Abstract
The objective of the present investigation was to design a sustained release floating microcapsules of theophylline using two polymers of different permeability characteristics; Eudragit RL 100 (Eu RL) and cellulose acetate butyrate (CAB) using the oil-in-oil emulsion solvent evaporation method. Polymers were used separately and in combination to prepare different microcapsules. The effect of drug-polymer interaction was studied for each of the polymers and for their combination. Encapsulation efficiency, the yield, particle size, floating capability, morphology of microspheres, powder X-ray diffraction analysis (XRD), and differential scanning calorimetry (DSC) were evaluated. The in vitro release studies were performed in PH 1.2 and 7.4. The optimized drug to polymer ratios was found to be 4:1 (F2) and 0.75:1 (F'2) with Eu RL and CAB, respectively. The best drug to polymer ratio in mix formulation was 4:1:1 (theophylline: Eu RL: CAB ratio). Production yield, loading efficiencies, and particle size of F2 and F’2 were found to be 59.14% and 45.39%, 73.93% and 95.87%, 372 and 273 micron, respectively. Microsphere prepared with CAB showed the best floating ability (80.3 ± 4.02% buoyancy) in 0.1 M HCl for over 12 h. The XRD and DSC showed that theophylline in the drug loaded microspheres was stable and in crystaline form. Microparticles prepared using blend of Eu RL and CAB polymers indicated more sustained pattern than the commercial tablet (P<0.05). Drug loaded floating microballoons prepared of combination of Eu RL and CAB with 1:1 ratio were found to be a suitable delivery system for sustained release delivery of theophylline which contained lower amount of polymer contents in the microspheres.
Keywords: Theophylline, Eudragit RL100, Cellulose acetate butyrate, Microparticles, Emulsion-solvent evaporation
INTRODUCTION
Floating systems are low-density systems having sufficient buoyancy to float over the gastric contents and remain in the stomach for a prolonged period of time. While the system floats over the gastric contents, the drug is released slowly at the desired rate, which results in increased gastro-retention time and reduced fluctuation in plasma drug concentration(1). Gastric emptying of dosage forms is extremely variable process to the extent that prolonging and controlling the emptying time is considered as a valuable asset of dosage forms which can reside in the stomach for a long period of time(2). Conventional oral dosage forms do not offer any control over drug delivery and cause great fluctuations in plasma drug concentrations. Single unit dosage forms have the disadvantage of a release all-or-nothing emptying process, while the multiple unit particulate systems pass uniformly through the GIT to avoid the variation of gastric emptying and thus release the drug more uniformly(3–5). The uniform distribution of these multiple unit dosage forms along the GIT could result in more reproducible drug absorption and reduced risk of local irritation; this gave rise to oral controlled drug delivery and led to development of gastro-retentive floating microspheres(5).
To prepare floating microspheres both natural and synthetic polymers have been used. Kawashima et al. prepared hollow microspheres or microballoons of ibuprofen using acrylic polymers by the emulsion-solvent diffusion method(6). Popular polymer solution systems that have been described in previous works to prepare floating micros-pheres are polycarbonate/dichloromethane(7,8), Eudragit S100/i-propanol(9) and CAB/Eu RL mixture in acetone(10). Moreover, methylcellulose and chitosan micropellets loaded with lansoprazole showed better encapsulation efficiencies with a lower density than gastric contents(11).
Srivastava et al. reported cimetidine-loaded floating microspheres of hydroxypropyl methylcellulose and ethyl cellulose(12). The prepared microspheres exhibited prolonged drug release (~8 h) and remained buoyant for >10 h(13). Sato et al. developed hollow microspheres or microballoons of riboflavin, aspirin, salicylic acid, ethoxybenzamide and indomethacin using Eudragit S100 as enteric polymer(14). Streubel et al. used polypropylene foam powder as porous carrier for the development of verapamil HCl-loaded floating microparticles(15). Development and comparative study of Eu RL and CAB microspheres containing theophylline with/without the addition of surfactant to the internal phase have been described in previous works(9).
Eu RL is a water-insoluble polymer and has been widely used in the microencapsulation as an enteric coating for tablets and capsules. Recently several workers have described using of Eu RL as a polymer employing aqueous or non- aqueous medium(16). The microencapsulation of drugs with CAB has been carried out successfully in either an aqueous or an organic vehicle. The high permeability of Eu RL gives the initial burst release, which is desirable from therapeutic point of view. CAB polymer exhibit slower rate of in vitro drug release initiated by lag time, which reduces the plasma drug fluctuations, as seen in conventional tablet dosage forms. There are several methods available which may be employed in the microencapsulation with CAB and Eu RL: they include coacervation-phase separation method, spraying-drying method and extrusion method(17).
In current study, an emulsion-solvent diffusion/evaporation technique was used to prepare a floating sustained-release system of theophylline. The influence of several factors on various physical characteristics, including particle size, drug loading, dissolution and floating properties of the resulting micro-spheres were investigated.
