Abstract
AIMS
Cannabis is the most prevalent illicit drug used worldwide and can be responsible for serious health defects in users. However, the risk related to cannabis consumption is not well established. The present study aimed to assess cannabis-related adverse events leading to hospitalization, and to estimate the corresponding annual risk for consumers.
METHODS
Participants were patients admitted to the public hospitals in the Toulouse area (France) between January 2004 and December 2007 in relation to the use of cannabis. Reasons for admission and other occurring events were identified through hospital discharge summaries. We described all observed adverse events (AEs) and estimated their regional incidence on the basis of cannabis consumption data.
RESULTS
We included 200 patients, and identified a total of 619 adverse events (AEs), one of which was lethal. Psychiatric disorders involved 57.7% of patients and accounted for 18.2% of AEs. Most frequent outcomes were central and peripheral nervous system disorders (15.8% of AEs), acute intoxication (12.1%), respiratory system disorders (11.1%) and cardiovascular disorders (9.5%). We estimated that in 2007 the incidence of cannabis-related AEs in the Midi-Pyrenees region ranged from 1.2 per 1000 regular cannabis users (95% confidence interval (CI) 0.7, 1.6) to 3.2 (95% CI 2.5, 3.9).
CONCLUSIONS
Cannabis use is associated with complications, considered to be serious since they lead to hospitalization. Beyond the well-known and widely investigated psychiatric events, serious cerebro and cardiovascular complications have been identified. These findings contribute to improve the knowledge of cannabis-related adverse events.
Keywords: adverse event, cannabis, hospitalization, pharmacoepidemiology
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The general knowledge on cannabis toxicity has improved, but quantitative data are still lacking.
Little is known about the somatic complications associated with cannabis exposure. Available data rest upon case reports and only a few studies have been conducted in this field.
Psychiatric disorders related to cannabis exposure are somehow still controversial.
WHAT THIS STUDY ADDS
Quantitative estimates are key to comprehending the risks of medical outcomes in cannabis users, in particular the somatic complications of use. Our study suggests that cannabis use has to be linked to serious health defects, particularly neurological and cardiovascular disorders.
Our study gives estimates of the annual incidence of cannabis-related hospitalizations.
Our study should contribute to enhancing the medical management of cannabis using patients.
Introduction
Cannabis remains the most prevalent illegal drug used worldwide, and the latest epidemiological data have reported 1.2 million regular cannabis users in France, half of whom were daily users [1, 2].
Available data provide keys to optimize the assessment and management of cannabis use disorders [3]. Studies have investigated cannabis-related psychiatric outcomes, whereas somatic complications remain unclear since a causal relationship is more difficult to establish. However, somatic events related to cannabis have been described over the past decade and case reports have underlined quite unexpected events, particularly cardiovascular events [4]. As early as the 1960s, sparse cases of arteriopathy, cerebral stroke and myocardial infarction were reported [5–7]. Such case reports have become more numerous since the early 2000s.
This study aimed to assess the serious adverse events related to the use of cannabis in a defined area (Midi-Pyrenees, France), between 2004 and 2007.
Methods
Premise
Any medical outcome that notably results in death or requires inpatient hospitalization or prolongation of existing hospitalization is considered a serious adverse event [8]. We can assume that hospitalizations associated with intoxication bear information related to the corresponding serious adverse events.
Design and procedures
This observational study was conducted at the six public hospitals of Toulouse (1 102 887 inhabitants), the capital city of Midi-Pyrenees (2 810 247 inhabitants), France, on hospitalizations recorded between January 1 2004 and December 31 2007, and potentially related to cannabis [9].
Setting
Data collection
Data from the national computer database for standardized hospital discharge summaries (PMSI, Programme de Médicalisation des Systèmes d'Information) were provided by the hospital department of medical information, with the approval of the national data protection committee, CNIL (approval number 909236, 09/08/2009). This database gathers basic patient characteristics (gender, date of birth and permanent identification number), days of admission and discharge, main and related diagnoses. Diagnoses are encoded according to the international classification of diseases, 10th revision (ICD-10) [10].
Population
Our population sample included inpatients of all medical and surgical wards of the six Toulouse hospitals, whose admission occurred during the study period and for whom diagnosis codes included the ICD-10 terms related to mental and behavioural disorders associated to the use of psychoactive drugs (F10 to F19 codes).
