Abstract
Galbanic acid, a sesquiterpene coumarin from Ferula szowitsiana, and conferol, another sesquiterpene coumarin from F. badrakema, were evaluated for their effects on the reversal of multi-drug resistance in clinical isolates of Staphylococcus aureus and Escherichia coli, respectively. Neither galbanic acid (up to 1000 μg/ml) nor conferol (up to 400 μg/ml) by itself shows any antibacterial activities against tested strains. The minimum inhibitory concentrations (MICs) of ciprofloxacin and tetracycline were determined using macrodilution technique in the presence and absence of sub-inhibitory concentrations of galbanic acid (31.25-1000 μg/ml) or conferol (50-400 μg/ml), however they caused no change in MICs of the antibiotics. Galbanic acid did not show any inhibitory effect on efflux phenomenon of E. coli. This can be related to the outer membrane of gram-negative bacteria which is impermeable to lipophilic compounds or another mechanism rather than efflux responsible for resistance in tested E. coli strains. An inhibitory effect of conferol on the efflux was compared with verapamil as a positive control. Because efflux is the only known mechanism of resistance to ethidium bromide (model efflux substrate) and verapamil reduced MIC of ethidium bromide, efflux mechanism can be considered as one of the resistance mechanisms in tested S. aureus strains. Conferol, however, did not enhance the antibiotic efficacy mediated by inhibiting efflux pumps in bacteria.
Keywords: Conferol, Escherichia coli, Ferula, Galbanic acid, Multi-drug resistance, Staphylococcus aureus
INTRODUCTION
Bacterial infections are becoming more challenging to treat, due to the emergence of multi-drug resistant (MDR) pathogenic bacteria(1). One of the resistance mechanisms is reduced drug uptake into the bacterial cell. There are several mechanisms through which the uptake of a drug into a cell can be reduced: changes in the structure of the cell membrane; loss or mutations of porins in the cell membranes; and active efflux of the drug from the cells(2). Efflux of antibiotics is a clinically important and common resistance mechanism for bacteria often endowing organisms with MDR phenotypes(3).
S. aureus is 0an important community- and major hospital-acquired pathogen. This organ-ism is a considerable concern, due to its ability to acquire resistance towards the newest antibacterial drugs(4). An analysis of the genome sequence of methicillin-resistant S. aureus N315 indicates that there are more than 20 open reading frames capable of encoding antibiotic efflux pumps(3). To date, more than 10 efflux pumps have been described for S. aureus, 0most of them are capable of extruding compounds of different chemical classes(5).
E. coli is recognized as a commensal organism and also the most common cause of urinary tract infections and diarrhea. In children and the immunocompromised pati-ents, it can cause more serious infections associated with higher morbidity and mortality(6). For gram-negative bacteria, the resistance nodulation and cell division (RND) efflux systems are major contributors to resistance such as AcrAB-TolC which is involved in the resistance of E. coli(1).
Fig. 1.
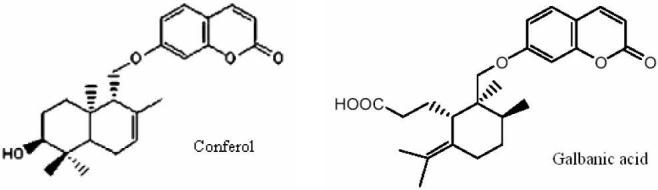
Chemical structure of conferol, a sesquiterpene-coumarin isolated from Ferula badrakemaand galbanic acid a sesquiterpene-coumarin isolated from Ferula szowitsiana.
Continued research in existing classes of antibiotics to identify derivatives that efflux minimally, and development of efflux pump inhibitors that could be used in combination with existing antibiotics to increase their potency, are strategies to combat efflux-mediated resistance(7).