MATERIALS AND METHODS
Theophylline (Merck, Germany), Eudragit RL 100 (RÖhm Pharma GMBh, Weiterstadt, Germany), cellulose acetate butyrate (17% butyryl, 29% acetyl and 1.5% hydroxyl contents, Aldrich, USA), Sucrose stearate (Crodesta F70) (Croda GmbH, Mettelal, Germany), Span 80 (sorbitan monolaurate), Tween 80 (polysorbate 80), methanol, acetone, liquid paraffin, n- hexane, n- heptanes, hydrochloric acid, potassium dihydrogen phosphate, sodium hydroxide (Merck, Germany). All solvents and reagents were of analytical grade.
Preparation of microparticles with Eu RL
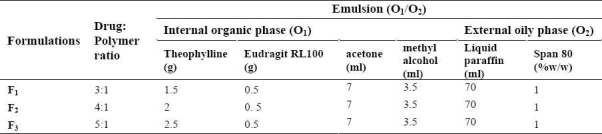
Microspheres were prepared by oil-in-oil (O1/O2) emulsion solvent evaporation method using different ratios of theophylline to Eu RL ratios (3:1, 4:1, and 5:1 as shown in Table 1). A mixed solvent system consisting of acetone and methanol in a 2:1 ratio and light liquid paraffin were chosen as primary and secondary oil phases, respectively. Span 80 was used as the surfactant for stabilizing the secondary oil phase. The drug suspension was emulsified in a liquid paraffin/Span 80 solution under stirring at 900 rpm (Model RZR-2000; Heidolph Electro, Kelheim, Germany) for 2 h. Then 50 ml of n-hexane (non-solvent) was added to harden the microspheres and stirring was continued for a further 1 h and the hardened microspheres were collected by filtration and washed with three portions of 50 ml of n-hexane and purified water air dried for 12 h. All microsphere formulations were prepared in triplicate.
Table 1.
Theophylline microsphere containing Eudragit RL formulations prepared by solvent evaporation method (o1/o2)
Preparation of microparticles with CAB
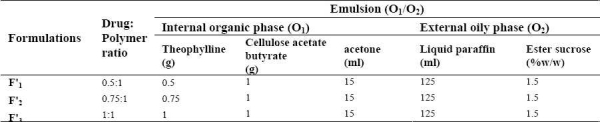
Microspheres were prepared by oil-in-oil (O1/O2) emulsion solvent evaporation method using different ratios of theophylline to CAB (0.5:1, 0.75:1 and 1:1) as shown in Table 2. Theophylline was dispersed in acetone (polymer solvent) containing CAB. The drug suspension was emulsified in a liquid paraffin/ester sucrose solution under stirring at 400 rpm (Model RZR-2000; Heidolph Electro, Kelheim, Germany) for 1 h in an ice bath. Then microspheres were collected, washed three times with 30 ml n-heptane to remove any remaining oily phase, air-dried for 12 h to obtain discrete microspheres. All microsphere formulations were prepared in triplicate.
Table 2.
Theophylline microsphere containing cellulose acetate butyrate formulations prepared by solvent evaporation method (o1/o2)
Preparation of microparticles with CAB:Eu RL combination
Microspheres were prepared using oil-in-oil (O1/O2) emulsion solvent evaporation method using theophylline to CAB and Eu RL ratio (4:1:1). The drug polymer dispersions completely dissolved in 10 ml acetone and were then slowly introduced into 75 ml liquid paraffin previously added with 1% Span 80, while stirring at 900 rpm for 2 h. Then, 50 ml of n-hexane (non-solvent) was added to solidify the microspheres and was stirred for 1 more h to allow complete evaporation of acetone. Microspheres were separated by filtration and washed thrice with 50 ml of n-hexane and purified water and air dried for 12 h. All microsphere formulations were prepared in triplicate.
Buoyancy percentage
The amount of 200 mg microspheres were spread over the surface of a USP dissolution apparatus (type II) filled with 900 ml 0.1 M acidic solution (HCl) containing 0.02% Tween 80(18). The medium was agitated with a paddle rotating at 100 rpm for 12 h. The floating and the settled portions of micro-spheres were recovered separately. The microspheres were dried and weighed. The buoyancy percentage was calculated by the following formula:
% buoyancy of microspheres = (weight of floating microspheres/initial weight of floating microspheres) × 100
Determination of percent loading efficiency and production yield
To 20 mg of each sample was added 10 ml methanol, stirred at 500 rpm for 30 min. The drug concentration was determined spectro-photometrically (UV-160, Shimadzu, Japan) at 286 nm. All experiments were done in triplicate. Loading efficiency was calculated according to the following equation:
Loading efficiency (%) = (actual drug content in microparticles/theoretical drug content) × 100
The prepared microspheres were collected and weighed. The measured weight was divided by the total amount of all non-volatile components which were used for the preparation of the microspheres. All of the experiments were performed in triplicate.