First, we selected hospitalizations during which ‘disorders due to use of cannabinoids’ (i.e. ICD-10 F12) or ‘disorders due to multiple drug use and use of other substances’ (i.e. ICD-10 F19) were reported (Figure 1). Then, we confirmed cannabis exposure through systematic review of medical history, discharge letters, or toxicological analysis results. We considered positive cannabis urinary dosage as validating evidence. Cannabis causal involvement was measured by two pharmacologists (EJ, MLM) through the method of causality assessment used to evaluate drug safety. Hospitalizations for which causality assessment was at least ‘possible’ made up the final study sample [11, 12].
Figure 1.
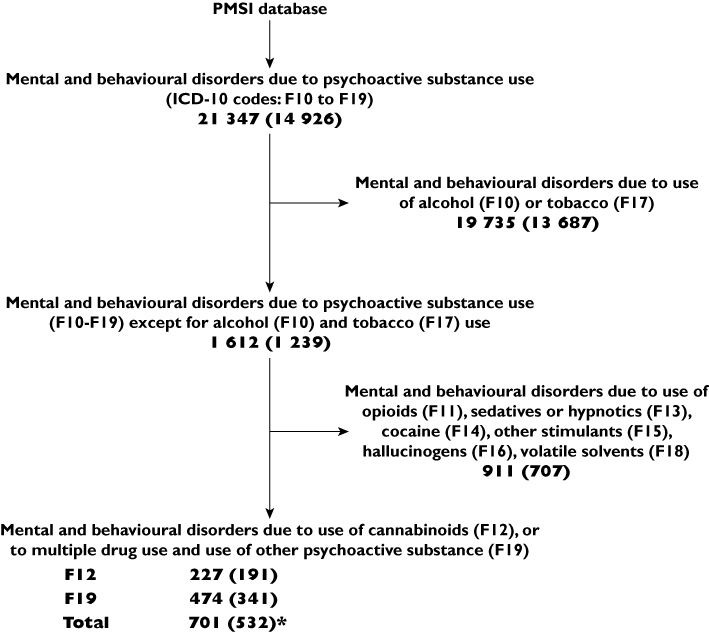
Selection of medical files potentially related to cannabis use (step 1, see § Methods). Bold figures correspond to the numbers of hospitalizations; the corresponding numbers of patients are in brackets. *For instance, we identified 701 hospitalizations related to the use of cannabinoids, which corresponded to a sample of 532 patients. ICD-10: WHO International classification of diseases, 10th revision. PMSI: Programme de médicalisation des systèmes d'information. F19: This category should be used when two or more psychoactive substances are known to be involved, but it is impossible to assess which substance is contributing most to the disorders. It should also be used when the exact identity of some or even all the psychoactive substances being used is uncertain or unknown, since many multiple drug users themselves often do not know the details of what they are taking
Therefore, a cannabis-related hospitalization was defined as a F12 or F19 coded hospitalization in which cannabis use was documented and identified as possibly related with the diagnosed outcomes.
Statistical methods
Descriptive analysis
We investigated three kinds of information: (i) patients (age, gender, personal and familial medico-surgical history), (ii) input services (medical discharge summaries and letters, and toxicological analyses) and (iii) events (categorized according to the World Health Organization adverse reaction terminology, WHO-ART) [13].
Estimates of incidence
It was not systematically possible to ascertain the degree of cannabis use in the identified adverse events. Therefore, we estimated the annual incidence among two reference populations of users provided by population based studies and considered: (i) all included patients as recent consumers (i.e. between one and nine consumptions over the previous 30 day period) and (ii) all included patients as regular consumers (i.e. 10 or more consumptions over the previous 30 day period). Data about cannabis consumption are provided by the national population based study ‘Baromètre Santé 2005’[14]. Incidence rates are expressed in a number of cases per 1000 (95% confidence interval (CI)). Data from the French national institute of statistics and economic surveys INSEE (Institut National de la Statistique et des Etudes Economiques) were used for annual demographic estimates [15].
Results
Participants
We confirmed and validated cannabis exposure in 41.9% (294/701) of the previously selected hospitalizations, which corresponded to 43.6% (232/532) of selected patients (Table 1).
Table 1.
