Abstract
AIMS
To evaluate whether good statin adherence is associated with a reduced incidence of major coronary events (MCEs) among diabetic patients with and without coronary heart disease (CHD).
METHODS
Using data derived by linkage of nationwide health databases in Finland, we conducted a nested case–control analysis of 3513 cases with an MCE, a composite of acute myocardial infarction and/or coronary revascularization, and 20 090 matched controls identified from a cohort of 60 677 statin initiators with diabetes. Cases and controls were matched according to gender, time of cohort entry and duration of follow-up and further classified to two risk groups according to the presence of CHD at statin initiation. The incidence of MCEs was compared between patients with good statin adherence (the proportion of days covered ≥80%) and patients with poor statin adherence (<80%). Odds ratios (OR) for MCEs were estimated by conditional logistic regression adjusting for several covariables.
RESULTS
Good statin adherence was associated with a reduced incidence of MCEs in those with prior CHD [OR 0.84 (95% CI 0.74–0.95)] and in those without it [OR 0.86 (95% CI 0.78–0.95)]. The association persisted among those followed up for 5 years or longer [OR 0.77 (95% CI 0.58–1.02) and OR 0.79 (95% CI 0.66–0.94) respectively]. In sensitivity analyses, a reduced MCE incidence was observed also in those without any documented cardiovascular disease (CVD) at statin initiation [OR 0.87 (95% CI 0.78–0.96) overall and OR 0.80 (95% CI 0.66–0.97) for those followed up 5 years or longer].
CONCLUSIONS
In patients with diabetes, good adherence to statins predicts reduced incidence of MCEs irrespective of the presence of CHD at statin initiation.
Keywords: adherence to medical regimen, diabetes mellitus, myocardial infarction, myocardial revascularization, statins
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Statin therapy is recommended in diabetes to lower the risk of coronary events. However, large randomized trials of statins performed specifically among diabetic patients have produced conflicting results. No long-term follow-up studies are available on the incidence of major coronary events and adherence to statin therapy in real-life patients with diabetes.
WHAT THIS STUDY ADDS
The study results demonstrate that good adherence to statins is associated with a reduced risk of major coronary events in patients with diabetes, irrespective of the presence of coronary heart disease at statin initiation. This study provides further justification for maintenance statin therapy in diabetes.
Introduction
Cardiovascular disease (CVD) is the major cause of morbidity and mortality in patients with diabetes. Thus, it is important to modify the multiple risk factors of CVD, including dyslipidaemia [1]. The most common pattern of dyslipidaemia in type 2 diabetes is the elevation of triglycerides combined with decreased high-density lipoprotein (HDL) cholesterol. As the absolute low-density lipoprotein (LDL) cholesterol concentration is usually not different from concentrations in non-diabetic persons type 2 diabetic patients tend to have higher levels of small, dense LDL possibly related to the high atherogenicity in diabetes [2]. Statins primarily reduce LDL cholesterol and to a lesser extent they also reduce triglycerides and elevate the HDL cholesterol concentration [3]. In general, the role of statins in the prevention of cardiac events is well established in patients with dyslipidaemia [4–8], especially in patients with coronary heart disease (CHD).
The risk of myocardial infarction in diabetic patients without prior CHD is similar to the risk in non-diabetic patients with CHD [9, 10]. The most recent European guideline recommended that statin therapy should be initiated in all patients with diabetes and overt CVD, and in the absence of CVD, it should be considered for all type 1 patients aged over 40 years and for all adult patients with type 2 diabetes whose total cholesterol is greater than 3.5 mmol L−1[11]. These recommendations are mainly based on subgroup analyses (and meta-analyses [12, 13]) of randomized controlled trials (RCTs) [4, 6, 8]. Few studies have been performed specifically in diabetic patients [14, 15]. Large RCTs in diabetic patients have produced conflicting results. In the CARDS study, atorvastatin prevented cardiovascular end points in patients with no history of CVD [14] whereas in the ASPEN study, no preventive effect of atorvastatin was noted irrespective of whether the patients had previous CHD [15].
Because of the selection and meticulous follow-up of the subjects, RCTs have limited external validity. In real life, suboptimal adherence to statin treatment has been noted in many patient groups [16–19], including patients with diabetes [20], which questions the effectiveness of statins. We performed a nationwide, register-based study to investigate whether good adherence to statins would be associated with a risk reduction of major coronary events (MCEs) in patients with diabetes. We predicted that prior CHD would act as a strong confounder and potentially an effect modifier. Therefore, we analysed separately those patients with and without documented CHD at statin initiation.
Methods
Data sources
We used data from administrative health databases generated through the universal healthcare and drug reimbursement systems covering all 5.3 million residents of Finland. We identified prescription records with the National Prescription Register, run since 1994 and managed by the Social Insurance Institution (SII) [21]. This Register contains records of prescription drug purchases reimbursed to residents in non-institutional settings. For each purchase, the data included the dispensing date, the Anatomical Therapeutic Chemical classification code [22], the strength and the quantity dispensed. Patients staying in a public nursing home or hospital without interruption for over 90 days are not eligible for drug reimbursement and their purchases are not registered. We identified these patients from the SII register. For identifying patients entitled to higher rates of reimbursement because of certain severe, chronic conditions, such as CHD, diabetes, pulmonary disorders, rheumatoid arthritis and organ transplantations, we used the SII Special Reimbursement Register introduced in 1964. To be eligible for special reimbursement, a patient's condition must meet explicit predefined criteria and a written certificate is required from the treating physician. Finally, we identified MCEs and other covariables from the Hospital Discharge Register that included individual clinical and administrative data on primary and secondary discharge diagnoses and surgical procedures, and the admission and discharge dates. The register covers all Finnish hospitals. The ninth revision of the International Classification of Diseases (ICD-9) was in use between 1986 and 1995 and the 10th revision (ICD-10) since 1 January 1996. Procedure codes follow the Finnish classification of diagnostic, therapeutic and surgical procedures. The validity of the Finnish Hospital Discharge Register for hard CHD events has been reported earlier [23]. The above databases were linked anonymously using encrypted personal identifiers.
Study design
We conducted a nested case–control analysis of the relationship between statin adherence and incidence of MCEs within a cohort of community-dwelling patients with diabetes initiating statin therapy between 1995 and 2007 in Finland. We chose a nested case–control design in order to increase efficiency of the study and to enhance equal assessment of adherence, operationalized as the proportion of days covered (PDC), for those cohort members experiencing an MCE and those not experiencing it.