% Yield = (Actual weight of product/Total weight of excipient and drug) × 100
Frequency distribution analysis
Samples of microspheres were analyzed for frequency distribution with calibrated optical microscope fitted with a stage and an ocular micrometer. Small quantities of microsphere were spread on a clean glass slide and the average size of 50 particles and the frequency distribution was determined in each batch.
Differential Scanning Colorimetry (DSC)
The physical state of drug in the microspheres was analyzed by Differential Scanning Calorimeter (Shimadzu, Japan). The thermo grams of the samples were obtained at a scanning rate of 10 °C/min conducted over a temperature range of 25-300 °C.
X-ray Powder diffractometry (X-RPD)
X-RPD of the theophylline microspheres were performed by a diffractometer using model (Siemens D5000, Munich, Germany) equipped with a graphite crystal monochromator (CuKα) (a voltage of 40 KV and a current of 20 mA) radiations to observe the physical state of drug in the microspheres at voltage of 40 KV and a current of 20 mA.
Dissolution studies
Drug release on the microspheres were carried out using a USP basket method for 24 h at a stirring speed of 100 rpm and temperature of 37 ± 0.5 °C. An amount of the microspheres equivalent to 200 mg of theophylline filled in a hard gelatin capsule (Size no.0) were placed in the dissolution medium containing 900 ml of hydrochloric acid (0.1 M) buffer solution (pH 1.2). After 2 h, 17 ml of 0.2 M phosphate buffer stock, pre-equilibrated at 37 °C, were added to the dissolution vessel. The pH was immediately adjusted, if necessary, with 0.2 N HCl or 0.2 N NaOH to pH 7.4(19). A quantity (3 ml) of the dissolution medium was sampled at predetermined time intervals and fresh dissolution medium was simultaneously used to replenish the dissolution medium on each occasion to keep the volume constant. The sample was filtered through filter disc (0.45 μm), and the drug concentration in the samples was assayed spectrophotometrically at 271 nm for both the acidic and enteric buffers. Each experiment was repeated three times.
RESULTS
Effect of drug-polymer ratios on the physical properties of the microparticles
One of the features of this process was the use of two solvents (termed as ’mixed solvent system’ or MSS here)(20) as a dispersed medium and suitable non-aqueous processing medium to enable formation of O1/O2 emulsion. Components of the MSS can be selected from any of the commonly available organic solvents such as dichloromethane, ethyl acetate, acetone, acetonitrile, methanol, etc(21,22). Having chosen oil as the processing medium, it is imperative that solvent for polymer be immiscible with oil. Acetone is a unique organic solvent which is polar, water-miscible and oil-immiscible. All other organic solvents like methanol, ethyl alcohol, ethyl acetate, acetone, dimethyl sulphoxide and tetrahydrofuran are oil-miscible and do not form emulsions of the polymer solution in oil(19,21). When the drug has some solubility in the acetone: ethanol solution, prolonged mixing caused an increas-ed amount of aggregation to occur. The range of surfactant concentration used was between 0 and 1%. Higher concentration promoted aggregation of the microcapsules. With oil as a processing medium, use of acetone alone as a dispersing medium did not ensure formation of a stable emulsion. Liquid paraffin containing 1% surfactant (Span 80/ester sucrose) and non-solvent (n-hexane) were used in the normal microencapsulation procedure.
Microspheres were formed after a series of steps like solvent evaporation and addition of non-solvent. Microspheres (CAB and Eu RL) were prepared using different drug-polymer ratios as shown in Tables 1 and 2. The drug-polymer ratio was varied by maintaining the amounts of polymer, surfactant and solvent constant in all preparations, and changing the amount of drug. The results of the effect of drug-polymer ratio (microspheres containing CAB/Eu RL) on production yield, drug loading efficiency and mean particle size are shown in Table 3. In all formulations, the mean amount of drug entrapped in the prepared microspheres was different from theoretical value. The drug loading efficiencies were in the range of 63-88% for microspheres prepared with Eu RL and 34.58-95.87% for microspheres containing CAB. The highest encapsulation efficiency (95.87%) was obtained with CAB polymer.
Table 3.
Effect of drug: polymer ratio on drug loading efficiency, production yield and particle size of theophylline microspheres
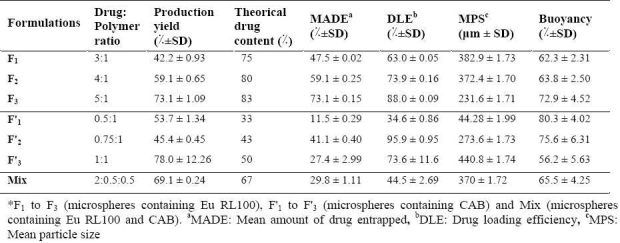
According to Table 3, increasing the drug to polymer ratios in microspheres prepared with both Eu RL 100 and CAB caused an increase in the production yield. In the case of microspheres containing Eu RL 100, increasing the drug to polymer ratios from 3:1 to 5:1 increased the production yield from 42.2 ± 0.93 to 73.1 ± 1.09. Similarly, increase drug to polymer ratios from 0.5:1 to 1:1 in microspheres containing CAB, the production yield increased from 45.39 to 78.02. The yield and loading efficiency of mix formulation (containing CAB and Eu RL) were 69.1 and 44.5, respectively. A volume-based size distribution of drug, polymer, and drug loaded microspheres indicated a log-probability distribution. Mean particle size of original theophylline, Eu RL and CAB was 429 ± 1.26 μm, 590.8 ± 1.73 μm and 131.3 ± 1.69 μm, respectively. The prepared floating micro-spheres containing of Eu RL were found to be discrete and spherical (Fig. 1). The mean diameter of microspheres composed of Eu RL1 and/or CAB were between 44.28 to 440.8 μm; CAB microspheres represented the least and largest size.,
Fig. 1.