Numbers of hospitalizations and patients at each stage of the method, depending on the type of hospitalization
| Numbers of hospitalizations (H) and patients (P) | ||||
|---|---|---|---|---|
| n (%) | ||||
| Use of cannabinoids | Use of multiple drugs | Total | ||
| Step 1: Selection (PMSI database) | H | 227 (32.4) | 474 (67.6) | 701 |
| P | 191 (35.9) | 341 (64.1) | 532 | |
| Step 2: Validation (Medical files and toxicology) | H | 200 (68.0) | 94 (32.0) | 294 |
| P | 168 (72.4) | 64 (27.6) | 232 | |
| Step 3: Inclusion (Imputability scores > Io) | H | 172 (76.8) | 52 (23.2) | 224 |
| P | 158 (79.0) | 42 (21.0) | 200 | |
Percentages were calculated at all steps among the respective total number of hospitalizations and patients.
The final sample included 224 hospitalizations (200 patients), corresponding to 75.8% of the 227 hospitalizations identified through F12 codes and to 11.0% of the 474 hospitalizations identified through F19 codes. Eleven hospitalizations (11 patients) were included on the basis of cannabinol positive urinary analyses, whereas there was no mention of cannabis use in the discharge letters.
Some hospitalizations (477) could not be included in the study. Validation was not possible for 407 hospitalizations (300 patients) because files lacked data to confirm the reasons for hospitalization (21.4%) or mentioned another reason rather than cannabis exposure (78.6%).
Inclusion was not possible for 70 hospitalizations (32 patients) because another diagnosis was identified (51.4%), or another drug was involved in the events (48.6%).
Main results
Among the 200 included patients, 153 (76.5%) were men. Mean age at admission was 28.0 years (95% CI 26.7, 29.3).
Annual incidence
The estimated incidence of cannabis-related hospitalizations in 2007 in Toulouse Urban Unit was 1.9 (95% CI 1.4, 2.3) per 1000 recent cannabis users and 3.2 (95% CI 2.5, 3.9) per 1000 regular cannabis users (Table 2).
Table 2.
Annual incidences in Toulouse urban unit (A) and region (B) of serious cannabis-related adverse events among recent and regular cannabis users
| A) Urban unit | ||||||
|---|---|---|---|---|---|---|
| Patients | Incidence (·10−3) | |||||
| Year | n | Recent users* | (95% CI) | Regular users† | (95% CI) | |
| 2004 | 25 | 0.67 | (0.41, 0.94) | 1.15 | (0.70, 1.60) | |
| 2005 | 41 | 1.08 | (0.75, 1.42) | 1.86 | (1.29, 2.43) | |
| 2006 | 61 | 1.59 | (1.19, 1.99) | 2.73 | (2.04, 3.41) | |
| 2007 | 73 | 1.87 | (1.44, 2.30) | 3.20 | (2.47, 3.94) | |
| B) Region | ||||||
|---|---|---|---|---|---|---|
| Hypothesis 1: ‘Cannabis users who needed to be hospitalized in Midi-Pyrenees were all admitted to a Toulouse teaching hospital.’ | ||||||
| Patients | Incidence (·10−3) | |||||
| Year | n | (95% CI) | Recent users* | (95% CI) | Regular users† | (95% CI) |
| 2004 | 25 | (5, 45) | 0.30 | (0.18, 0.42) | 0.52 | (0.31, 0.72) |
| 2005 | 41 | (21, 61) | 0.49 | (0.34, 0.63) | 0.83 | (0.58, 1.09) |
| 2006 | 61 | (41, 81) | 0.71 | (0.53, 0.89) | 1.22 | (0.92, 1.53) |
| 2007 | 73 | (53, 93) | 0.84 | (0.65, 1.03) | 1.44 | (1.11, 1.77) |
| Hypothesis 2: ‘Annual incidence is constant and observations made at urban unit scale can be proportionally reported to Midi-Pyrenees region.’ | ||||||
|---|---|---|---|---|---|---|
| Patients | Incidence (·10−3) | |||||
| Year | n | (95% CI) | Recent users* | (95% CI) | Regular users† | (95% CI) |
| 2004 | 56 | (13, 99) | 0.67 | (0.41, 0.94) | 1.15 | (0.70, 1.60) |
| 2005 | 91 | (47, 135) | 1.08 | (0.75, 1.42) | 1.86 | (1.29, 2.43) |
| 2006 | 136 | (92, 181) | 1.59 | (1.19, 1.99) | 2.73 | (2.04, 3.41) |
| 2007 | 162 | (117, 207) | 1.87 | (1.44, 2.30) | 3.20 | (2.47, 3.94) |
Recent use: Between one and nine uses during the last 30 days.
