Abstract
OBJECTIVE
Neuroprotective peptides (NAP+SAL) can prevent some alcohol-induced damage in fetal alcohol syndrome(FAS). Fractalkine, a chemokine constitutively expressed in the CNS reduces neuronal death from activated microglia. Using a model of FAS we evaluated if fractalkine is altered and if NAP+SAL work through fractalkine.
STUDY DESIGN
Using a FAS model, C57BL6/J-mice were treated on gestational day 8 with alcohol (0.03 mL/g), placebo or alcohol+peptides. Embryos were harvested after 6h(E8) and 10 days later(E18). Fractalkine was measured in the protein lysate (Luminex xMAP). Statistical analysis included Kruskal-Wallis.
RESULTS
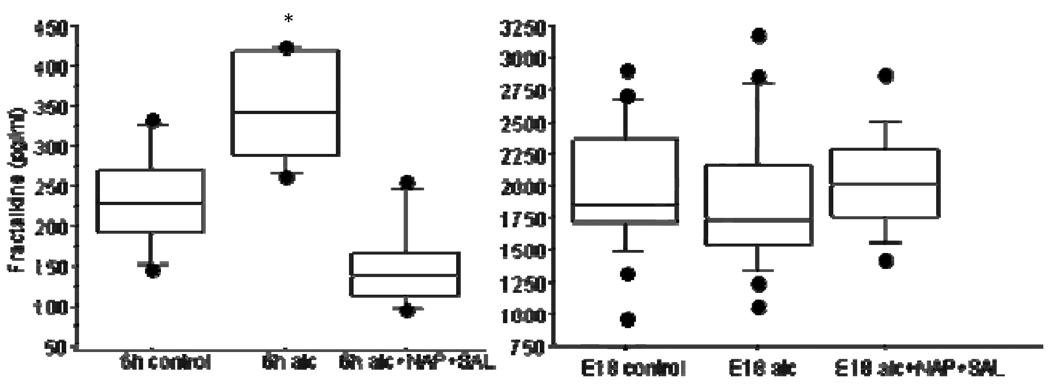
Fractalkine was significantly elevated at 6h (median 341pg/ml, range 263–424pg/ml) vs. controls (median 228pg/ml, range 146–332pg/ml; P<.001). NAP+SAL prevented the alcohol-induced increase (median 137, range 97–255 pg/ml, P<.001). At E18, fractalkine levels were similar in all groups (P=0.7).
CONCLUSION
Prenatal alcohol exposure acutely elevates fractalkine, perhaps in an effort to counter the alcohol toxicity. Pre-treatment with NAP+SAL prevents the acute increase in fractalkine.
Introduction
Prenatal alcohol exposure may result in fetal alcohol syndrome (FAS), the most prevalent non-inheritable cause of mental retardation or the less severe fetal alcohol spectrum disorder (FASD). The latter is characterized by a less severe phenotype and typically includes neurodevelopmental and neurobehavioral deficits that persist through adulthood. Gestational timing, dose, frequency and genetic predisposition as well as not clearly elucidated factors impact the confounding effects of alcohol on a fetus. Therefore, although not all children prenatally alcohol exposed will develop fetal alcohol syndrome, it is estimated that in the United States 1 in 100 children are born with FASD resulting in a substantial number of children susceptible to cognitive learning impairment (1,2). To better understand the complex mechanisms underlying FAS and FASD we have utilized well-defined animal models that mimic the human condition with careful consideration to gestational timing, brain structure vulnerability, dose and duration of alcohol exposure to induce the dysfunctional regulatory mechanisms of neuronal plasticity, a direct measure of cognitive learning. Previously, we demonstrated that treatment with novel peptides, SALLRSIPA (ADNF-9) and NAPVSIPQ (NAP), prevented alcohol-induced fetal growth restriction, microcephaly, oxidative damage, inflammatory cytokine release and learning dysfunction in the mouse model of FAS(3,4). Using the FAS animal model, our lab demonstrated that prenatal alcohol increased tumor necrosis factor–α (TNFα) and interleukin-6 (IL-6) levels in the embryos versus control, which was attenuated with NAP+SAL treatment (5). These proinflammatory cytokines (TNFα and IL6) affect long term potentiation, a molecular model for learning (6,7). Given the importance of oxidative stress and inflammatory-mediated alcohol induced injury in FAS and FASD, we report here on the role of fractalkine. Fractalkine is a chemokine with neuroprotective properties that acts as an anti-inflammatory molecule in vitro by attenuating the secretion of IL-6 and TNFα and the up regulation of inducible nitric oxide synthase (iNOS) in LPS-activated microglia(8). Fractalkine is constitutively expressed in neurons throughout the CNS, has a role in neuroprotection reducing neuronal death from activated microglia. Our objective was to evaluate if fractalkine is altered in FAS and if the mechanism of action of the neuroprotective peptides NAP+SAL includes fractalkine.