The exclusively old world genus Ferula belongs to the family Umbelliferae distributed throughout the Mediterranean area and central Asia, especially in the former USSR and neighboring countries such as Iran. This genus is well documented as a good source of biologically active compounds such as sesquiterpene derivatives(8). Some sesqui-terpenoid compounds act as enhancers of the nonspecific bacterial permeability to anti-biotics resulting from disruption of the cytoplasmic membrane. There are also some evidence on the reversal of MDR in tumor cells via inhibition of P-glycoprotein by these compounds(9,10). In this study, the possible effects of two sesquiterpene coumarin compounds, galbanic acid and conferol, on improvement of the antibiotics activity in resistant strains of E. coli and S. aureus, respectively, were investigated.
MATERIALS AND METHODS
Galbanic acid and conferol
Galbanic acid and conferol were isolated from F. szowitsiana and F. badrakema as described previously(11,12).
Bacterial strains
Six isolated samples of S. aureus and seven isolated samples of E. coli were obtained from Imam Reza and Ghaem University Hospitals, Mashhad, Iran, as resistant isolates. They were already subjected to disk diffusion method in the hospitals to obtain resistant strains. The antibiotic disks used were methicillin (30 μg), tetracycline (30 μg) and ciprofloxacin (5 μg) purchased from Padtan Teb, Iran. S. aureus ATCC 29737 and E. coli ATCC 8739 were used as standard strains.
Determination of the MICs of antibiotics and galbanic acid for E.coli strains, and antibiotics and, conferol, ethidium bromide and verapamil for S. aureus strains
MICs were determined by macrodilution technique using 24 well plates, in triplicate. Using two-fold broth dilution method, 0.1 ml aliquots of the bacterial cell suspension (106cfu/ml) was added into each well containing 1 ml of serial two-fold dilutions of tetracycline (Ningxia Qiyuan, China), cipro-floxacin (Temad, Iran), galbanic acid, ethidium bromide (CinnaGen, Iran), verapamil (Recordati, Italy) or conferol in MHB (Mueller-Hinton Broth) (Himedia, India). Galbanic acid was dissolved in ethanol before dilution into MHB, at final concentration of 5% which had no antibacterial effect on its own. Also conferol was dissolved in dimethyl sulfoxide (Merck, Germany) at final concentration of 2%(13), and diluted in MHB supplemented with 0.5-1% Tween 80 (Merck, Germany)(14). In each plate, inoculated and uninoculated wells of tested-material-free broth were included (the first well controls the adequacy of the broth to support the growth of the organism and the second is a check of sterility). The plates were incubated overnight at 37 °C(15).
To indicate bacterial growth, 0.5 ml of 2,3,5-triphenyltetrazolium chloride (TTC) 5 mg/ml (Sigma, UK) were added to the wells and incubated at 37 °C for 10-30 min(16).
Combination effects of galbanic acid or conferol with antibiotics
Combination studies were performed using the broth chequerboard assay; sub-inhibitory concentrations of galbanic acid (31.25-1000 μg/ml) were added to serial two-fold dilutions of ciprofloxacin or tetracycline; and sub-inhibitory concentrations of conferol (50-400 μg/ml) or verapamil as the positive control (25-200 μg/ml) were added to serial two-fold dilutions of ciprofloxacin, tetracycline or ethidium bromide as a test substrate. After inoculating the wells with 0.1 ml of the bacterial cell suspension (106cfu/ml), the plates were incubated overnight at 37 °C. The growth of microorganisms was assessed by TTC assay as described above(15,17).
RESULTS
The MICs of ciprofloxacin and tetracycline against E. coli were ≥40 and ≥80 μg/ml, respectively. MICs of antibiotics against S. aureus were 10-80 μg/ml for ciprofloxacin and 80-160 μg/ml for tetracycline. S. aureus and E. coli isolates were considered resistant to ciprofloxacin and tetracycline when the MICs (μg/ml) were ≥4 and ≥16, respectively, according to National Committee on Clinical Laboratory Standards breakpoint criteria(18).