Regular use: 10 or more uses during the last 30 days.
Characteristics of observed adverse events (AEs)
Overall, there were 619 adverse events (Table 3). One of these led to death. Psychiatric disorders accounted for 19.2% of total AEs (119/619) and involved 57.5% of patients (115/200). Incidence of psychiatric outcomes was 2.9 per 1000 (95% CI 2.4, 3.5) among recent cannabis users and 5.0 per 1000 (4.1, 6.0) among regular cannabis users (Table 4). Central and peripheral nervous system disorders were the second most identified system-organ class, with 15.8% of total AEs (98/619) and 44.0% of patients (88/200) (Table 3). The term ‘intoxication’ was quoted 75 times (12.1% of total AEs and 37.5% of patients), nine of which were unintentional intoxications. Respiratory system disorders (11.1%) were observed among 31.0% of patients (62/200) and consisted of dyspnoea (16), haemoptysis (10) and spontaneous pneumothorax (7). The corresponding incidence was 1.6 per 1000 (95% CI 1.2, 2.0) among recent cannabis users. Cardiovascular disorders accounted for 9.5% of total AEs (59/619) and involved 29.0% of patients (58/200). Incidence of cardiovascular disorders was 1.5 per 1000 (95% CI 1.1, 1.9) among recent cannabis users and 2.6 per 1000 (95% CI 1.9, 3.2) among regular users. We recorded 17 extracardiac vascular disorders, four of which were cerebrovascular accidents. Two of them involved men aged 26 and 28 years, with neither personal nor familial cardiovascular history. The only cardiovascular risk factor identified was a long past cannabis consumption. One of these two patients has died. Although complete assessment was systematically carried out, no aetiology could be retained except for cannabis use. We also recorded 15 myocardial, endocardial, pericardial and valve disorders, seven of which were myocardial infarctions. There were two cases of thrombosis and one case of thromboangitis obliterans. A 36-year-old female patient was admitted for an atypical Buerger disease syndrome, with oligoarthritis that had been evolving for a year. She had a 10 pack-year smoking history and a 2 year history of chronic cannabinoid intoxication at the rate of two joints a day. The examinations highlighted a bilateral arteriopathy of the lower limbs.
Table 3.
Classification of all quoted adverse events (AEs) according to the WHO-ART
| AEs | Patients per AE | |||
|---|---|---|---|---|
| (n = 619) | (n = 200) | |||
| WHO-ART system-organ classes | n | % | n | % |
| Psychiatric disorders | 119 | 19.2 | 115 | 57.5 |
| Central and peripheral nervous system disorders | 98 | 15.8 | 88 | 44.0 |
| Poison specific terms | 75 | 12.1 | 75 | 37.5 |
| Respiratory system disorders | 69 | 11.1 | 62 | 31.0 |
| Cardiovascular disorders | 59 | 9.5 | 58 | 29.0 |
| Cardiovascular disorders, general | 27 | 4.4 | 26 | 13.0 |
| Vascular (extracardiac) disorders | 17 | 2.7 | 17 | 8.5 |
| Myo-, endo-, pericardial and valve disorders | 15 | 2.4 | 15 | 7.5 |
| Gastro-intestinal system disorders | 55 | 8.9 | 53 | 26.5 |
| Body as a whole – general disorders | 52 | 8.4 | 52 | 26.0 |
| Autonomic nervous system disorders | 39 | 6.3 | 36 | 18.0 |
| Secondary terms – events | 20 | 3.2 | 20 | 10.0 |
| Vision disorders | 11 | 1.8 | 11 | 5.5 |
| Resistance mechanism disorders | 5 | 0.8 | 5 | 2.5 |
| Musculo-skeletal system disorders | 3 | 0.5 | 3 | 1.5 |
| Obstetrics | 3 | 0.5 | 3 | 1.5 |
| Reproductive disorders, female | 2 | 0.3 | 2 | 1.0 |
| Neonatal and infancy disorders | 1 | 0.2 | 1 | 0.5 |
| Other system-organ classes | 11 | 1.8 | 11 | 5.5 |
| Liver and biliary system disorders | 3 | 0.5 | 3 | 1.5 |
| Red blood cell disorders | 2 | 0.3 | 2 | 1.0 |
| Urinary system disorders | 2 | 0.3 | 2 | 1.0 |
| Skin and appendages disorders | 1 | 0.2 | 1 | 0.5 |
| Metabolic and nutritional disorders | 1 | 0.2 | 1 | 0.5 |
| Platelet, bleeding and clotting disorders | 1 | 0.2 | 1 | 0.5 |
| Other | 1 | 0.2 | 1 | 0.5 |
Column 1 (‘AEs’) gives the numbers of all AEs whereas column 2 (‘Patients per AE’) gives the number of patients who have developed these AEs. A higher number in column 1 than in the corresponding column 2 means that some patients have developed the same AE several times. For instance, if a given patient was hospitalized four times for the same reason, there will be four AEs counted in column 1 and one patient per AE counted in column 2.