Cohort definition
The population considered eligible for the study cohort comprised diabetic patients (type 1 or 2) aged 45 to 75 years, as identified in the Special Reimbursement Register prior to statin initiation, who were dispensed their first statin prescription between 1 January 1995 and 31 December 2007 (Figure 1). The first statin dispensation refers to having no statins dispensed during the preceding 365 days. We defined the date of the first dispensing of any statin as the cohort entry date. Patients with a history of organ transplantation according to the Special Reimbursement Register before cohort entry were excluded because of the complexity of their pharmacotherapy and increased tendency for hospitalizations. We followed up cohort members until they experienced an MCE or were censored because of liver disease (discharge diagnosis of ICD-10 K70-77 or ICD-9 070X, 0706D, 0988B, 1305A, 570X-573X), organ transplantation, long-term institutionalization, 76th birthday, death or 31 December 2007, whichever came first.
Figure 1.
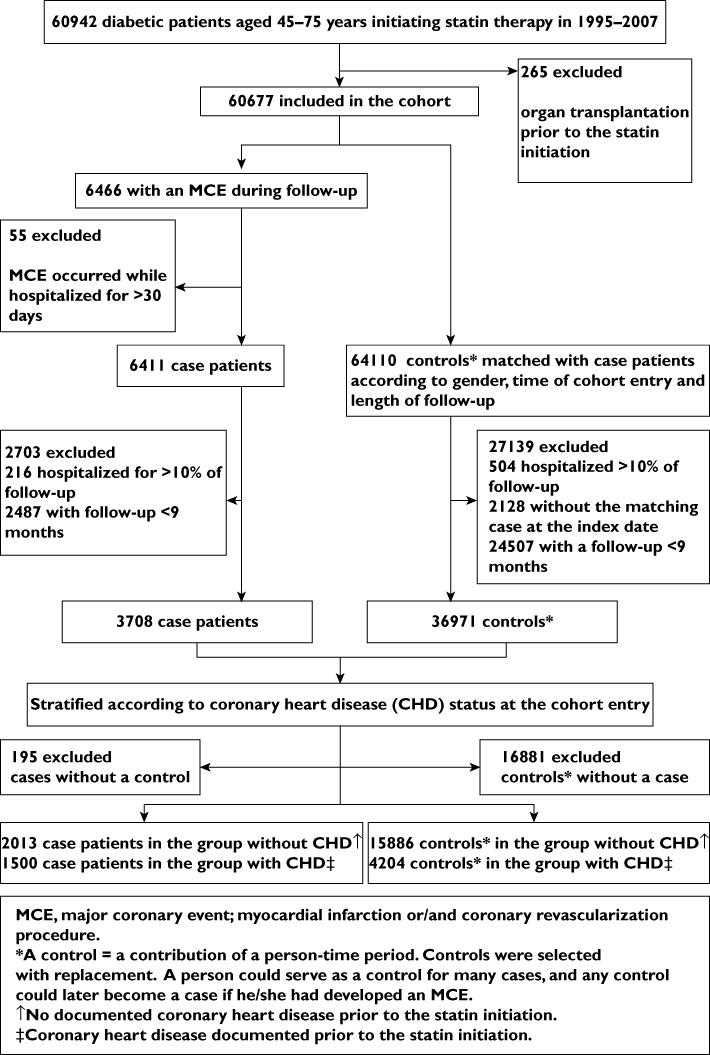
Study flow chart
Case ascertainment
Cases were defined as those who experienced a composite of MCEs, including hospitalization for an acute myocardial infarction (a primary or secondary discharge diagnosis of ICD-10 I21, I22, or ICD-9 410.x) and/or for coronary revascularization (procedure codes for coronary artery bypass grafting, angioplasty or stenting). We defined the date of the first MCE as the index date. As the Prescription Register does not capture drugs used in hospitals, we excluded cases with an MCE occurring during a hospital stay that lasted over 30 days in order to reduce the potential for immeasurable time bias [24].
Controls
Using incidence density sampling, we randomly selected 10 controls for each MCE case. We matched controls to cases for gender and, to control for secular trends, for time of cohort entry (±60 days). We selected controls from the risk set for each case consisting of cohort members who had not experienced an MCE by the date that resulted in the same duration of follow-up as for the case and assigned the above date as the index date for the controls. We selected controls with replacement; a patient could serve as a control for many cases and any control could later become a case if he/she had developed an MCE.
To reduce exposure misclassification [24], we excluded cases and controls who had been hospitalized for more than 10% of their follow-up time (Figure 1). In a pilot study, we observed that monthly MCE rates, especially those of revascularization procedures, were high during the first months of statin therapy, then declined steeply and stabilized at around 9 months [25]. We interpreted this as a sign for protopathic bias [26] and excluded all cases and controls with a follow-up of less than 9 months from the analysis.
We classified cases and controls into two risk groups according to the presence of CHD at statin initiation. Those patients who had a hospital discharge diagnosis of myocardial infarction, angina or a coronary revascularization procedure performed during a 7-year period prior to the cohort entry as well as those having ever been eligible to special reimbursement for CHD (since 1986) were assigned to the group with prior CHD. We categorized cases and controls who did not meet any of these criteria into the group without prior CHD.
Assessment of adherence to statins
We identified reimbursed statin purchases from the Prescription Register and assessed statin adherence, defined as PDC [27, 28], for the follow-up period from the cohort entry to the index date. Assuming a dosage of one tablet per day, we calculated the PDC with a statin by dividing the number of days on which the patient had a statin available by the number of days of follow-up multiplied by 100. During the last 90 days of the follow-up, we took into account the number of tablets covering the time from the dispensing date to the index date. The period for calculating PDC was the same for each matched set because of matching for the length of follow-up. Using a conventional cut-off value of 80% [18, 29, 30], we defined adherence a priori as a two-category variable: good (PDC ≥ 80%) and poor adherence (PDC < 80%). We analysed statins as a group, and switching between various statins was considered as continuation of therapy. To further characterize exposure to statins, we calculated the equivalent dose of the initiating statin as a simvastatin equivalent as presented by Law et al. [31].