Methods
In this study, a well defined FAS mouse model which exposes fetal mice to ethanol during a critical period of organogenesis and neurogenesis and renders a high incidence of anomalies and demise was used (9). C57Bl6/J female mice (Jackson Laboratories, Bar Harbor, Maine) were kept under a 12-hour light, 12-hour dark regimen, with food and water available at all times. The mice received humane animal care in compliance with the National Institutes of Health (NIH) guidelines for care and use of experimental animals. The protocol was approved by National Institute of Child Health and Human Development Animal Care and Use Committee. Six-week-old females (21–24 g) were mated with C57Bl6/J males for 4 hours. Presentation of the vaginal copulation plug was considered day 0 of pregnancy There were three treatment groups: alcohol, placebo and alcohol+NAP+SAL. On gestational day 8, we treated pregnant mice intraperitoneally with 25% ethyl alcohol in saline (vol/vol) or vehicle alone at 0.03 mL/g body weight.
The peptides, NAP and SAL (20 mg in 0.2 mL), were administered immediately prior to the alcohol/placebo treatment. NAP was diluted in 50 mL DMSO and diluted in filtered Dulbecco’s phosphate-buffered saline solution. SAL was dissolved and diluted in filtered Dulbecco’s phosphate-buffered saline solution. The peptides concentration reflect previously established protective levels for each peptide in the prevention of alcohol-induced fetal death, growth restriction, and microcephaly (3,4). At 6 hours after injection, embryos were explanted using micro-dissection and homogenized in a buffer containing 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 0.05% Tween-20, and a cocktail of protease inhibitors (Roche). At 10 days after injection (E18), brain tissues were isolated from the embryo and homogenized in a buffer containing 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 0.05% Tween-20, and a cocktail of protease inhibitors (Roche). The homogenates were centrifuged at 10,000g for 5 minutes. The supernatant was removed, analyzed for protein concentration and LUMINEX analysis for measurement of fractalkine. Each gestational time point included at least 4 samples with each sample representing 2–7 litters (a gestation typically includes 8–10 embryos).
Fractalkine levels were normalized to total protein. Fractalkine concentrations was determined using antibodies for the analyte covalently immobilized to a set of microspheres according to protocol developed and validated at LINCO Research, Inc. The analyte on the surface of microspheres were then detected by a cocktail of biotinylated antibodies. Following binding of streptavidin–phycoerythrin conjugate, the reporter fluorescent signal was measured with a Luminex100 reader (Luminex Corp., Austin, TX 78727, USA). Data were calculated using a calibration curve obtained in each experiment using the respective recombinant proteins diluted in kit matrix for plasma samples and lysis buffer for tissue samples. Concentrations of fractalkine was calculated using StatLIAs software (Brendan Scientific Corp., Carlsbad, CA 92008, USA) with a five-parameter logistic curve-fitting method, and normalized to the amount of protein in each sample. Statistical analysis included Kruskal-Wallis for comparison of fractalkine levels with P<0.05 considered significant. Data are reported as median (range).