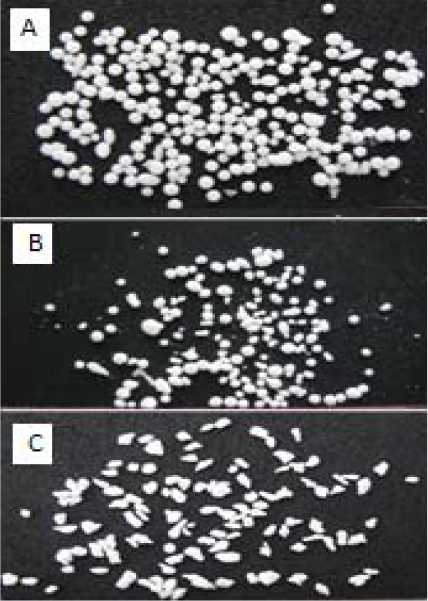
Optical microscopic photograph of a spherical microspheres F1 (theo: Eu RL ratio 4:1), F’2 (theo: CAB ratio 0.75:1) and Mix (theo: Eu RL: CAB ratio 1:0.5:0.5) formulations at 10x.
Percentage Buoyancy
Good in vitro buoyancy was observed for all microsphere formulations (Table 3). Microspheres prepared using CAB showed the optimized floatability (88.3 ± 4.02 buoyancy) in 0.1 M HCl. A floating time of 12 h may be considered a satisfactory performance of the prepared formulations. Eu RL is more permeable than cellulose acetate butyrate. Eu RL has 10% of functional quaternary ammonium groups. Density of Eu RL and CAB are 0.815-0.835 g/cm 3 and 1.16-1.3 g/cm 3 , respectively. Eu RL will give rise to an initial burst release which is essential from therapeutic point of view, while CAB will control the drug release by maintaining the buoyancy, which renders drug more permeable. It was evident that addition of Eu RL 100 increased the permeability of microcapsules to the surrounding dissolution medium due to the swelling nature of the polymer(23). In addition to this, the porous nature of microcapsules produces an upward motion of the dosage form to float on the gastric contents.
DSC
Pure theophylline exhibits a sharp melting endotherm around 271.4 °C (Fig. 2A, f). It is obvious from thermograms that the DSC curves of physical mixtures of drug with polymers as well as the microsphere formulations are almost the same. This endotherm of the drug is present in most of the thermograms at 269 to 270 °C (Fig. 2A). The intensity of the drug fusion peak, however, for the microsphere formulation was lower than that of the pure drug and physical mixtures.
Fig. 2.
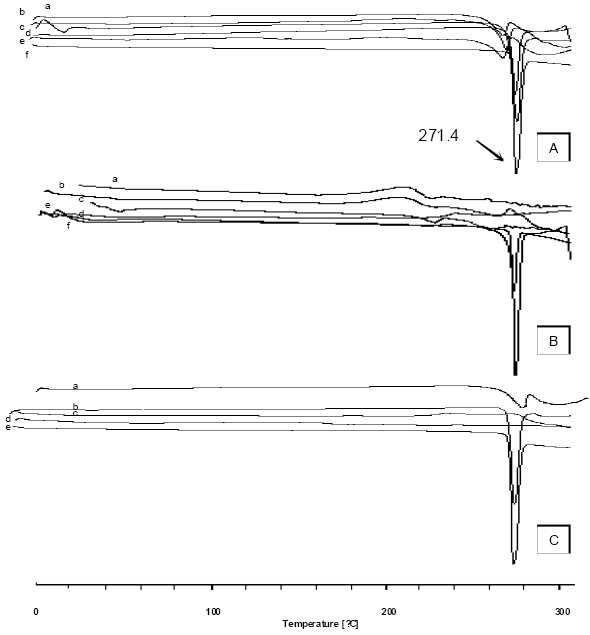
DSC thermogram of (A) microspheres of Eu RL; a) physical mixture F2, b) F1 (3:1 ratio) , c) theophylline d) F2 (4:1 ratio), e) F3 (5:1 ratio), f) Eu RL ( B) microspheres of CAB; a) F’1 (0.5:1 ratio) , b) F’2 (0.75:1 ratio), c) F’3? (1:1 ratio), d) physical mixture F’2, e) CAB, f) theophylline and (C) microspheres of mixture; a) Mix (1:0.5:0.5), b) physical mixture, c) Eu RL, d) CAB , e) theophylline.