Statistical analysis
We characterized cases and controls using descriptive statistics. We weighted the descriptive statistics for controls by the inverse of the number of controls per case in order to account for the variable number of controls in each matched set. This corresponded to standardizing the number of controls to one per case. We used conditional logistic regression to estimate age-adjusted odds ratios (OR) and multivariable-adjusted odds ratios (AOR) with their 95% confidence intervals (CI) separately for the two risk groups.
For the group without prior CHD, the multivariable logistic regression model included age and duration of diabetes (continuous variables) at index date, type of antidiabetic medication [oral only (biguanides, sulfonylureas, rosiglitazone, pioglitazone, guar gum, repaglinide and/or nateglinide), insulin only, both oral therapy and insulin, or no pharmacotherapy] and other medications [fibrates, warfarin, hormone replacement therapy, antihypertensive medication (diuretics, β-adrenoceptor blockers, calcium channel blockers, ACE inhibitors or angiotensin receptor blockers) and the number of different cardiovascular drugs (antithrombotic therapy, digoxin/antiarrhythmic drugs/nitrates, miscellaneous antihypertensive drugs, diuretics, pentoxifylline, β-adrenoceptor blockers, calcium channel blockers and ACE inhibitors/angiotensin receptor blockers)] during the 365 days prior to cohort entry and comorbidities during the 7 years prior to cohort entry. These comorbidities were cerebrovascular disease or peripheral atherosclerosis, congestive heart failure and microangiopathic complications (retinopathy, neuropathy, nephropathy or dialysis) indicated by the applicable ICD codes in the Hospital Discharge Register and moderate to severe hypertension and rheumatoid arthritis identified with the Special Reimbursement Register. The final model for the group with prior CHD included the above variables. In this model, however, we included duration of CHD (<2 years or ≥2 years) and treated age (5-year grouping) and duration of diabetes (<10 years or ≥10 years) as categorical variables. Additionally, we considered the presence of familial hypercholesterolaemia and pulmonary disorders (asthma or chronic obstructive pulmonary disease) according to the Special Reimbursement Register, use of thiazolidinediones, clopidogrel, spironolactone and antidepressants, and socioeconomic status (nine-category variable based on employment and occupation) as potential confounders in both risk groups. They did not affect the ORs for the main exposure (change <2%) when added to the final models and were thus excluded.
Within the two risk groups, we examined the association between statin adherence and incidence of an MCE separately for those aged less than 65 years and those 65 years and older, men and women, and those with <3 years, 3–<5 years and 5 years or more of follow-up. We tested whether the ORs for the last two periods were statistically significantly different from the OR for the time window <3 years according to Altman & Bland [32], without adjustment for multiple comparisons. We analysed the modifying effects of gender and age (<65 years or ≥65 years) in the same way. We tested statistical significance of the modifying effect of the risk status (with prior CHD vs. without prior CHD) by including a product term between the risk status and statin adherence in the model, using the data from both risk groups.
For evaluating dose–response relationships, we defined adherence as a three-category variable (PDC < 40%, 40–79% or ≥80%) and further as a continuous variable for calculating P-values for trend. We used sas software (version 9.1; SAS Institute, Inc., Cary, NC, USA) for statistical analyses.
Ethical considerations
The SII, the National Data Protection Agency and the National Institute for Health and Welfare, Helsinki, Finland approved our study protocol. There was no legal requirement for ethics committee approval because we used only unidentifiable patient data and did not contact the patients.
Results
Of the 60 677 diabetic patients included in the cohort, an MCE occurred in 6466 patients from whom 3708 qualified as cases (Figure 1). Of these cases, 2031 were classified into the group without prior CHD and compared with 15 886 matched controls (median eight controls per case, interquartile range seven to nine). The corresponding figures in the group with prior CHD were 1500 cases and 4204 controls (median three controls, interquartile range two to four) respectively. Half of the cases experienced a myocardial infarction: 49.4 % in those without prior CHD and 51.7% in those with CHD.
Patients experiencing an MCE tended to be older, and had more comorbidities and a longer duration of diabetes than controls (Table 1). Statin adherence was good (PDC ≥ 80%) for over 50% of patients. The maximum duration of follow-up since statin initiation was 12.6 years in those without prior CHD and 12.2 years in those with CHD. In order to establish if the patients with good statin adherence differed from those with poor adherence with respect to risk factors for an MCE, we examined the distributions of these factors in the controls. There was no difference in the mean duration of diabetes between the two adherence groups but advanced age, female gender, presence of hypertension, and use of hormone replacement therapy and several cardiovascular drugs were associated with good adherence (Table 2).
