produced very similar reductions in aliskiren AUC (Figure 3), suggesting that they interact with aliskiren via the same mechanism.
The effect of grapefruit juice on CYP3A4 is well established, but the effects of fruit juices on intestinal transporters are less extensively characterized. Grapefruit, orange and apple juice have reduced the Cmax of fexofenadine, a substrate of OATP1A2 and possibly of OATP2B1 [7, 13, 14, 16, 19], by 62%, 67% and 72%, and its AUC by 63%, 69% and 73%, respectively [7]. Grapefruit juice and orange juice have also decreased the Cmax of celiprolol, a substrate of OATP1A2 [17], by 95% and 89% and its AUC by 87% and 83%, respectively [9, 10]. In addition, orange juice has reduced the Cmax of the OATP1A2 substrates, atenolol and ciprofloxacin, by 49% and 23% and their AUC values by 40% and 22% [8, 11, 15, 17]. In the present study, orange juice and apple juice were roughly equipotent in reducing the absorption of aliskiren from the gut lumen. Interestingly, the effects of orange and apple juice on aliskiren pharmacokinetics were also of a similar magnitude to those of grapefruit juice (81% decrease in Cmax and 61% decrease in AUC) [25]. As aliskiren is a substrate of OATP2B1, with a Km of 72 µmol L−1[27], the most likely mechanism of these interactions is inhibition of the OATP2B1-mediated intestinal uptake of aliskiren by orange juice and apple juice.
The duration of the inhibitory effect of grapefruit juice on intestinal OATP transporters seems to be much shorter than that on CYP3A4 [16]. In a previous study, ingestion of grapefruit juice concomitantly with or 2 h before fexofenadine decreased the AUC of fexofenadine by 52% and 38%, respectively, but grapefruit juice ingested 4 h before the drug had no effect [16]. In another study, ingestion of a single 300-ml dose of grapefruit juice concomitantly with talinolol decreased the AUC of talinolol to a similar extent as ingestion of 300 ml of grapefruit juice three times daily for 6 days, prior to talinolol administration [12]. It is likely that the inhibitory effect of orange juice and apple juice on OATP2B1 is of similar duration. Thus, it is possible that ingestion of only a single 200-ml dose of orange juice or apple juice concomitantly or a few hours before aliskiren would decrease the AUC of aliskiren to an extent similar to that seen in the present study with ingestion of 200 ml of these juices three times daily.
Several constituents of fruit juices have been screened for inhibitory activity on intestinal OATP transporters. It should be noted that the contents of juices may vary depending on fruit species and juice brands. In addition, the flavonoids in these juices are usually found as glycoside conjugates that require hydrolysis to active aglycones in vivo, making the in vitro–in vivo prediction of the interactions challenging [33, 34]. The major flavonoid in orange juice, hesperidin, inhibits OATP1A2 in vitro with an IC50 of 2.7 µmol L−1 for fexofenadine uptake [35], which is much lower than the concentration normally occurring in orange juice (106–1965 µmol L−1) [36–38]. There are no published studies on the possible effects of hesperidin on OATP2B1. However, tangeritin and nobiletin, also found in orange juice (at concentrations of 0.35–1.6 µmol L−1 and 2.5–11 µmol L−1, respectively) [39, 40], have been shown to inhibit the OATP2B1-mediated uptake of estrone-3-sulfate in HEK293 cells at 1 µmol L−1 concentration [23]. The flavonoid constituents of apple juice include phlorizin (6–450 µmol L−1), epicatechin (41 µmol L−1), quercetin (4–23 µmol L−1) and kaempferol (0.5 µmol L−1) [37, 41, 42], of which quercetin and kaempferol have been found to inhibit the OATP2B1-mediated uptake of bromosulphophthalein in HEK293 cells with inhibition constants of 8.7 µmol L−1 and 15.1 µmol L−1, respectively [24]. Moreover, extracts of green tea, containing catechin and epicatechin, and ginkgo leaf, containing kaempferol and quercetin, have been found to inhibit OATP2B1 in vitro[43]. In addition, certain constituents of orange juice, e.g. 3,3',4',5,6,7,8-heptamethoxyflavone, tangeritin and nobiletin, have been shown to inhibit P-glycoprotein in vitro[44–46]. However, inhibition of the P-glycoprotein should increase, not decrease, the plasma concentrations of aliskiren [31]. Therefore, it appears unlikely that inhibition of the P-glycoprotein would play an important role in the interaction between orange juice and aliskiren. Apple juice (up to 5% of normal strength), on the other hand, has had no effect on P-glycoprotein activity in vitro[7].