Table 4.
Incidence of adverse events of the nine most represented WHO-ART system-organ classes
| Incidence (·10−3) | |||||
|---|---|---|---|---|---|
| WHO-ART system-organ classes | Patients (n) | Recent users | (95% CI) | Regular users | (95% CI) |
| Psychiatric disorders | 115 | 2.94 | (2.41, 3.48) | 5.05 | (4.13, 5.97) |
| Central and peripheral nervous system disorders | 88 | 2.25 | (1.78, 2.72) | 3.86 | (3.06, 4.67) |
| Poison specific terms | 75 | 1.92 | (1.49, 2.35) | 3.29 | (2.55, 4.04) |
| Respiratory system disorders | 62 | 1.59 | (1.19, 1.98) | 2.72 | (2.04, 3.40) |
| Cardiovascular disorders | 58 | 1.48 | (1.10, 1.87) | 2.55 | (1.89, 3.20) |
| Gastro-intestinal system disorders | 53 | 1.36 | (0.99, 1.72) | 2.33 | (1.70, 2.95) |
| Body as a whole – general disorders | 52 | 1.33 | (0.97, 1.69) | 2.28 | (1.66, 2.90) |
| Autonomic nervous system disorders | 36 | 0.92 | (0.62, 1.22) | 1.58 | (1.06, 2.10) |
| Secondary terms – events | 20 | 0.51 | (0.29, 0.74) | 0.88 | (0.49, 1.26) |
Incidence was calculated among the estimated population of recent cannabis users and among the population of regular cannabis users in a Toulouse urban unit.
We identified two female reproductive disorders and one intra-uterine growth restriction (IUGR).
Discussion
Cannabis consumption is associated with a large range of adverse events. More than half of patients presented psychiatric disorders, and almost a third presented respiratory system disorders or cardiovascular disorders. Cannabis-related hospitalizations rose steadily during the study period. In 2007, the annual incidence of cannabis-related serious adverse events was estimated to be 3.2 per 1000 regular users (95% CI 2.5, 3.9).
Interpretation
Sample characteristics
Age and gender characteristics were similar to those observed in other studies, except for the proportion of patients over 45 years (7.0% vs. 2.2% in French population-based studies) [14]. Older people are more likely to be hospitalized due to impairment of their health status with age, and that could explain this difference.
Serious cannabis-related disorders
Psychiatric disorders were expected to be the most frequent disorders since cannabis is mainly used for its psychoactive effects, in a recreational context [16].
We observed a total of seven myocardial infarctions (MI). In one case, diagnosis was asserted in view of several painful episodes which systematically occurred within an interval of 30 min after the use of cannabis. Similar cases of MI in patients with moderate tabagism and chronic use of cannabis are reported in the literature [17]. Cannabis has been shown to trigger the occurrence of angina pectoris symptoms after physical effort among patients with a history of coronary disease or stable angina pectoris, earlier than the use of tobacco [18]. In another study, risk of MI was almost 5-fold greater in patients who had acknowledged cannabis use (4.8, 95% CI 2.4, 9.5) [19]. We observed three cases of thrombosis, one of which was a thromboangitis obliterans. Cases of youthful arteritis with no precisely identified aetiology are numerous in the literature. Such arteritis was reported as early as the 1960s but it had not been attributed to cannabis until 1999 [20]. We observed four cerebrovascular accidents in patients under 40 years. This differed from the expected mean age of stroke patients in France, which ranged between 75 and 80 years in 2007 [21, 22]. Two of these cerebrovascular accidents occurred in males aged less than 30 years and one was fatal. There is no similar case reported in the literature. Strokes are more common in young adults who use stimulant drugs like cocaine or methamphetamine, and such users are more likely to be found among regular cannabis users. However, a thorough investigation of the included cases revealed neither consumption nor risk factor (family or personal history) other than heavy cannabis use. The responsibility of cannabis in the occurrence of coronary arteritis and cerebral strokes is still uncertain. However, in young adults, practitioners must think of possible chronic cannabis intoxication.