Results
Six hours after alcohol exposure, fractalkine levels were significantly higher (median 341pg/ml, range 263–424pg/ml) as compared to control levels (median 228pg/ml, range 146–332pg/ml; P<.001; Figure 1). The peptides NAP+SAL prevented the alcohol induced increase in fractalkine in the embryos (median 137 pg/ml, range 97–255 pg/ml, P<.001). Ten days after treatment, on E18, fractalkine levels were similar in all groups (medians 1821, 1852 and 2013 pg/ml respectively, P=0.7).
Figure 1.
Fractalkine levels in animals exposed to control (placebo), alcohol or alcohol+NAP+SAL six hours (6h) after treatment (left) and 10 days after treatment, on embryonic day 18 (E18) on right. Fractalkine levels are shown in box plot.
Comment
Fractalkine levels are acutely altered in a model of fetal alcohol syndrome, with significantly higher levels after alcohol exposure, this effect does not persist as ten days later, on E18, the levels are similar. The neuroprotective peptides NAP+SAL prevented the acute rise in fractalkine. In a previous study our lab demonstrated that prenatal alcohol exposure significantly increased the acute levels of the pro-inflammatory cytokines (TNF-a and IL-6) in embryos (5) and found spatial learning deficits in alcohol-exposed offspring. These studies suggest that cytokine and chemokine dysregulation in fetal alcohol syndrome may be in part responsible for the impaired spatial learning and memory formation seen and at least in part identifies a mechanism of neuroprotection of NAP and SAL.
Synaptic plasticity, the strengthening and alteration of the synapse, is directly correlated to learning and long term memory formation. Long-term potentiation (LTP), the underlying neuronal molecular mechanism for learning and memory, through synaptic transmission, enhances synaptic plasticity. In recent years molecules related to the immune system specifically chemokines have been also shown to effect CNS plasticity. Fractalkine is localized in neurons and is constitutively expressed in the CNS. (10). Like most chemokines, fractalkine activates multiple intracellular signaling pathways, most notably the influx of calcium, and mediates cell adhesion through binding to a seven transmembrane domain G protein–coupled receptor (GPCR) CX3CR1 which is exclusively expressed in brain microglia (11). Fractalkine is described as the “molecular messenger in neuron-microglia communication.”
Studies have shown that an up regulation of fractalkine results in microglia dysfunction and is associated with neurologic dysfunction (12). This is consistent with our work with NAP and SAL that were able to prevent alterations in a model of Down syndrome where there is a glial defect and learning deficits in the offspring (13). The up regulation of fractalkine after alcohol exposure may underlie the glial deficit and thus the subsequent learning deficit. Importantly also, microglia begin to develop at embryonic day 8 and these are responsible for over 90% of adult microglia. This overlaps with the timing of our alcohol exposure, and may explain why the effects of the alcohol induced damage are life long(14). Thus the damage to the microglia, especially at this critical time point, via fractalkine, may explain some of the damages associated with fetal alcohol syndrome. This may also explain, at least in part, a mechanism of how the peptides NAP and SAL prevent the alcohol induced damage, by preventing the rise in fractalkine.
Long term potentiation in CA1 region of the hippocampus is impaired by the soluble form of fractalkine. (15). The deficits in cognitive learning and memory observed in the alcohol exposed animals are thought to be the result of direct or indirect actions of alcohol within the CA1 region of the hippocampus (16). A similar study (17) demonstrated physiologic dysregulation of FKN/CX3CR signaling using a fractalkine antibody in young adult rats decreased hippocampus neurogenesis and normal age-related changes in FKN/CX3CR that decreased hippocampal neurogenesis in aged rats. The authors noted in the latter experiment that they could not rule out increased microglia activation observed in normal aging as the cause for or consequence of the dysregulation of fractalkine. However in this present study we can rule out the aging paradigm as the direct or indirect causative effect for the apparent dysregulation of fractalkine and from a recently published study(14et al) we may be able to attribute the alcohol-induced learning impairment to a long-standing microglial dysfunction from fractalkine up regulation.