The interactions of fruit juices with aliskiren might also be explained by other mechanisms, such as formation of insoluble complexes between fruit juice constituents and aliskiren. In addition, alteration of intestinal pH by organic acids might slightly increase the ionization of basic aliskiren (pKa 9.49) and thus decrease its already low passive permeability [26, 28, 29]. On the other hand, the transport activity of OATP2B1 has been found to be enhanced at acidic pH at least for some substrates [21]. Moreover, increase in intestinal fluid volume by an osmotic effect might decrease intestinal aliskiren concentration and thus slow its absorption. Furthermore, the carbohydrates of fruit juices might delay gastric emptying and therefore delay drug absorption. However, this mechanism seems unlikely, because the tmax of aliskiren was not extended. Grapefruit juice, orange juice and apple juice are all relatively acidic, with pH values of 3.3, 3.8 and 3.6, respectively, for the brands used in our previous grapefruit juice and aliskiren study [25] and the present study. The main organic acid in grapefruit juice and orange juice is citric acid, but both juices also contain traces of succinic and malic acids [47]. Apple juice contains mainly malic acid, but also lactic and citric acids [47]. Citric acid and lactic acid have not inhibited OATP2B1 at 10 mmol L−1 concentration [21], but it is not known whether malic acid or succinic acid inhibits OATP2B1. The carbohydrates sucrose, fructose and glucose are the main osmotic components of fruit juices, with grapefruit, orange and apple juice containing about 8 g, 9 g and 10 g of carbohydrates, respectively, per 100 g of juice in the brands used. Further studies are required to clarify the exact mechanisms of the pharmacokinetic interactions between fruit juices and aliskiren.
Because orange juice and apple juice markedly reduced the plasma concentrations of aliskiren and reduced its effect on plasma renin activity, it is possible that ingestion of aliskiren continuously with either of these juices may reduce the antihypertensive efficacy of aliskiren, at least in some patients. In the present study, no differences in haemodynamic effects were observed between the phases after a single dose of aliskiren in healthy volunteers. This can be explained by the delayed start of the blood pressure-lowering effect of aliskiren, which gradually reaches its maximum after 2 weeks of treatment [48, 49]. Marked interindividual variability existed in these interactions, even among this homogenous group of healthy volunteers. The variability is probably even larger among patients using aliskiren. Interestingly, a high-fat meal also reduces the AUC of aliskiren by about 70% [29]. To minimize intraindividual variability in aliskiren exposure because of the food effect, aliskiren is recommended to be taken in a routine pattern with regard to meals [48, 49].
In conclusion, both orange juice and apple juice markedly reduce the plasma concentrations and renin-inhibiting effect of aliskiren, probably by reducing its absorption from the gastrointestinal tract. The interactions may be caused by inhibition of the OATP2B1-mediated intestinal absorption of aliskiren by constituents of these juices, but further studies are required to clarify the mechanisms involved.
Acknowledgments
We thank Ms Kaisa Kurkinen, MSc, for aliskiren concentration measurements and Ms Eija Mäkinen-Pulli, Ms Lisbet Partanen and Mr Jouko Laitila for skilled assistance. This study was supported by grants from the Helsinki University Central Hospital Research Fund (Helsinki, Finland) and the Sigrid Jusélius Foundation (Helsinki, Finland).
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Bailey DG, Dresser GK. Interactions between grapefruit juice and cardiovascular drugs. Am J Cardiovasc Drugs. 2004;4:281–97. doi: 10.2165/00129784-200404050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bailey DG. Fruit juice inhibition of uptake transport: a new type of food-drug interaction. Br J Clin Pharmacol. 2010;70:645–55. doi: 10.1111/j.1365-2125.2010.03722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey DG, Spence JD, Munoz C, Arnold JM. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–9. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 4.Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, Brown MB, Guo W, Watkins PB. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–53. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yee GC, Stanley DL, Pessa LJ, Dalla Costa T, Beltz SE, Ruiz J, Lowenthal DT. Effect of grapefruit juice on blood cyclosporin concentration. Lancet. 1995;345:955–6. doi: 10.1016/s0140-6736(95)90700-9. [DOI] [PubMed] [Google Scholar]