Respiratory disorders related to cannabis consumption are similar to those of tobacco and result in cough, expectoration, respiratory tract inflammation and bronchial cell growth modification that can lead to chronic bronchitis or cancer. Concomitant use of cannabis precipitates the occurrence of tobacco-induced respiratory complications [23]. We observed 10 cases of haemoptysis, probably related to adulterants rather than cannabinoids. Literature reports one case of fatal alveolar haemorrhage in an exclusive cannabis user, and it was attributed to the anhydride acids released during the combustion of artisanal pipe plastics [24]. Inhaled cannabis has been shown to induce a concentration of carboxyhaemoglobin 5-fold greater than tobacco and thus it induces more important cellular hypoxaemia [25].
We identified a case of intra-uterine growth restriction. Available data show that cannabinoids pass through the fetoplacental barrier and act directly on the embryo as early as the blastocyst stage [26]. Chronic and substantial exposure to cannabis may reduce newborn size and weight as well as the gestation period, and increase risk of childbirth complications. Cardiac malformations were reported as congenital diseases potentially induced by cannabis [27].
Cannabis toxicity varies depending on the type of exposure (acute or chronic intoxication, route of administration, quantity), and upon the quality of cannabis preparations which may vary extensively depending on time and place of supply [16, 28]. It is not possible to determine the origin and composition of cannabis preparations, which plays a confusing role.
Strengths and limitations
Detection of cannabis-related hospitalizations
Data are likely to represent an incomplete but accurate capture of cannabis-related hospital admissions. Indeed, lack of cannabis-related diagnoses in the computer database made files undetectable. Besides, validation could only be assessed when cannabis use was mentioned in the discharge summaries. Patients may have failed to report cannabis use and their physicians to inquire about, to test for, or to connect it to the reason for hospitalization. We observed that cannabis screening had been performed in only 19.6% of the hospitalizations which were reported as cannabis-related. These factors operate to under-estimate the incidence. Furthermore, only proven cases were included, which was not the case for hospitalizations connected to traffic accidents, considering the lack of evidence to connect them to cannabis use. Similarly, in the case of polyconsumption, we only included the hospitalizations in which cannabis was clearly pointed out. Some hospitalizations were set aside because of missing discharge letters, probably due to anticipated or not allowed patient discharge. However, only hospitalizations associated with events that had physical and psychological impact were relevant and a patient capable of leaving the hospital on his own probably did not suffer from seriously incapacitating events. For these reasons, captured data are most likely to represent serious cannabis-related hospitalizations.
Reference population
It is difficult to assess precise epidemiological reference data in a context of illicit drug consumption. Moreover, it is also difficult to compute the population that is likely to be admitted to the public hospitals of the Toulouse area.
Estimates made among a Toulouse urban unit were complemented with regional estimates, following two assumptions (Table 2): (i) firstly, we considered that all hospitalized cannabis users living in the region had been admitted to at least one of the six participating hospitals (hypothesis 1) and (ii) secondly, we considered that hospitalized cannabis users were admitted to Midi-Pyrenees health services with constant prevalence and we applied urban unit incidence rates to the whole region, proportionally (hypothesis 2).
We estimated the population of cannabis users in the Toulouse urban unit on the basis of cannabis consumption national rates. In addition, although cannabis use was clearly identified as daily use in most analysed medical files, we chose recent or regular users rather than daily users as the reference population, because frequency of use was not systematically specified. In so doing, we probably overestimated our reference populations of cannabis users.
Are regional estimates relevant?
Although our study was conducted in a restricted geographical area, its results are consistent with those reported by the regional emergency observatory of Midi-Pyrenees. In 2007, we included 88.5% (92.2% in 2006) of the hospitalizations recorded in all of Midi-Pyrenees emergency departments. In the French healthcare system, emergency departments (EDs) are graduated depending on their reception and treatment capabilities; and the EDs attached to Toulouse teaching hospitals have the highest grade. Given that Toulouse is one of the most densely populated French cities, as well as the capital city of Midi-Pyrenees, which is the largest region of France, we can reasonably assume that our estimates are relevant [22].