Table 1.
Characteristics of cases and their matched controls
| Patients without prior CHD | Patients with prior CHD | |||
|---|---|---|---|---|
| Characteristic | Cases (n = 2013) | Controls* (n = 15886) | Cases (n = 1500) | Controls* (n = 4204) |
| Age, mean (SD; years) | 64.4 (7.1) | 62.0 (2.5) | 65.8 (6.9) | 65.1 (3.9) |
| 45–54, n (%) | 215 (10.7) | 2606 (16.6) | 136 (9.1) | 381 (8.1) |
| 55–64, n (%) | 723 (35.9) | 6970 (44.0) | 429 (28.6) | 1441 (33.7) |
| 65–75, n (%) | 1075 (53.4) | 6310 (39.4) | 935 (62.3) | 2382 (58.2) |
| Men, n (%) | 1302 (64.7) | 9998 (64.7) | 1034 (68.9) | 3088 (68.9) |
| Duration of follow-up, mean (SD; years) | 3.7 (2.4) | 3.7 (0.9) | 3.2 (2.3) | 3.2 (1.4) |
| Duration of diabetes, mean (SD; years) | 13.5 (9.7) | 11.5 (3.1) | 12.9 (9.1) | 11.7 (5.2) |
| Duration of CHD, mean (SD; years) | NA | NA | 8.4 (5.1) | 8.1 (2.9) |
| Statin equivalent at cohort entry, median (interquartile range)† | 0.5 (0.25–1.0) | 0.5 (0.25–1.0) | 0.25 (0.25–0.50) | 0.25 (0.25–0.50) |
| Statin adherence, mean (SD; %) | 68.2 (31.8) | 69.8 (11.0) | 71.2 (30.9) | 72.5 (18.2) |
| Statin adherence, n (%) | ||||
| 1–19 | 255 (12.7) | 1716 (11.0) | 166 (11.0) | 421 (9.7) |
| 20–39 | 208 (10.3) | 1527 (9.7) | 127 (8.5) | 341 (8.0) |
| 40–59 | 234 (11.6) | 1988 (12.6) | 175 (11.7) | 542 (12.9) |
| 60–79 | 304 (15.1) | 2342 (14.7) | 222 (14.8) | 559 (13.0) |
| ≥80 | 1012 (50.3) | 8313 (52.1) | 810 (54.0) | 2341 (56.4) |
| Medical characteristics at cohort entry, n (%) | ||||
| Cerebrovascular disease or peripheral atherosclerosis | 245 (12.2) | 1027 (6.8) | 213 (14.2) | 491 (10.9) |
| Congestive heart failure | 129 (6.4) | 570 (3.7) | 292 (19.5) | 766 (17.6) |
| Microangiopathic complications (retinopathy, neuropathy, nephropathy or dialysis) | 166 (8.3) | 790 (5.2) | 142 (9.5) | 278 (6.4) |
| Moderate to severe hypertension | 1149 (57.1) | 8406 (53.1) | 791 (52.7) | 2110 (51.4) |
| Rheumatoid arthritis | 67 (3.3) | 344 (2.1) | 46 (3.1) | 101 (2.4) |
| Pulmonary disorders (asthma or COPD) | 136 (6.8) | 918 (5.6) | 91 (6.1) | 252 (6.4) |
| Antidiabetic medication, n (%)‡ | ||||
| Only oral blood glucose-lowering drugs§ | 956 (47.9) | 8715 (54.6) | 700 (46.7) | 2195 (52.1) |
| Only insulin | 464 (23.1) | 3255 (20.7) | 350 (23.3) | 788 (18.4) |
| Both oral and insulin | 551 (27.4) | 3613 (22.7) | 410 (27.3) | 1100 (26.8) |
| No antidiabetic drug therapy | 33 (1.6) | 303 (1.9) | 40 (2.7) | 121 (2.7) |
| Concomitant medications, n (%)‡ | ||||
| Fibrates | 71 (3.5) | 639 (4.3) | 71 (4.7) | 237 (5.1) |
| Thiazolidinediones | 16 (0.8) | 186 (1.1) | 5 (0.3) | 8 (0.4) |
| Warfarin | 112 (5.6) | 637 (4.1) | 167 (11.1) | 548 (12.6) |
| Clopidogrel | 7 (0.4) | 9 (0.1) | 46 (3.1) | 62 (2.3) |
| Spironolactone | 23 (1.1) | 135 (0.8) | 21 (1.4) | 52 (1.5) |
| Antidepressives | 157 (7.8) | 1423 (8.8) | 134 (8.9) | 346 (9.0) |
| HRT (% according to the female population in the risk group) | 137 (19.3) | 1342 (22.7) | 82 (17.6) | 225 (20.6) |
| Antihypertensive medication | 1466 (72.8) | 10557 (66.3) | 1443 (96.2) | 3986 (95.3) |
| Number of cardiovascular drugs‡ | ||||
| 0–1 | 943 (46.9) | 9136 (57.6) | 132 (8.8) | 532 (13.0) |
| 2 | 473 (23.5) | 3642 (22.8) | 313 (20.9) | 974 (23.3) |
| 3 | 357 (17.7) | 1902 (12.1) | 429 (28.6) | 1132 (27.0) |
| 4 | 175 (8.7) | 892 (5.7) | 399 (26.6) | 923 (22.0) |
| 5 | 55 (2.7) | 234 (1.5) | 183 (12.2) | 516 (12.1) |
| 6–8 | 10 (0.5) | 53 (0.3) | 44 (2.9) | 127 (2.7) |
CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; HRT, hormone replacement therapy.
Descriptive statistics for controls weighted by the inverse of the number of controls in the matched set.
Statin equivalent in simvastatin dose: simvastatin 20 mg = lovastatin 20 mg = pravastatin 80 mg = fluvastatin 80 mg = atorvastatin 5 mg = rosuvastatin <5 mg.
Dispensed in the 365 days prior to cohort entry.
Including biguanides, sulfonylureas, thiazolidinediones, guar gum, repaglinide and nateglinide.
Table 2.
Characteristics of controls according to adherence level
| Patients without prior CHD | Patients with prior CHD | |||
|---|---|---|---|---|
| Characteristic | Poor adherence (<80%; n = 7573) | Good adherence (≥80%; n = 8313) | Poor adherence (<80%; n = 1863) | Good adherence (≥80%; n = 2341) |
| Age, mean (SD; years) | 61.6 (2.5) | 62.3 (2.5) | 64.6 (4.0) | 65.4 (3.8) |
| 45–54, n (%) | 1364 (18.3) | 1242 (15.1) | 203 (10.1) | 178 (6.6) |
| 55–64, n (%) | 3335 (44.2) | 3645 (43.8) | 636 (33.3) | 805 (34.0) |
| 65–75, n (%) | 2874 (37.5) | 3436 (41.1) | 1024 (56.7) | 1358 (59.4) |
| Men, n (%) | 4862 (66.1) | 5136 (63.4) | 1417 (71.0) | 1671 (67.3) |
| Duration of follow-up, mean (SD; years) | 3.8 (0.9) | 3.6 (0.9) | 3.2 (1.4) | 3.2 (1.4) |
| Duration of diabetes, mean (SD; years) | 11.4 (3.1) | 11.5 (3.2) | 11.8 (5.2) | 11.6 (5.2) |