- 6.Edwards DJ, Bellevue FH, 3rd, Woster PM. Identification of 6',7'-dihydroxybergamottin, a cytochrome P450 inhibitor, in grapefruit juice. Drug Metab Dispos. 1996;24:1287–90. [PubMed] [Google Scholar]
- 7.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 8.Neuhofel AL, Wilton JH, Victory JM, Hejmanowsk LG, Amsden GW. Lack of bioequivalence of ciprofloxacin when administered with calcium-fortified orange juice: a new twist on an old interaction. J Clin Pharmacol. 2002;42:461–6. [PubMed] [Google Scholar]
- 9.Lilja JJ, Backman JT, Laitila J, Luurila H, Neuvonen PJ. Itraconazole increases but grapefruit juice greatly decreases plasma concentrations of celiprolol. Clin Pharmacol Ther. 2003;73:192–8. doi: 10.1067/mcp.2003.26. [DOI] [PubMed] [Google Scholar]
- 10.Lilja JJ, Juntti-Patinen L, Neuvonen PJ. Orange juice substantially reduces the bioavailability of the beta-adrenergic-blocking agent celiprolol. Clin Pharmacol Ther. 2004;75:184–90. doi: 10.1016/j.clpt.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Lilja JJ, Raaska K, Neuvonen PJ. Effects of orange juice on the pharmacokinetics of atenolol. Eur J Clin Pharmacol. 2005;61:337–40. doi: 10.1007/s00228-005-0930-9. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz UI, Seemann D, Oertel R, Miehlke S, Kuhlisch E, Fromm MF, Kim RB, Bailey DG, Kirch W. Grapefruit juice ingestion significantly reduces talinolol bioavailability. Clin Pharmacol Ther. 2005;77:291–301. doi: 10.1016/j.clpt.2004.11.111. [DOI] [PubMed] [Google Scholar]
- 13.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–71. [PubMed] [Google Scholar]
- 14.Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004;308:438–45. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 15.Maeda T, Takahashi K, Ohtsu N, Oguma T, Ohnishi T, Atsumi R, Tamai I. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharmacol. 2007;4:85–94. doi: 10.1021/mp060082j. [DOI] [PubMed] [Google Scholar]
- 16.Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–70. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Miyazaki T, Kano T, Sugiura T, Kubo Y, Tsuji A. Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009;98:2529–39. doi: 10.1002/jps.21618. [DOI] [PubMed] [Google Scholar]
- 18.Shirasaka Y, Kuraoka E, Spahn-Langguth H, Nakanishi T, Langguth P, Tamai I. Species difference in the effect of grapefruit juice on intestinal absorption of talinolol between human and rat. J Pharmacol Exp Ther. 2010;332:181–9. doi: 10.1124/jpet.109.159756. [DOI] [PubMed] [Google Scholar]
- 19.Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8:787–802. doi: 10.2217/14622416.8.7.787. [DOI] [PubMed] [Google Scholar]
- 20.Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–60. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–8. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- 22.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Wah Yee S, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–23. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- 24.Mandery K, Bujok K, Schmidt I, Keiser M, Siegmund W, Balk B, König J, Fromm MF, Glaeser H. Influence of the flavonoids apigenin, kaempferol and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem Pharmacol. 2010;80:1746–53. doi: 10.1016/j.bcp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Tapaninen T, Neuvonen PJ, Niemi M. Grapefruit juice greatly reduces the plasma concentrations of the OATP2B1 and CYP3A4 substrate aliskiren. Clin Pharmacol Ther. 2010;88:339–42. doi: 10.1038/clpt.2010.101. [DOI] [PubMed] [Google Scholar]
- 26.Waldmeier F, Glaenzel U, Wirz B, Oberer L, Schmid D, Seiberling M, Valencia J, Riviere GJ, End P, Vaidyanathan S. Absorption, distribution, metabolism, and elimination of the direct renin inhibitor aliskiren in healthy volunteers. Drug Metab Dispos. 2007;35:1418–28. doi: 10.1124/dmd.106.013797. [DOI] [PubMed] [Google Scholar]
- 27.Vaidyanathan S, Camenisch G, Schuetz H, Reynolds C, Yeh CM, Bizot MN, Dieterich HA, Howard D, Dole WP. Pharmacokinetics of the oral direct renin inhibitor aliskiren in combination with digoxin, atorvastatin, and ketoconazole in healthy subjects: the role of P-glycoprotein in the disposition of aliskiren. J Clin Pharmacol. 2008;48:1323–38. doi: 10.1177/0091270008323258. [DOI] [PubMed] [Google Scholar]
- 28.Wood JM, Maibaum J, Rahuel J, Grütter MG, Cohen NC, Rasetti V, Rüger H, Göschke R, Stutz S, Fuhrer W, Schilling W, Rigollier P, Yamaguchi Y, Cumin F, Baum HP, Schnell CR, Herold P, Mah R, Jensen C, O'Brien E, Stanton A, Bedigian MP. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003;308:698–705. doi: 10.1016/s0006-291x(03)01451-7. [DOI] [PubMed] [Google Scholar]