In addition, the incidence of health complications related to illicit drugs can hardly be assessed generally. Until now, most large-scale epidemiological studies which evaluated the extent of adverse drug reactions related to hospitalizations were restricted to prescription drugs [29, 30]. Studying serious health outcomes related to illicit drugs requires a case by case analysis and does not allow automatic data processing of heavy computer databases. Moreover, currently, no nationwide database is available or easily workable in France.
In conclusion, our results suggest that the risk to develop serious adverse events is high in cannabis using populations. Although somatic complications are less frequent than psychiatric complications, they are serious disorders. Neurological, cardiovascular and respiratory disorders are particularly involved.
We probably underevaluated the incidence of cannabis-related serious outcomes but we have provided the first available estimates. We have looked beyond the well-known psychiatric adverse reactions and provided estimates of rates of serious cardiovascular and respiratory events.
These results should encourage similar and perhaps better control research studies in other settings. Furthering the knowledge in the field of cannabis-related complications is necessary. The awareness thus generated should contribute to improve case detection and lead to care adjustments. The education of people at large is another point to consider.
Acknowledgments
Financial support was provided by the French Mission Interministérielle de Lutte contre les Drogues et Toxicomanies (MILDT) and by the French drug agency AFSSaPS (Agence Française de Sécurité Sanitaire des Produits de Santé). This work was awarded first prize in the young researchers' award at the 13th French annual congress of physiology, pharmacology and therapeutics (P2T) by the French pharmacology and therapeutics society SFPT (Marseille, 15–17 April 2009). We thank Pascale Morandi for her help in reviewing the English.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.United Nations Office of Drugs and Crime (UNODC) World drug report 2009. United Nations publications, 314 p. Available at http://www.unodc.org/documents/wdr/WDR_2009/WDR2009_eng_web.pdf (last accessed 6 August 2010)
- 2.Beck F, Legleye S, Spilka S. Niveaux d'usage et profils des usagers en France en 2005. In: Costes JM, Cannabis, Données Essentielles, editors. Martineau H, Ades JE. Saint-Denis: Observatoire français des drogues et toxicomanies (OFDT); 2007. pp. 20–9. Available at http://www.ofdt.fr/BDD/publications/docs/cdecomp.pdf (last accessed 6 August 2010) [Google Scholar]
- 3.Winstock AR, Ford C, Witton J. Assessment and management of cannabis use disorders in primary care. BMJ. 2010;340:c1571. doi: 10.1136/bmj.c1571. Review. [DOI] [PubMed] [Google Scholar]
- 4.[No authors listed] Cannabis and the cardiovascular system. Br Med J. 1978;1:460–1. [PMC free article] [PubMed] [Google Scholar]
- 5.Sterne J, Ducastaing C. [Arteritis caused by Cannabis indica] Arch Mal Coeur Vaiss. 1960;53:143–7. French. [PubMed] [Google Scholar]
- 6.Mohan H, Sood GC. Conjugate deviation of the eyes after Cannabis indica intoxication. Br J Ophthalmol. 1964;48:160–1. doi: 10.1136/bjo.48.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles R, Holt S, Kirkham N. Myocardial infarction and marijuana. Clin Toxicol. 1979;14:433–8. doi: 10.3109/15563657909010604. [DOI] [PubMed] [Google Scholar]
- 8.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) London: European Medecines Agency; 1996. ICH topic E6 (R1): Guideline for good clinical practice (CPMP/ICH/135/95) [Google Scholar]
- 9.Institut National de la Statistique et des Etudes Economiques (INSEE) [Internet]. Statistiques locales, chiffres clés sur un territoire [updated 01/12/2009] 2009. INSEE. Available at http://www.insee.fr/fr/bases-de-donnees/default.asp?page=statistiques-locales.htm (last accessed 6 August 2010)
- 10.World Health Organization [Internet] International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Version for 2007; c1994/2006 [update 05/04/2006] World Health Organization. Available at: http://apps.who.int/classifications/apps/icd/icd10online/ (last accessed 6 August 2010)