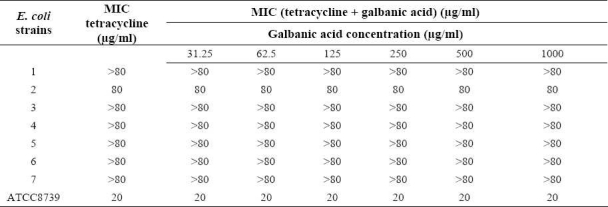
Galbanic acid alone had no inhibitory effect on E. coli isolates (up to 1000 μg/ml) and did not change the MICs of the antibiotics against E. coli (Tables 1 and 2).
Table 1.
MIC of ciprofloxacin in the absence and the presence of galbanic acid against standard and resistant hospital isolates of E. coli.
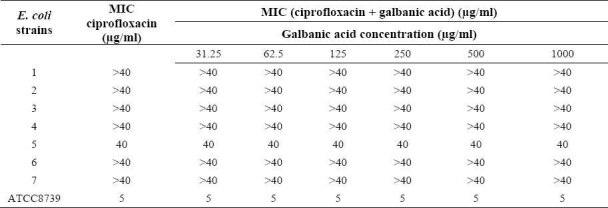
Table 2.
MIC of tetracycline in the absence and the presence of galbanic acid against standard and resistant hospital isolates of E. coli.
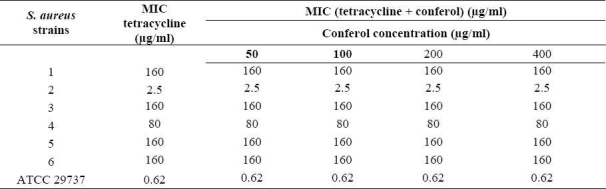
Conferol and verapamil (up to 400 μg/ml) by itself did not show any antibacterial activities against S. aureus. A synergistic study between verapamil and antibiotics (or ethidium bromide) led to a 2- to 8-fold reduction in MICs in clinical strains of S. aureus. The MICs of the ciprofloxacin, tetra-cycline and ethidium bromide in the presence of verapamil at the concentrations of 50, 100 and 200 μg/ml were reduced 2- and 4-fold in almost all S. aureus strains, and 8-fold in 50% of S. aureus strains. Verapamil exposure resulted in 2- to 4-fold reduction in MICs, against susceptible standard strains of S. aureus (Tables 3–5). However conferol caused no changes in MICs of antibiotics or ethidium bromide against any strains of S. aureus studied (Tables 6–8).
Table 3.
MIC of ciprofloxacin in the absence and the presence of verapamil against standard and resistant hospital isolates of S. aureus.
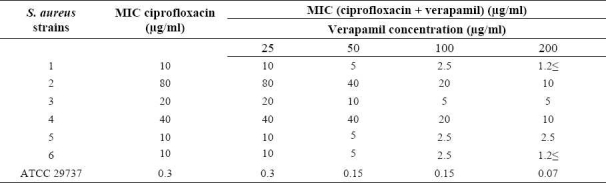
Table 5.
MIC of ethidium bromide in the absence and the presence of verapamil against standard and resistant hospital isolates of S. aureus.
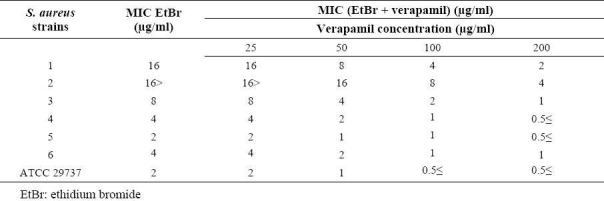
Table 6.
MIC of ciprofloxacin in the absence and the presence of conferol against standard and resistant hospital isolates of S. aureus.
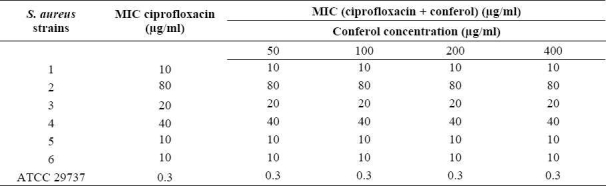
Table 8.