X-RPD
The X-ray diffraction patterns show that the pure drug is crystalline in nature (Fig. 3A, a). However, when it was incorporated into the polymer matrix the principal peaks of the drug was appeared with lower intensity (Fig. 3).
Fig. 3.
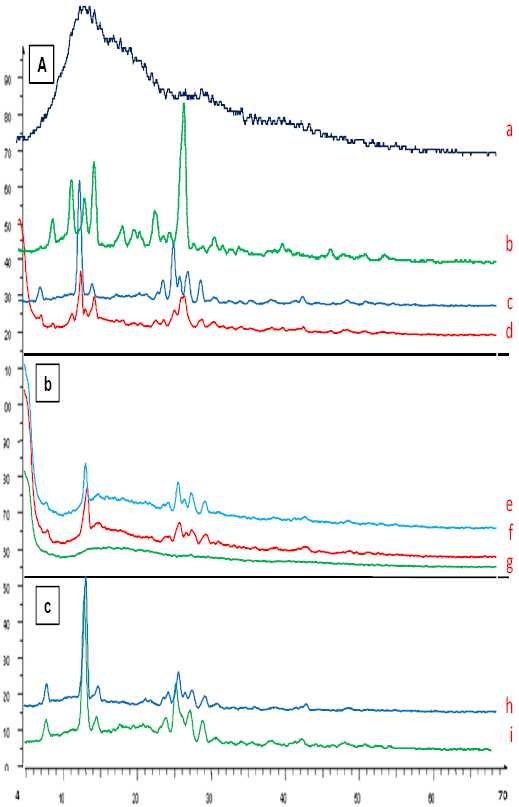
X-ray diffraction of A) theophylline (a), F2 (4:1 ratio) (b), physical mixture F2 (4:1 ratio) (c), Eu RL100 (d), B) CAB (e), physical mixture F’2 (f), F’2 (g), C) mix (1: 0.5: 0.5 ratio) (h), and physical mixture (i) formulations.
In vitro release studies
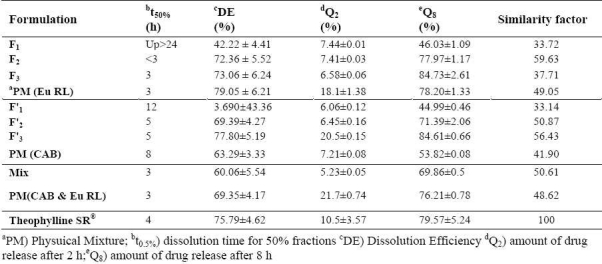
Fig. 4 shows the release profile of the drug from the microparticles. The in vitro release of theophylline from microspheres containing Eu RL exhibited initial burst effect which may be due to the presence of some drug particles on the surface of the microspheres. The release profiles are illustrated in Fig. 4A. In order to have better comparison between the dissolution profiles, dissolution efficiency (the area under the dissolution curve at a given time which is expressed as percentage of the area of the rectangle described by 100% dissolution at the same time), t50% (dissolution time for 50% fractions of drug), and f2 (used to compare multipoint dissolution profiles), Q2h and Q8h were calculated. Microspheres with high loading efficiency or high drug entrapment (F3 formulations) showed faster dissolution rate. Fig. 4 and Table 4 show that the initial drugs release for some of microsphere formulations are slightly high. Fig. 4 also shows that in most cases a biphasic dissolution pattern exist. This is the point where pH of the dissolution medium was altered from 1.2 to 7.4.
Fig. 4.
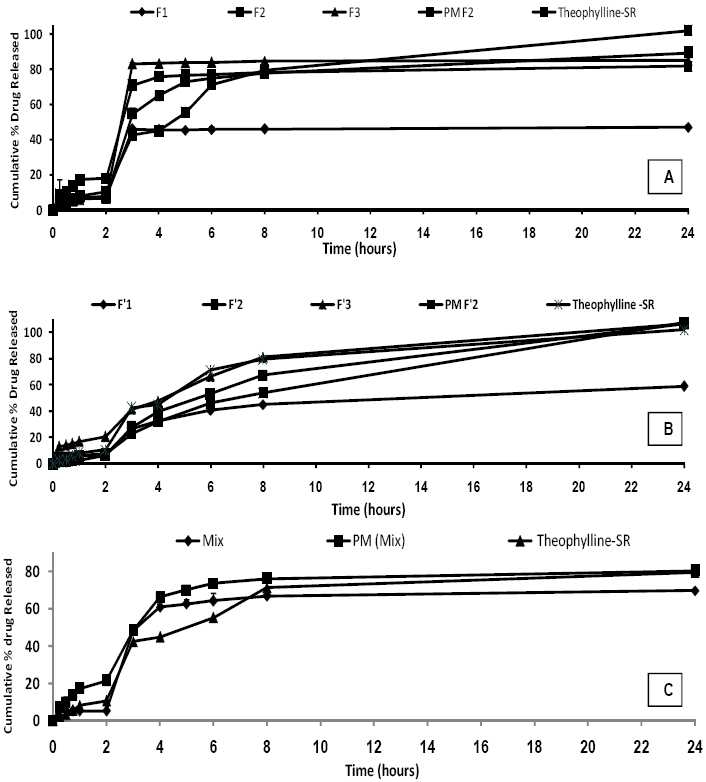
Percent release of theophylline from microspheres prepared with different polymer-to-drug ratio containing Eudragit RL (A), cellulose acetate butyrate (B), combination cellulose acetate butyrate and Eudragit RL (C), physical mixture and commercial theophylline SR®.