- 11.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 12.Wiholm BE, Olsson S, Moore N, Waller P. Spontaneous reporting systems outside the US. In: Strom BL, editor. Pharmacoepidemiology. 3rd edn. Chichester: Wiley; 2000. pp. 175–92. [Google Scholar]
- 13.WHO. International Monitoring of Adverse Reactions to Drugs: Adverse Reaction Terminology. Uppsala: WHO Collaborating Centre for International Drug Monitoring; 1992. [Google Scholar]
- 14.Beck F, Legleye S, Spilka S. Cannabis, cocaïne, ecstasy: entre expérimentation et usage régulier. In: Cormand MF, editor. Baromètre santé 2005. Attitudes et comportement de santé. Saint-Denis: Institut national de prévention et d'éducation pour la santé (INPES), Baromètres santé; 2007. pp. 169–221. Available at http://www.inpes.sante.fr/CFESBases/catalogue/pdf/1109.pdf (last accessed 6 August 2010) [Google Scholar]
- 15.Institut National de la Statistique et des Etudes Economiques (INSEE) [Internet] Estimation de population par sexe et âge quinquennal, Années 1990–2007 [updated 05/2008] 2008. INSEE. Available at: http://www.insee.fr/fr/themes/detail.asp?reg_id=99&ref_id=estim-pop (last accessed 6 August 2010)
- 16.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–91. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 17.Aryana A, Williams MA. Marijuana as a trigger of cardiovascular events: speculation or scientific certainty? Int J Cardiol. 2007;118:141–4. doi: 10.1016/j.ijcard.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Aronow WS, Cassidy J. Effect of marihuana and placebo-marihuana smoking on angina pectoris. N Engl J Med. 1974;291:65–7. doi: 10.1056/NEJM197407112910203. [DOI] [PubMed] [Google Scholar]
- 19.Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–9. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- 20.Combemale P, Consort T, Denis-Thelis L, Estival JL, Dupin M, Kanitakis J. Cannabis arteritis. Br J Dermatol. 2005;152:166–9. doi: 10.1111/j.1365-2133.2005.06340.x. [DOI] [PubMed] [Google Scholar]
- 21.Bonnaud I, Giraudeau B, Julie V, Soulat L, Beaufils JM, Brock T, Goralski M, Perrotin D. les médecins référents membres du GEUC. [Epidemiology and management of stroke patients in emergency departments of the Centre region of France] Rev Neurol. 2005;161:311–7. doi: 10.1016/s0035-3787(05)85037-4. French. [DOI] [PubMed] [Google Scholar]
- 22.Ducasse A. (Dir), Rapport annuel sur l'activité des structures d'urgence en Midi-Pyrénées 2008, Observatoire Régional des Urgences Midi-Pyrénées (ORU-MIP) 2009. 169p. Available at http://www.oru-mip.fr/docs/urg2008.pdf (last accessed 6 August 2010)
- 23.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221–8. doi: 10.1001/archinte.167.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert O, Mathieu D, Hanquet O, Lardinois I, Cornut P, Pierard P, Van Meerhaeghe A. [Hemoptysis in a young man] Rev Mal Respir. 2006;23:471–6. doi: 10.1016/s0761-8425(06)71820-4. [DOI] [PubMed] [Google Scholar]
- 25.Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101–6. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- 26.Park B, McPartland JM, Glass M. Cannabis, cannabinoids and reproduction. Prostaglandins Leukot Essent Fatty Acids. 2004;70:189–97. doi: 10.1016/j.plefa.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Steinberger EK, Ferencz C, Loffredo CA. Infants with single ventricle: a population-based epidemiological study. Teratology. 2002;65:106–15. doi: 10.1002/tera.10017. [DOI] [PubMed] [Google Scholar]
- 28.Delourme J, Delattre C, Godard P, Steenhouwer F, Just N. [Respiratory consequences of inhalation of adulterated cannabis] Rev Mal Respir. 2009;26:552–6. doi: 10.1016/s0761-8425(09)74675-3. French. [DOI] [PubMed] [Google Scholar]
- 29.van der Hooft CS, Sturkenboom MC, van Grootheest K, Kingma HJ, Stricker BH. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf. 2006;29:161–8. doi: 10.2165/00002018-200629020-00006. [DOI] [PubMed] [Google Scholar]
- 30.Patel H, Bell D, Molokhia M, Srishanmuganathan J, Patel M, Car J, Majeed A. Trends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998–2005. BMC Clin Pharmacol. 2007;7:9. doi: 10.1186/1472-6904-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