MIC of ethidium bromide in the absence and the presence of conferol against standard and resistant hospital isolates of S. aureus.
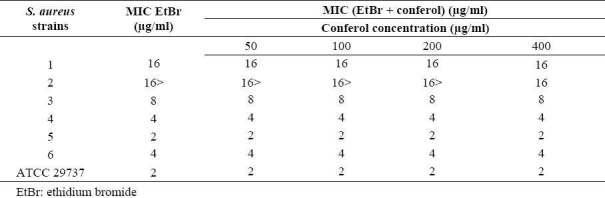
Table 4.
MIC of tetracycline in the absence and the presence of verapamil against standard and resistant hospital isolates of S. aureus.
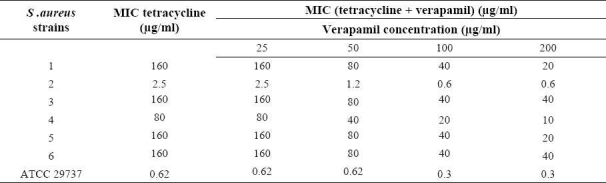
Table 7.
MIC of tetracycline in the absence and the presence of conferol against standard and resistant hospital isolates of S. aureus.
DISCUSSION
Multi-drug efflux is an increasingly reported phenomenon and has been described for many organisms, including bacteria, fungi and protozoa, and as a mechanism of resistance in mammalian tumor cells(19,20). One natural role of efflux pumps in prokaryotic and eukaryotic cells is to remove toxins from the interior of the cell. This protective function enables bacterial cells to survive in the presence of antibiotics during the treatment of infections. Many academic and pharmaceutical programs have focused on identifying inhibitors of gram-negative and gram-positive efflux systems that could potentially be used in combination with antibiotics to improve efficacy and to suppress resistance(3).
It has been suggested that plants could provide a rich source of MDR efflux pump inhibitors(6). Compounds such as sesqui-terpene-coumarin ether and driportlandin have been identified as promising modulators of MDR in mammalian cells(10). Galbanic acid a sesquiterpene coumarin isolated from F. szowitsiana, enhances the activity of penicillin G, cephalexin(21), ciprofloxacin and tetracy-cline against S. aureus possibly by inhibiting the efflux mechanism(22,23). In this study, the effect of galbanic acid on the reversal of resistance in gram-negative bacteria, E. coli was investigated. The results show that various concentrations of this compound did not have any modulating activity. Such effect can be explained by the following reasons:
-In gram-negative bacteria the outer membrane significantly slows down the entry of both lipophilic and hydrophilic agents. The former, are hindered by the lipopolysaccharide components of the outer leaflet of the outer membrane bilayer. Hydrophilic agents cross the outer membrane through water-filled porins whose size prevents rapid diffusion(7). Outer membrane as an additional permeability barrier, explains the greater resistance obser-ved by gram-negative bacteria as opposed to gram-positive organisms(4). Galbanic acid, probably could not cross the outer membrane to inhibit efflux proteins because of its hydrophobic structure.
-Another mechanism other than efflux might be responsible for resistance in tested E. coli isolates.
-It is also possible that highly resistant strains have acquired too many target-based mutations, making it difficult, even with an ideal efflux inhibitor, to achieve sufficient intracellular antibiotic concentrations to over-come the reduction in target binding affinity(3).
-In E. coli, MDR pumps that confer resistance to fluoroquinolones and tetracycline belong to the RND (Resistance Nodulation Cell Division) family. In S. aureus, by contrast, resistance to the aforementioned compounds is mainly due to the activity of pumps belonging to MFS (Major Facilitator Superfamily). It is therefore less likely that a single inhibitor will potentiate fluoroqui-nolones and tetracycline in both E. coli and S. aureus(7).