Table 4.
Comparision of various release characteristics of theophylline from different microsphere formulations, and physical mixture.
Comparing the drug release from micro-spheres containing CAB (Fig. 4B) shows that the release of drug from these microspheres is slower than the release of the drug from microspheres containing Eu RL (compare F3 with F’3). However, no significant difference was observed between the percentages of drug released at 8h (Q8h) between microspheres containing Eu RL or CAB (P > 0.05). Combination of CAB and Eu RL correspon-ding to lower level of the polymer with theophylline in the formulation (Mix) resulted in a sustained fashion in the drug release rate and reduced the initial release (Fig. 4C). Combination of CAB and Eu RL corresponding to lower level of the polymer with theophylline in the formulation (Mix) resulted in sustaining the drug release rate and reduced the initial release (Fig. 4C).
When microspheres come in contact with gastric fluid the gel formers, polysaccharides, and polymers hydrate to form a colloidal gel barrier that controls the rate of fluid penetration into the device and consequent drug release. As the exterior surface of the dosage form dissolves, the gel layer is maintained by the hydration of the adjacent hydrocolloid layer. The air trapped by the swollen polymer lowers the density and confers buoyancy to the microspheres. However, a minimal gastric content needed to allow proper achievement of buoyancy(1,5). Hollow microspheres of acrylic resins, Eudragit, polyethylene oxide, and cellulose acetate; polystyrene floatable shells; polycarbonate floating balloons and gelucire floating granules are the recent developments.
DISCUSSION
The encapsulation efficiency of the drug depended on the solubility of the drug in the solvent and continuous phase. The high entrapment efficiency of theophylline in microspheres may be attributed to its poor aqueous solubility. Encapsulation efficiency rose with increase in theophylline concen-tration in the microspheres containing Eu RL and CAB. This could be due to the high permeability characteristics of polymer which would facilitate the diffusion of part of the entrapped drug to the surrounding medium during the preparation of the microparticles. The reason for increased production yield at high drug-polymer ratios could be due to decreased diffusion rate of solvents (acetone and/or methanol) from concentrated solutions into emulsion. The extent of loading influenced the particle size distribution of microspheres. Decreasing of microspheres size can be attributed to the fact that with the higher diffusion rate of non-solvent to polymer solution the smaller size of microcapsules is easily obtained(24). The mean particle size of CAB microspheres was greater than that of Eu RL microspheres, and this may be viscosity related.
The nature of the polymer influenced the floating behavior of the microspheres. Micros-pheres with the highest levels of CAB and Eu RL were least buoyant. It is likely that the surfactant incorporated in the formulations would have increased their wettability and hence, hydration, more than in the other microspheres which had lower levels of the permeable Eu RL and CAB. Consequently, the increased amount of absorbed liquid medium replaced the air inside the floating microspheres, thus rendering them less buoyant(5,12,13).
The drug could be either dispersed in crystalline/amorphous form or dissolved in the polymeric matrix during the process of microencapsulation(25,26). Any abrupt or drastic change in the thermal behavior of rather the drug or polymer may indicate a possible drugpolymer interaction(27). DSC thermograms did not reveal any interaction between drug and excipients in prepared microspheres.
Low intensity of peaks could be ascribed to the crystalline state of the drug in the microparticles. This confirms the results obtained from DSC experiments.