- 29.Vaidyanathan S, Jarugula V, Dieterich HA, Howard D, Dole WP. Clinical pharmacokinetics and pharmacodynamics of aliskiren. Clin Pharmacokinet. 2008;47:515–31. doi: 10.2165/00003088-200847080-00002. [DOI] [PubMed] [Google Scholar]
- 30.Tapaninen T, Neuvonen PJ, Niemi M. Rifampicin reduces the plasma concentrations and the renin-inhibiting effect of aliskiren. Eur J Clin Pharmacol. 2010;66:497–502. doi: 10.1007/s00228-010-0796-3. [DOI] [PubMed] [Google Scholar]
- 31.Tapaninen T, Backman JT, Kurkinen K, Neuvonen PJ, Niemi M. Itraconazole, a P-glycoprotein and CYP3A4 inhibitor, markedly raises the plasma concentrations and enhances the renin-inhibiting effect of aliskiren. J Clin Pharmacol. doi: 10.1177/0091270010365885. doi: 10.1177/0091270010365885. [DOI] [PubMed] [Google Scholar]
- 32.Laitinen A, Niemi M. Frequencies of single nucleotide polymorphisms of SLCO1A2, SLCO1B3 and SLCO2B1 genes in a Finnish population. Basic Clin Pharmacol Toxicol. 2011;108:9–13. doi: 10.1111/j.1742-7843.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- 33.Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med. 2004;36:829–37. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J Nutr. 2005;135:48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81:495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 36.Ameer B, Weintraub RA, Johnson JV, Yost RA, Rouseff RL. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin Pharmacol Ther. 1996;60:34–40. doi: 10.1016/S0009-9236(96)90164-2. [DOI] [PubMed] [Google Scholar]
- 37.Tomás-Barberán FA, Clifford MN. Flavanones, chalcones and dihydrochalcones – nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1073–80. [Google Scholar]
- 38.Vanamala J, Reddivari L, Yoo KS, Pike LM, Patil BS. Variation in the content of bioactive flavonoids in different brands of orange and grapefruit juices. J Food Compost Anal. 2006;19:157–66. [Google Scholar]
- 39.Rouseff RL, Ting SV. Quantitation of polymethoxylated flavones in orange juice by high-performance liquid chromatography. J Chromatogr. 1979;176:75–87. doi: 10.1016/s0021-9673(00)92088-0. [DOI] [PubMed] [Google Scholar]
- 40.Sendra JM, Navarro JL, Izquierdo L. C18 solid-phase isolation and high-performance liquid chromatography/ultraviolet diode array determination of fully methoxylated flavones in citrus juices. J Chromatogr Sci. 1988;26:443–8. doi: 10.1093/chromsci/26.9.443. [DOI] [PubMed] [Google Scholar]
- 41.Young JF, Nielsen SE, Haraldsdóttir J, Daneshvar B, Lauridsen ST, Knuthsen P, Crozier A, Sandström B, Dragsted LO. Effect of fruit juice intake on urinary quercetin excretion and biomarkers of antioxidative status. Am J Clin Nutr. 1999;69:87–94. doi: 10.1093/ajcn/69.1.87. [DOI] [PubMed] [Google Scholar]
- 42.Schieber A, Keller P, Carle R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J Chromatogr A. 2001;910:265–73. doi: 10.1016/s0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- 43.Fuchikami H, Satoh H, Tsujimoto M, Ohdo S, Ohtani H, Sawada Y. Effects of herbal extracts on the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2006;34:577–82. doi: 10.1124/dmd.105.007872. [DOI] [PubMed] [Google Scholar]
- 44.Takanaga H, Ohnishi A, Yamada S, Matsuo H, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. Polymethoxylated flavones in orange juice are inhibitors of P-glycoprotein but not cytochrome P450 3A4. J Pharmacol Exp Ther. 2000;293:230–6. [PubMed] [Google Scholar]
- 45.Ikegawa T, Ushigome F, Koyabu N, Morimoto S, Shoyama Y, Naito M, Tsuruo T, Ohtani H, Sawada Y. Inhibition of P-glycoprotein by orange juice components, polymethoxyflavones in adriamycin-resistant human myelogenous leukemia (K562/ADM) cells. Cancer Lett. 2000;160:21–8. doi: 10.1016/s0304-3835(00)00549-8. [DOI] [PubMed] [Google Scholar]
- 46.Honda Y, Ushigome F, Koyabu N, Morimoto S, Shoyama Y, Uchiumi T, Kuwano M, Ohtani H, Sawada Y. Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2-mediated drug efflux. Br J Pharmacol. 2004;143:856–64. doi: 10.1038/sj.bjp.0706008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunha SC, Fernandes JO, Ferreira I. HPLC/UV determination of organic acids in fruit juices and nectars. Eur Food Res Technol. 2002;214:67–71. [Google Scholar]
- 48.Novartis. Tekturna Prescribing Information. Available at http://www.pharma.us.novartis.com/product/pi/pdf/tekturna.pdf (last accessed 20 January 2011)
- 49.European Medicines Agency. European Public Assessment Report for Rasilez. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/rasilez/rasilez.htm (last accessed 20 January 2011)