| Duration of CHD, mean (SD; years) | NA | NA | 8.2 (2.7) | 8.0 (2.9) |
| Statin equivalent at cohort entry, median (interquartile range)* | 0.5 (0.25–1.0) | 0.5 (0.25–1.0) | 0.25 (0.25–0.5) | 0.25 (0.25–0.5) |
| Medical characteristics at cohort entry, n (%) | ||||
| Cerebrovascular disease or peripheral atherosclerosis | 422 (5.9) | 605 (7.6) | 203 (9.8) | 288 (11.7) |
| Congestive heart failure | 271 (3.6) | 299 (3.7) | 323 (17.6) | 443 (17.7) |
| Microangiopathic complications (retinopathy, neuropathy, nephropathy or dialysis) | 375 (5.2) | 415 (5.2) | 118 (6.2) | 160 (6.5) |
| Moderate to severe hypertension | 3825 (50.5) | 4581 (55.5) | 892 (48.7) | 1218 (53.5) |
| Rheumatoid arthritis | 161 (2.1) | 183 (2.2) | 41 (2.1) | 60 (2.6) |
| Pulmonary disorders (asthma or COPD) | 441 (5.7) | 477 (5.6) | 119 (7.0) | 133 (6.0) |
| Antidiabetic medication, n (%)† | ||||
| Only oral blood glucose-lowering drugs‡ | 4095 (53.9) | 4620 (55.3) | 972 (52.3) | 1223 (52.0) |
| Only insulin | 1614 (21.6) | 1641 (20.0) | 383 (20.3) | 405 (17.0) |
| Both oral and insulin | 1722 (22.7) | 1891 (22.8) | 451 (24.7) | 649 (28.4) |
| No antidiabetic drug therapy | 142 (1.9) | 161 (2.0) | 57 (2.7) | 64 (2.6) |
| Concomitant medications, n (%)† | ||||
| Fibrates | 246 (3.6) | 393 (5.0) | 92 (4.7) | 145 (5.4) |
| Thiazolidinediones | 79 (0.9) | 107 (1.2) | 3 (0.3) | 5 (0.5) |
| Warfarin | 272 (3.7) | 365 (4.4) | 225 (11.9) | 323 (13.2) |
| Clopidogrel | 4 (0.1) | 5 (0.1) | 12 (1.0) | 50 (3.4) |
| Spironolactone | 54 (0.7) | 81 (0.9) | 22 (1.5) | 30 (1.5) |
| Antidepressives | 674 (8.7) | 749 (8.8) | 139 (8.4) | 207 (9.5) |
| HRT (% according to the female population in the adherence group) | 549 (20.3) | 793 (24.8) | 88 (21.0) | 137 (20.3) |
| Antihypertensive medication | 4777 (62.7) | 5780 (69.7) | 1748 (94.9) | 2238 (95.7) |
| Number of cardiovascular drugs† | ||||
| 0–1 | 4604 (60.8) | 4559 (54.6) | 274 (15.0) | 258 (11.4) |
| 2 | 1680 (21.9) | 1962 (23.5) | 494 (25.9) | 480 (21.2) |
| 3 | 798 (10.6) | 1104 (13.4) | 482 (26.0) | 650 (27.7) |
| 4 | 389 (5.3) | 503 (6.2) | 370 (20.0) | 553 (23.6) |
| 5 | 85 (1.2) | 149 (1.8) | 196 (10.6) | 320 (13.2) |
| 6–8 | 17 (0.2) | 36 (0.4) | 47 (2.5) | 80 (2.8) |
CHD, coronary heart disease, COPD, chronic obstructive pulmonary disease, HRT, hormone replacement therapy. All descriptive statistics weighted by the inverse of the number of controls in the matched set.
Statin equivalent in simvastatin dose: simvastatin 20 mg = lovastatin 20 mg = pravastatin 80 mg = fluvastatin 80 mg = atorvastatin 5 mg = rosuvastatin <5 mg.
Dispensed in the 365 days prior to cohort entry.
Including biguanides, sulfonylureas, thiazolidinediones, guar gum, repaglinide and nateglinide.
Compared with patients with poor statin adherence, those with good adherence had a reduced incidence of MCEs in both those without [AOR for the composite end point 0.86 (95% CI 0.78–0.95] and those with prior CHD [OR 0.84 (95% CI 0.74–0.95), P = 0.24 for interaction; Table 3]. The AORs for myocardial infarction were 0.82 (95% CI 0.71–0.94) and 0.67 (95% CI 0.56–0.81) respectively.
Table 3.
Odds ratios of major coronary events for good vs. poor statin adherence according to risk status
| Patients without prior CHD | Patients with prior CHD | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | |||||
| Sample | Unadjusted* | P-value | Adjusted† | P-value | Unadjusted* | P-value | Adjusted‡ | P-value |
| Total | 0.90 (0.82, 0.99) | 0.02 | 0.86 (0.78, 0.95) | 0.002 | 0.87 (0.77, 0.99) | 0.03 | 0.84 (0.74, 0.95) | 0.01 |
| Follow-up time (years) | 0.47§;0.19¶ | 0.06§; 0.21¶ | ||||||
| <3 | 0.96 (0.84, 1.10) | 0.57 | 0.91 (0.79, 1.05) | 0.21 | 0.99 (0.84, 1.16) | 0.90 | 0.95 (0.80, 1.12) | 0.53 |
| 3–5 | 0.87 (0.72, 1.05) | 0.15 | 0.84 (0.69, 1.02) | 0.07 | 0.67 (0.50, 0.88) | 0.004 | 0.69 (0.51, 0.91) | 0.01 |
| ≥5 | 0.82 (0.69, 0.98) | 0.03 | 0.79 (0.66, 0.94) | 0.01 | 0.81 (0.62, 1.06) | 0.12 | 0.77 (0.58, 1.02) | 0.07 |
| Age (years) | 0.48** | 0.18** | ||||||
| <65 | 0.88 (0.76, 1.02) | 0.08 | 0.84 (0.73, 0.98) | 0.02 | 0.99 (0.77, 1.27) | 0.92 | 0.98 (0.76, 1.28) | 0.88 |
| ≥65 | 0.93 (0.81, 1.07) | 0.31 | 0.91 (0.78, 1.05) | 0.19 | 0.82 (0.68, 0.98) | 0.03 | 0.79 (0.65, 0.95) | 0.01 |
| Gender | 0.27†† | 0.49†† | ||||||
| Women | 0.84 (0.71, 0.98) | 0.02 | 0.80 (0.68, 0.94) | 0.01 | 0.81 (0.64, 1.02) | 0.07 | 0.79 (0.62, 1.00) | 0.046 |
| Men | 0.93 (0.83, 1.05) | 0.25 | 0.89 (0.79, 1.01) | 0.06 | 0.90 (0.78, 1.04) | 0.16 | 0.87 (0.75, 1.01) | 0.07 |
CHD, coronary heart disease.
Adjusted for gender, time of cohort entry and length of follow-up (by study design), and age at index date.
Adjusted for gender, time of cohort entry and length of follow-up (by study design), age and the duration of diabetes at index date, medical characteristics (i.e. cerebrovascular disease or peripheral atherosclerosis, congestive heart failure, microangiopathic complications, moderate to severe hypertension and rheumatoid arthritis), type of antidiabetic medication and concomitant medications (i.e. fibrates, warfarin, hormone replacement therapy, antihypertensive medication and the number of cardiovascular drugs) at cohort entry.