In vitro release
As more drugs are released from the microspheres, more channels are probably produced, contributing to faster drug release rates. F1 , F2 and F3 formulations showed the lowest burst release in comparison with theophylline SR and physical mixture formulations and the percentage of burst release reduced as the ratio of drug to polymer in the preparation of microsphere decreased. The burst release could be attributed to the presence of some theophylline particles on the surface of microspheres. When particles are prepared by O1 /O2 method, water-soluble drugs do not have tendency to migrate to the non-polar medium, thereby concentrating on the surface of the microspheres leading to burst effect(28). Moreover, the burst release could also be explained by the imperfect encapsulation of the drug inside microparticles, resulting from the unstable nature of the emulsion droplets during the solvent removal step. This potential instability may cause a part of the loaded drug to relocate at the microparticle surface, thereby rapidly released(29). The first portion of the biphasic dissolution curves is due to theophylline dissolution which starts immediately after the beginning of the dissolution process. For the release of the drug in the second phase combination of the diffusion of the remaining dispersed drug into the bulk medium, formation of pores within the matrix due to the initial drug dissolution and swelling which enhances the permeability of the polymer to the drug might be involved(30). Fig. 4 illustrates that different theophylline microspheres exhibited different dissolution profiles. In order to find out which release profiles is more suitable for oral administration, the release data were compared with those of commercial theophylline extended release formulations. The theophylline microspheres prepared in this study could be embedded into soft gelatin capsules for peroral administration. According to the US pharmacopoeia not less than 70-80% of the theophylline should be released within 8 h(21). The similarity factor showed that microsphere formulations F2 (containing Eu RL), F’2 , F’3 (containing CAB) and mix (containing Eu RL and CAB) exactly matches the release profile of commercial formulations (Table 4) and there was no significant difference between these two dissolution profiles (f2 = 50.61-59.63%). CAB has a low permeability to drug which results from its high intermolecular attraction. Hydrogen bonding between the hydroxyl groups of the carboxylic moiety and the carbonyl oxygen of ester group increases the degree of solidity of the polymer and decreases its porosity and permeability. However, Eu RL is a copolymer of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups. The ammonium groups present as salts promotes permeability and act as a channeling agent for the entrance of the liquid medium through the floating microsphere wall, causing it to swell. This facilitates the diffusion of the dissolved drug out of the microsphere into the dissolution medium. Thus, by varying the ratio of CAB and Eu RL in the theophylline microspheres, the rate of release of theophylline can be controlled.
CONCLUSION
Theophylline microspheres were prepared using the solvent evaporation method. This method has been applied for the preparation of microballoons system with other polymers and drugs(5). The high permeability of Eu RL gives the initial burst release, which is desirable from therapeutic point of view. CAB polymer exhibit slower rate of in vitro drug release initiated by lag time, which reduces the plasma drug fluctuations, as seen in conven-tional tablet dosage forms. In the present study, controlled release without initial peak level achieved with these formulations may reduce dose frequency and side effects as well as improve patient compliance.
Acknowledgments
The financial support of research office of Tabriz University of Medical Sciences is greatly acknowledged.
REFERENCES
- 1.Gaba P. Floating microspheres: a review. 2008. Available from URL: URL:http://www.pharmainfo.net/reviews /floating-microspheres-review .
- 2.Hirtz J. The gut absorption of drugs in man: a review of current concepts and methods of investigation. Br J Clin Pharmacol. 1985;19:77–83. doi: 10.1111/j.1365-2125.1985.tb02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien YW. Controlled and modulated release drug delivery systems. In: Swarbrick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. New York: Marcel Dekker; 1990. pp. 280–285. [Google Scholar]
- 4.Jain NK. 1st ed. New Delhi: CBS Publishers and Distributors; 2002. Controlled novel drug delivery; pp. 236–255. [Google Scholar]
- 5.Kouchak M, Badrian A. Preparation and in vitro evaluation of a microballon delivery system for theophylline. Iran J Pharm Res. 2007;6:35–42. [Google Scholar]
- 6.Kawashima Y, Niwa T, Takechi H, Hino T, Itoh Y. Hollow microspheres for use as floating controlled drug delivery systems in the stomach. J Pharm Sci. 1992;81:35–140. doi: 10.1002/jps.2600810207. [DOI] [PubMed] [Google Scholar]
- 7.Soppimath KS, Kulkarni AR, Rudzinski WE, Aminabhavi TM. Microspheres as floating drug-delivery systems to increase gastric retention of drugs. Drug Metab Rev. 2002;33:149–160. doi: 10.1081/dmr-100104401. [DOI] [PubMed] [Google Scholar]