We found that prenatal alcohol exposure acutely results in elevation of the neuroprotective chemokine fractalkine, perhaps in an effort to counter the alcohol toxicity. Treatment with the peptides NAP+SAL along with the alcohol prevents the acute increase in fractalkine. Since alterations in fractalkine affect microglia activation, this may explain at least some of the deleterious effects of alcohol in fetal alcohol syndrome. Furthermore, the ability of NAP and SAL to prevent the rise in fractalkine may explain the mechanism of their neuroprotection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE: Through work at the NIH, Dr. Spong is named on patent applications for these two peptides.
References
- 1.Centers for Disease Control and Prevention. [Retrieved September 2008];Behavioral risk factor surveillance system. Available at: http://www.cdc.gov/brfss [Context Link]
- 2.Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- 3.Spong CY, Abebe DT, Gozes I, Brenneman DE, Hill JM. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J Pharmacol Exp Ther. 2001;297:774–779. [PubMed] [Google Scholar]
- 4.Vink J, Auth J, Abebe DT, Brenneman DE, Spong CY. Novel peptides prevent alcohol-induced spatial learning deficits and proinflammatory cytokine release in a mouse model of fetal alcohol syndrome. Am J Obstet Gynecol. 2005;193:825–829. doi: 10.1016/j.ajog.2005.02.101. [DOI] [PubMed] [Google Scholar]
- 5.Vink J, Auth J, Abebe DT, Brenneman DE, Spong CY. Novel peptides prevent alcohol-induced spatial learning deficits and proinflammatory cytokine release in a mouse model of fetal alcohol syndrome. Am J Obstet Gynecol. 2005 Sep;193(3 Pt 1):825–829. doi: 10.1016/j.ajog.2005.02.101. [DOI] [PubMed] [Google Scholar]
- 6.Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB J. 2004 Nov;18(14):1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Wu J, Rowan MJ, Anwyl R. Beta-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. European Journal of Neuroscience. 2005;22:2827–2832. doi: 10.1111/j.1460-9568.2005.04457.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol Interv. 2010 Oct;10(5):263–270. doi: 10.1124/mi.10.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster WS, Walsh DA, Lipson AH, McEwen SE. Teratogenesis after acute alcohol exposure in inbred and outbred mice. Neurotoxicol Teratol. 1980;2:227–234. [Google Scholar]
- 10.Sunnemark D, Eltayeb S, Nilsson M, Wallström E, Lassmann H, Olsson T, Berg AL, Ericsson-Dahlstrand A. CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. J Neuroinflammation. 2005 Jul 29;2:17. doi: 10.1186/1742-2094-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006 Jul;9(7):917–924. doi: 10.1038/nn1715. Epub 2006 Jun 18. [DOI] [PubMed] [Google Scholar]
- 12.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010 Apr;126(1):56–68. doi: 10.1016/j.pharmthera.2010.01.002. Epub 2010 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toso L, Cameroni I, Roberson R, Abebe D, Bissell S, Spong CY. Prevention of developmental delays in a Down syndrome mouse model. Obstet Gynecol. 2008 Dec;112(6):1242–1251. doi: 10.1097/AOG.0b013e31818c91dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010 Nov 5;330(6005):841–845. doi: 10.1126/science.1194637. Epub 2010 Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maggi L, Trettel F, Scianni M, Bertollini C, Eusebi F, Fredholm BB, Limatola C. LTP impairment by fractalkine/CX3CL1 in mouse hippocampus is mediated through the activity of adenosine receptor type 3 (A3R) J Neuroimmunol. 2009 Oct 30;215(1–2):36–42. doi: 10.1016/j.jneuroim.2009.07.016. Epub 2009 Aug 26. [DOI] [PubMed] [Google Scholar]
- 16.Shors TJ, Mathew PR. NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learn Mem. 1998 Jul–Aug;5(3):220–230. [PMC free article] [PubMed] [Google Scholar]
- 17.Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX(3)CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2009 Dec 15; doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]