Adjusted for gender, time of cohort entry and length of follow-up (by study design), age, the duration of diabetes (<10 years or ≥10 years) and the duration of CHD (<2 years or ≥2 years) at index date, medical characteristics (i.e. cerebrovascular disease or peripheral atherosclerosis, congestive heart failure, microangiopathic complications, moderate to severe hypertension and rheumatoid arthritis), type of antidiabetic medication and concomitant medications (i.e. fibrates, warfarin, hormone replacement therapy, antihypertensive medication and the number of cardiovascular drugs) at cohort entry.
P-value for interaction when comparing the odds ratios for the follow-up times 3–5 years and <3 years.
P-value for interaction when comparing the odds ratios for the follow-up times ≥5 years and <3 years.
P-value for interaction when comparing the odds ratios between age groups.
P-value for interaction when comparing the odds ratios between genders.
The beneficial effect of good statin adherence persisted beyond 5 years of follow-up, although the risk reduction was not statistically significant among those with CHD at statin initiation. In the group without prior CHD, the AOR for MCEs among those followed up for 5 years or more was 0.79 (95% CI 0.66–0.94) and 0.77 (95% CI 0.58–1.02) in the group with prior CHD (Table 3). Age did not modify the association in those without prior CHD; AOR was 0.84 (95% CI 0.73–0.98) in those aged less than 65 years and 0.91 (95% CI 0.78–1.05) in those 65 years and older. In those with prior CHD, however, good adherence was associated with a lower risk for MCEs only in patients aged 65 years and older [AOR 0.78 (95% CI 0.64–0.94)] but not in the younger patients [AOR 0.98 (95% CI 0.75–1.28), P = 0.18 for interaction]. Finally, the favourable effect of good statin adherence tended to be weaker among men than women in both risk groups.
When comparing with PDC < 40%, we observed a reduced MCE incidence both for PDC 40–79% [AOR 0.85 (95% CI 0.74–0.97)] and PDC ≥ 80% [AOR 0.78 (95% CI 0.69–0.88)] in the group without prior CHD. The respective AORs were 0.90 (95% CI 0.74–1.07) and 0.82 (95% CI 0.70–0.97) in the group with prior CHD. Per each additional 10% unit increase in PDC, the incidence of MCEs reduced by 3% in both risk groups (AOR 0.97 per 10% units, P < 0.001 and P = 0.02 for trend respectively).
Discussion
The main finding of our study is that in real life, the incidence of MCEs is significantly lower in diabetic patients with good adherence to statin therapy (≥80%) when compared with those patients with poor adherence. Furthermore, we showed that the relative risk reduction is of a similar magnitude (∼15−20%) in patients with and without CHD. In addition, the reduction in MCE incidence was still evident when we further restricted the study population to those with no documented CVD, defined as no documented CHD, cerebrovascular disease nor peripheral atherosclerosis, at statin initiation [AOR 0.87 (95% CI 0.78–0.96) overall and AOR 0.80 (95% CI 0.66–0.97) in those followed up for 5 years or longer, data not shown].
Instead of comparing the incidence of MCEs between users and non-users of statins, we restricted our study population to statin users with different levels of statin adherence. We predicted that the risk of MCEs would be different in those patients with both diabetes and an indication for statins compared with those with diabetes only. Poor adherence means lower cumulative statin exposure over time, leading to less effective or even sub-therapeutic drug use, whereas good adherence increases the likelihood of optimal treatment, resembling the situation in RCTs [33–35]. In RCTs, statin adherence above 80% is considered optimal but in real life adherence to statins may be lower [16–20, 36]. Older age, presence of CHD and comorbidities are associated with optimal statin persistence or adherence [16–18, 20, 36] and were therefore controlled for in our analysis.
Studies on statin effectiveness have demonstrated that optimal adherence may reduce coronary artery disease risk by 18–19% [30, 37] and mortality by approximately 50% [38, 39] when compared with less optimal adherence. Separate analyses for diabetic populations had follow-up times limited to 2 years, and they assessed all-cause mortality [40, 41] or the risk of all-cause hospitalizations [42], with results favouring adherent statin use. We were able to demonstrate a beneficial effect of statin therapy on coronary morbidity, a more specific target of statin therapy.
We may have introduced a healthy-adherer bias when using the adherence level as an exposure [43]. Patients with good adherence to statins may have different health-seeking behaviour and lifestyle, making them less susceptible to MCEs. In US register-based studies, patients with better adherence to statins were more likely to use screening services [44] and were less prone to accidents than patients with lower adherence [45]. In order to assess the relationship between adherence and the lifestyle indicators not available in our data, we used data from a nationwide cross-sectional health examination survey carried out in Finland in 2000–2001 [46]. Of the 8028 persons in the survey, we identified 854 statin users, including individuals both with and without diabetes. Individuals with good statin adherence reported less current smoking than those with poor adherence (11% vs. 15%), had less frequently a BMI > 25 kg m−2 (63% vs. 71%) and had less frequently blood pressure ≥140/90 mmHg (47% vs. 57%). The proportion of women who had participated in screening mammography was 55% vs. 51% respectively. Leisure-time physical activity was less common among those with good in comparison with those with poor adherence (49% vs. 65%). Thus, we cannot rule out lifestyle as a factor contributing to our findings.
A sensitivity analysis with the rule-out approach [47] was performed to evaluate how strong an association between an unmeasured confounder and adherence, and the confounder and outcome (MCE) would be required to explain the decreased incidence of MCEs in good adherence groups solely by this unmeasured confounder. The analysis suggested that if the OR between an unmeasured confounder and adherence is <1.5, then ORs of 4 or greater between the confounder and incidence of an MCE would be required for the ORs observed in this study to be explained completely by confounding (data not shown). We considered smoking as the most important risk factor for an MCE [48] for which we could not control. As the reported increase in CVD risk associated with smoking is two to threefold [48] and the prevalence of smoking in persons with good and poor adherence to statins was of similar magnitude in the health examination survey cited above, it seems unlikely that inclusion of smoking in the analysis would have changed our conclusions.
In our main analyses, we wanted to avoid controlling for intermediate variables on the causal pathway between adherence to statins and an MCE (i.e. overadjustment bias, [49]) and therefore considered use of other medications prior to statin initiation only. Re-analyses adjusting for the use of other medications and the number of cardiovascular drugs within 365 days prior to the index date did not change our conclusions, although the estimated risk reductions with good adherence were somewhat greater than in the original analyses: 20% (vs. 14%) in the group without prior CHD and 21% (vs. 16%) in the group with CHD (data not shown).