- 8.Muthusamy K, Govindarazan G, Ravi TK. Preparation and evaluation of lansoprazole floating micropellets. Ind J Pharm Sci. 2005;67:75–79. [Google Scholar]
- 9.Thanoo BC, Sunny MC, Jayakrishnan A. Oral sustained-release drug delivery systems using polycarbonate microspheres capable of floating on the gastric fluid. J Pharm Pharmacol. 1993;45:21–24. doi: 10.1111/j.2042-7158.1993.tb03672.x. [DOI] [PubMed] [Google Scholar]
- 10.Joseph NJ, Lakshmi S, Jayakrishnan A. A floating-type oral dosage form for piroxicam based on hollow polycarbonate microspheres: in vitro and in vivo evaluation in rabbits. J Control Release. 2002;79:71–79. doi: 10.1016/s0168-3659(01)00507-7. [DOI] [PubMed] [Google Scholar]
- 11.Stithit S, Chen W, Price JC. Development and characterization of buoyant theophylline microspheres with near zero order release kinetics. J Microencaps. 1998;19:725–737. doi: 10.3109/02652049809008255. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Park TG, Choi HK. Development of oral drug delivery system using floating microspheres. J Microencaps. 1999;16:715–729. doi: 10.1080/026520499288663. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava AK, Ridhurkar DN, Wadhwa S. Floating microspheres of cimetidine: formulation, characterization and in vitro evaluation. Acta Pharm. 2005;55:277–285. [PubMed] [Google Scholar]
- 14.Jain AK, Jain CP, Tanwar YS, Naruka PS. Formulation, characterization and in vitro evaluation of floating microspheres of famotidine as a gastro retentive dosage form. 2009;3:222–226. [Google Scholar]
- 15.Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. Physicochemical properties to determine the buoyancy of hollow microspheres (microballons) prepared by the emulsion solvent diffusion method. Eur J Pharm Biopharm. 2003;55:297–304. doi: 10.1016/s0939-6411(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 16.Streubel A, Siepmann J, Bodmeier R. Floating microparticles based on low density foam powder. Int J Pharm. 2002;241:279–292. doi: 10.1016/s0378-5173(02)00241-7. [DOI] [PubMed] [Google Scholar]
- 17.Joseph W, Nairn JG. Some factors affecting the microencasulation of pharmaceuticals with cellulose acetate phthalate. J Pharm Sci. 1986;75:573–78. doi: 10.1002/jps.2600750610. [DOI] [PubMed] [Google Scholar]
- 18.Wan LSC, Chui WK. Deviation of the ratio of drugs in a two-component mixture encapsulated in cellulose phthalate microspheres. J Microencapsul. 1995;12:417–23. doi: 10.3109/02652049509087254. [DOI] [PubMed] [Google Scholar]
- 19.24th ed. Vol. 1. Rockville: United States: Pharmacopoeial Convention; 2000. The United States Pharmacopoeia; pp. 1941–1943. [Google Scholar]
- 20.Kilicarslan M, Baykara T. The effect of the drug/polymer ratio on the properties of verapamil HCL loaded microspheres. Int J Pharm. 2003;252:99–109. doi: 10.1016/s0378-5173(02)00630-0. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan NB, Thomas PA, Pandit JK, Kulkarni MG, Mashelkar RA. Preparation of non-porous microspheres with high entrapment efficiency of proteins by a (water-in-oil)-in-oil emulsion technique. J Control Release. 1999;55:9–20. doi: 10.1016/s0168-3659(98)00140-0. [DOI] [PubMed] [Google Scholar]
- 22.Kim BK, Hwang SJ, Park JB, Park HJ. Preparation and characterization of drug-loaded poly-methacrylate microspheres by an emulsion solvent evaporation method. J Microencapsul. 2002;19:811–822. doi: 10.1080/0265204021000022770. [DOI] [PubMed] [Google Scholar]
- 23.Nath B, Nath LK, Mazumdar B, Sharma N, Sarkar M. Design and development of metformin HCl floating microparticles using two polymers of different permeability characteristics. Int J Pharm Sci Nanotech. 2009;2:627–637. [Google Scholar]
- 24.Bhardwaj SB, Shukla AJ, Collins CC. Effect of varying drug loading on particle size distribution and drug release kinetics of verapamil hydrochloride microspheres prepared with cellulose esters. J Microencapsul. 1995;12:71–81. doi: 10.3109/02652049509051128. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann J, Bodmeier R. Biodegradable, somatostatin acetate containing microspheres prepared by various aqueous and non-aqueous solvent evaporation methods. Eur J Pharm Biopharm. 1998;45:75–82. doi: 10.1016/S0939-6411(97)00125-2. [DOI] [PubMed] [Google Scholar]
- 26.Bodmeier R, Chen H. Preparation and characterization of microspheres containing the anti-inflammatory agents, indomethacin, ibuprofen, and ketoprofen. J Control Release. 1989;10:167–175. [Google Scholar]
- 27.Jones DS, Pearce KJ. Investigation of the effects of some process variables on microencapsulation of propranolol HCl by solvent evaporation method. Int J Pharm. 1995;118:199–205. [Google Scholar]
- 28.Jameela SR, Suma N, Jayakrishnan A. Protein release from poly (ε-caprolactone) microspheres prepared by melt encapsulation and solvent evaporation techniques: a comparative study. J Biomater Sci Polym. 1997;8:457–466. doi: 10.1163/156856297x00380. [DOI] [PubMed] [Google Scholar]
- 29.Lu W, Park TG. Protein release from poly (lactic-coglycolic acid) microspheres: protein stability problems. J Pharm Sci Technol. 1995;49:13–19. [PubMed] [Google Scholar]
- 30.Pignatello R, Consoli P, Puglisi G. In vitro release kinetics of tolmetin from tabletted Eudragit microparticles. J Microencapsul. 2000;7:373–383. doi: 10.1080/026520400288337. [DOI] [PubMed] [Google Scholar]