Conferol is a sesquiterpene coumarin isolated from F. badrakema. There is a report that conferol can enhance the cytotoxicity of chemotherapeutic agents in tumor cells(12). While there is limited structural homology between bacterial and mammalian efflux systems, there is significant substrate overlap. Therefore, it is not surprising that many mammalian MDR inhibitors also affect bacterial efflux(3). On the other hand, conferol is structurally similar to galbanic acid, ineffective on E. coli strains, but acting as modulator of MDR in S. aureus strains. Thus, in other part of this study, the possible effect of conferol on the improvement of antibiotic activity in resistant strains of S. aureus was examined. The calcium channel antagonist verapamil, a known inhibitor of efflux pumps, was used as positive control(1,24).
Conferol and verapamil (up to 400 μg/ml) by itself did not show any antibacterial activity against any of the S. aureus strains studied (both clinical resistant isolates and standard). A synergistic study between verapamil and antibiotics (or ethidium bromide) led to a 2- to 8-fold reduction in MICs in resistant clinical isolates and 2- to 4-fold reductions in MICs in standard strain of S. aureus.
Ethidium bromide is a model efflux substrate that the only known mechanism of resistance to it is via efflux(3). Since verapamil caused a 2- to 8-fold reduction in ethidium bromide MIC, efflux mechanism can be considered as one of the resistance mechanisms in tested S. aureus isolates(1,6). The fact that verapamil also reduced the MICs of antibiotics and ethidium bromide in S. aureus ATCC 29737 suggests basal intrinsic efflux activity that confers a baseline low level of intrinsic resistance to structurally unrelated compounds considered toxic to the bacterial cell(5,24).
Conferol can significantly enhance the cytotoxicity of chemotherapeutic drugs in tumor cells by interaction with the trans-membrane domains of P-glycoprotein(12); however according to the results obtained in the synergistic studies in bacteria, it did not inhibit bacterial efflux proteins to enhance antibiotics efficacy. This could be related to structural differences between bacterial and mammalian efflux systems; the mammalian P-glycoprotein whose over expression confers resistance to cytotoxic compounds belongs to ABC transporters (ATP-binding cassette), whereas in S. aureus, MDR is mainly conferred by MFS efflux systems such as NorA(1).
CONCLUSION
Galbanic acid, a sesquiterpene coumarin from F. szowitsiana, and conferol, a sesqui-terpene coumarin from F. badrakema, did not show any effect as modulators of antibiotic resistance on clinical isolates of E. coli or S. aureus, respectively.
Acknowledgments
This work was supported financially by Vice Chancellery for Research of Mashhad University of Medical Sciences, Mashhad, Iran.
REFERENCES
- 1.Marquez B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie. 2005;87:1137–1147. doi: 10.1016/j.biochi.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Langton KP, Henderson PJF, Herbert RB. Antibiotic resistance: multidrug efflux proteins, a common transport mechanism? Nat Prod Rep. 2005;22:439–451. doi: 10.1039/b413734p. [DOI] [PubMed] [Google Scholar]
- 3.Mullin S, Mani N, Grossman TH. Inhibition of antibiotic efflux in bacteria by the novel multidrug resistance inhibitors biricodar (VX-710) and timcodar (VX-853) Antimicrob Agents Chemother. 2004;48:4171–4176. doi: 10.1128/AAC.48.11.4171-4176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stavri M, Piddock LJ, Gibbons S. Bacterial efflux pump inhibitors from natural sources. J Antimicrob Chemother. 2007;59:1247–1260. doi: 10.1093/jac/dkl460. [DOI] [PubMed] [Google Scholar]
- 5.Couto I, Costa SS, Viveiros M, Martins M, Amaral L. Efflux-mediated response of Staphylococcus aureus exposed to ethidium bromide. J Antimicrob Chemother. 2008;62:504–513. doi: 10.1093/jac/dkn217. [DOI] [PubMed] [Google Scholar]