There are a few other unmeasured confounders. We had no information on aspirin use because this drug is sold over-the-counter. However, the efficacy of aspirin in the prevention of vascular disease among patients with diabetes has recently been questioned [50, 51]. We were not able to identify the type of diabetes in our data, while the majority of the cases and controls seem to have type 2 diabetes based on the low number of patients on insulin only (Table 1). Finally, lack of data on glycaemic control may have distorted our results [52, 53].
Some misclassification in measures of exposure, covariates and outcomes may affect our results because of the reliance on administrative data. Because of excluding patients hospitalized for more than 10% of the follow-up and cases with their MCE recorded when hospitalized for more than 30 days, underestimation of adherence seems unlikely. Physicians prefer simple drug regimes and splitting of statin tablets seems rare [54]. Statins are available on tablet strengths covering a wide dosage range, making a one tablet per day dosage regimen possible. We therefore consider this regimen as a valid basis for calculation of adherence. On the other hand, dispensed prescribing is not a guarantee for actual drug use. Also, overlapping prescriptions [55] may overestimate adherence, especially in a short-term follow-up. We excluded the first 9 months of follow-up that decreases this problem. Instead of using a time-varying exposure measure [56], we calculated the mean PDC over the follow-up, assuming a stable drug-purchasing pattern. We evaluated the validity of this assumption among patients who had entered the cohort in 1997 and had been followed up for more than 1 year. In the group without prior CHD, the adherence level (<80% or ≥80%) remained the same for every year of the follow-up for 59% of the cases (n = 206) and for 58% of the controls (n = 1484). The respective proportions in the group with CHD were 69% (n = 177) and 71% (n = 551).
In summary, our findings on the association between good adherence to statins and reduced risk of MCEs based on nearly all community-dwelling subjects with diabetes in Finland provide further justification for maintenance statin therapy in diabetes.
Acknowledgments
For their data management skills, we thank Hilkka Ruuska, Secondary school graduate, and Kristiina Tyrkkö, MScSoc (Social Insurance Institution), Simo Pelanteri, MSc, and Sirkka Rinne, Secondary school graduate (National Institute for Health and Welfare) and Jouni Junnila, MSc (4Pharma Ltd). We thank Jaana Martikainen, LicSc (Pharm) (Social Insurance Institution) for help in data interpretation. Of the acknowledged, Jouni Junnila received financial compensation for his work.
Competing Interests
This work was partly funded by a research grant from the Social Insurance Institution (26 October 2007) (PR, MJK, A-HS).
PR was employed by the University of Turku, Finland to conduct randomized controlled trials sponsored by Orion Pharma and Élan. RH has participated as an employee of the University of Turku in designing, performing and reporting of industry-driven randomized controlled trials. RH is a member of the advisory board in social medicine of the Social Insurance Institution. No other authors have any potential or real conflicts of interest to declare.
REFERENCES
- 1.American Diabetes Association. Standards of medical care in diabetes – 2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2003;26(Suppl 1):S83–S86. doi: 10.2337/diacare.26.2007.s83. [DOI] [PubMed] [Google Scholar]
- 3.Jones P, Davidson M, Stein E, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW, STELLAR Study Group Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92:152–60. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 4.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 5.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 7.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 8.Heart protection Study Collaborative Group. MRC/BHF heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 9.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 10.Schramm T, Gislason G, Køber L, Rasmussen S, Rasmussen J, Abildstrøm S, Hansen M, Folke F, Buch P, Madsen M, Vaag A, Torp-Pedersen C. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117:1945–54. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 11.Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, Priori S, Östergren J, Tuomilehto J, Thrainsdottir I. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 12.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 13.Costa J, Borges M, David C, Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ. 2006;332:1115–24. doi: 10.1136/bmj.38793.468449.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HAW, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 15.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–85. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 16.Mantel-Teeuwisse AK, Goettsch WG, Klungel OH, de Boer A, Herings RMC. Long term persistence with statin treatment in daily medical practice. Heart. 2004;90:1065–6. doi: 10.1136/hrt.2003.026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perreault S, Blais L, Lamarre D, Dragomir A, Berbiche D, Lalonde L, Laurier C, St-Maurice F, Collin J. Persistence and determinants of statin therapy among middle-aged patients for primary and secondary prevention. Br J Clin Pharmacol. 2005;59:564–73. doi: 10.1111/j.1365-2125.2005.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benner J, Glynn R, Mogun H, Neumann P, Weinstein M, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 19.Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, LeLorier J. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–62. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly LA, Doney ASF, Morris AD, Palmer CNA, Donnan PT. Long-term adherence to statin treatment in diabetes. Diabet Med. 2008;25:850–5. doi: 10.1111/j.1464-5491.2008.02476.x. [DOI] [PubMed] [Google Scholar]
- 21.Furu K, Wettermark B, Andersen M, Martikainen J, Almarsdottir A, Sørensen H. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106:86–94. doi: 10.1111/j.1742-7843.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 22.The National Agency for Medicines. Classification of Medicines and the Defined Daily Doses 2007. Helsinki: National Agencies for Medicines; 2007. [Google Scholar]
- 23.Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Raiha P, Karja-Koskenkari P, Mähönen M, Niemelä M, Kuulasmaa K, Palomäki P, Mustonen J, Lehtonen A, Arstila M, Vuorenmaa T, Lehto S, Miettinen H, Torppa J, Tuomilehto J, Kesäniemi YA, Pyörälä K, Salomaa V. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:132–7. doi: 10.1097/00149831-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am J Epidemiol. 2008;168:329–35. doi: 10.1093/aje/kwn135. [DOI] [PubMed] [Google Scholar]
- 25.Korhonen M, Huupponen R, Ruokoniemi P, Helin-Salmivaara A. Protopathic bias in observational studies on statin effectiveness. Eur J Clin Pharmacol. 2009;65:1167–8. doi: 10.1007/s00228-009-0701-0. [DOI] [PubMed] [Google Scholar]
- 26.Tamim H, Monfared AAT, LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol Drug Saf. 2007;16:250–8. doi: 10.1002/pds.1360. [DOI] [PubMed] [Google Scholar]
- 27.Caetano PA, Lam JM, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: examples from research on statin and antihypertensive utilization. Clin Ther. 2006;28:1411–24. doi: 10.1016/j.clinthera.2006.09.021. Discussion 1410. [DOI] [PubMed] [Google Scholar]
- 28.Andrade S, Kahler K, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–74. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 29.Schneeweiss S, Patrick A, Maclure M, Dormuth C, Glynn R. Adherence to beta-blocker therapy under drug cost-sharing in patients with and without acute myocardial infarction. Am J Manag Care. 2007;13:445–52. [PMC free article] [PubMed] [Google Scholar]
- 30.Perreault S, Dragomir A, Blais L, Brard A, Lalonde L, White M, Pilon D. Impact of better adherence to statin agents in the primary prevention of coronary artery disease. Eur J Clin Pharmacol. 2009;65:1013–24. doi: 10.1007/s00228-009-0673-0. [DOI] [PubMed] [Google Scholar]
- 31.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423–30. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman D, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 34.Colhoun HM, Thomason MJ, Mackness MI, Maton SM, Betteridge DJ, Durrington PN, Hitman GA, Neil HAW, Fuller JH, CARDS Investigators Design of the Collaborative AtoRvastatin Diabetes Study (CARDS) in patients with type 2 diabetes. Diabet Med. 2002;19:201–11. doi: 10.1046/j.1464-5491.2002.00643.x. [DOI] [PubMed] [Google Scholar]
- 35.Sacks FM, Pfeffer MA, Moye L, Brown L, Rouleau JL, Hartley LH, Rouleau J, Grimm R, Sestier F, Wickemeyer W, Cole TG, Braunwald E, The Care Investigators Rationale and design of a secondary prevention trial of lowering normal plasma cholesterol levels after acute myocardial infarction: the Cholesterol and Recurrent Events trial (CARE) Am J Cardiol. 1991;68:1436–46. doi: 10.1016/0002-9149(91)90276-q. [DOI] [PubMed] [Google Scholar]
- 36.Helin-Salmivaara A, Lavikainen P, Ruokoniemi P, Korhonen M, Huupponen R. Persistence with statin therapy in diabetic and non-diabetic persons: a nation-wide register study in 1995–2005 in Finland. Diabetes Res Clin Pract. 2009;84:e9–e11. doi: 10.1016/j.diabres.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Bouchard M, Dragomir A, Blais L, Bérard A, Pilon D, Perreault S. Impact of adherence to statins on coronary artery disease in primary prevention. Br J Clin Pharmacol. 2007;63:698–708. doi: 10.1111/j.1365-2125.2006.02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann A. Continuation of statin treatment and all-cause mortality: a population-based cohort study. Arch Intern Med. 2009;169:260–8. doi: 10.1001/archinternmed.2008.552. [DOI] [PubMed] [Google Scholar]
- 39.McGinnis BD, Olson KL, Delate TMA, Stolcpart RS. Statin adherence and mortality in patients enrolled in a secondary prevention program. Am J Manag Care. 2009;15:689–95. [PubMed] [Google Scholar]
- 40.Zhang Q, Safford M, Miller D, Crystal S, Rajan M, Tseng C, Pogach L. Short-term statin exposure is associated with reduced all-cause mortality in persons with diabetes. Med Care. 2007;45:308–14. doi: 10.1097/01.mlr.0000250227.94196.f0. [DOI] [PubMed] [Google Scholar]
- 41.Ho PM, Magid D, Masoudi F, McClure D, Rumsfeld J. Adherence to cardioprotective medications and mortality among patients with diabetes and ischemic heart disease. BMC Cardiovasc Disord. 2006;6 doi: 10.1186/1471-2261-6-48. Available at: http://www.biomedcentral.com/1471-2261/6/48 (last accessed 1 December 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho PM, Rumsfeld J, Masoudi F, McClure D, Plomondon M, Steiner J, Magid D. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–41. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 43.Simpson S, Eurich D, Majumdar S, Padwal R, Tsuyuki R, Varney J, Johnson J. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brookhart MA, Patrick A, Dormuth C, Avorn J, Shrank W, Cadarette S, Solomon D. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–54. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 45.Dormuth C, Patrick A, Shrank W, Wright J, Glynn R, Sutherland J, Brookhart MA. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119:2051–7. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aromaa A, Koskinen S. Health and Functional Capacity in Finland. Baseline Results of the Health 2000 Health Examination Survey. Helsinki: National Public Health Institute; 2002. Publications of the National Public Health Institute B3/2002 (accessed 1 May 2009) [Google Scholar]
- 47.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 48.Pencina M, D'Agostino R, Larson M, Massaro J, Vasan R. Predicting the 30-year risk of cardiovascular disease: the Framingham heart study. Circulation. 2009;119:3078–84. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schisterman E, Cole S, Platt R. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–95. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cubbon R, Gale C, Rajwani A, Abbas A, Morell C, Das R, Barth JH, Grant PJ, Kearney MT, Hall AS. Aspirin and mortality in patients with diabetes sustaining acute coronary syndrome. Diabetes Care. 2008;31:363–5. doi: 10.2337/dc07-1745. [DOI] [PubMed] [Google Scholar]
- 51.Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Rosenson RS, Williams CD, Wilson PW, Kirkman MS, American Diabetes Association, American Heart Association, American College of Cardiology Foundation Aspirin for primary prevention of cardiovascular events in people with diabetes. J Am Coll Cardiol. 2010;55:2878–86. doi: 10.1016/j.jacc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Ray KK, Seshasai SRK, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–72. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 53.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- 54.Dormuth C, Schneeweiss S, Brookhart A, Bassett K, Wright J. Factors related to splitting tablets of HMG-CoA reductase inhibitors: a population-based analysis in British Columbia. Pharmacoepidemiol Drug Saf. 2007;16:S137. [Google Scholar]
- 55.Hachem C, Morgan R, Johnson M, Kuebeler M, El-Serag H. Statins and the risk of colorectal carcinoma: a nested case-control study in veterans with diabetes. Am J Gastroenterol. 2009;104:1241–8. doi: 10.1038/ajg.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filion K, Schneider-Lindner V, Karp I, Lvesque L, Brophy J, Suissa S. Continuation of statin treatment and mortality: a note of caution on excessive benefits. Arch Intern Med. 2009;169:1080. doi: 10.1001/archinternmed.2009.157. [DOI] [PubMed] [Google Scholar]