- 6.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomovskaya O, Watkins WJ. Efflux pumps: their role in antibacterial drug discovery. Current Med Chem. 2001;8:1699–1711. doi: 10.2174/0929867013371743. [DOI] [PubMed] [Google Scholar]
- 8.Abd El-Razek MH, Ohta S, Hirata T. Terpenoid coumarins of the genus Ferula. Heterocycles. 2003;60:689–716. [Google Scholar]
- 9.Brehm-Stecher BF, Johnson EA. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob Agents Chemother. 2003;47:3357–3360. doi: 10.1128/AAC.47.10.3357-3360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madureira AM, Molnar A, Abreu PM, Molnar J, Ferreira MJ. A new sesquiterpene-coumarin ether and a new abietane diterpene and their effects as inhibitors of P-glycoprotein. Planta Med. 2004;70:828–833. doi: 10.1055/s-2004-827231. [DOI] [PubMed] [Google Scholar]
- 11.Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, et al. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochem. 2007;68:554–561. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Rassouli FB, Matin MM, Iranshahi M, Bahrami AR, Neshati V, Mollazadeh S, et al. Mogoltacin enhances vincristine cytotoxicity in human transitional cell carcinoma (TCC) cell line. Phytomedicine. 2009;16:181–187. doi: 10.1016/j.phymed.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Zgoda JR, Porter JR. A convenient microdilution method for screening natural products against bacteria and fungi. Pharmaceut Biol. 2001;39:221–225. [Google Scholar]
- 14.Kuroda M, Nagasaki S, Ohta T. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to (β-lactams in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2007;59:425–432. doi: 10.1093/jac/dkl519. [DOI] [PubMed] [Google Scholar]
- 15.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 16.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Tanaka H, Yamaguchi R, Kato K, Etoh H. Synergistic effects of mupirocin and an isoflavanone isolated from Erythrina variegata on growth and recovery of methicillin-resistant Staphylococcus aureus. IJAA. 2004;24:241–246. doi: 10.1016/j.ijantimicag.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Wayne: NCCLS Publisher; 2004. Performance standards for antimicrobial susceptibility testing; pp. M100–S14. 14th informational supplement. [Google Scholar]
- 19.Gibbons S, Oluwatuyi M, Kaatz GW. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J Antimicrob Chemother. 2003;51:13–17. doi: 10.1093/jac/dkg044. [DOI] [PubMed] [Google Scholar]
- 20.Zioh M, Kaatz GW, Gibbons S. Inhibitors of multidrug resistance (MDR) have affinity for MDR substrates. Bioorg Med Chem Lett. 2004;14:881–885. doi: 10.1016/j.bmcl.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Shahverdi AR, Fakhimi A, Zarrini G, Dehghan G, Iranshahi M. Galbanic acid from Ferula szowitsiana enhanced the antibacterial activity of penicillin G and cephalexin against Staphylococcus aureus. Biol Pharm Bull. 2007;30:1805–1807. doi: 10.1248/bpb.30.1805. [DOI] [PubMed] [Google Scholar]
- 22.Fazly Bazzaz BS, Memariani Z, Khashiarmanesh Z, Iranshahi M, Naderinasab M. Effect of galbanic acid, a sesquiterpene coumarin from Ferula szowitsiana, as an inhibitor of efflux mechanism in resistant clinical isolates of Staphylococcus aureus. Braz J Microbiol. 2010;41:574–580. doi: 10.1590/S1517-83822010000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazly Bazzaz BS, Rezaei Du A, Iranshahi M, Naderinasab M, Khajeh Karamadini M. Evaluating the potentiating effect of galbanic acid from Ferula szowitsiana on three common antibiotics against resistant hospital isolates of Staphylococcus aureus. Iran J Pharm Res. 2009;8:217–221. [Google Scholar]
- 24.Aeschlimann JR, Dresser LD, Kaatz GW, Rybak MJ. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:335–340. doi: 10.1128/aac.43.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]


