Abstract
Purpose
The effect of statin medication use on risk of prostate cancer is unknown.
Materials and Methods
We examined data from a longitudinal, population-based cohort of 2447 men between the ages of 40 and 79 that were followed from 1990 to 2007. Information on statin use was self-reported and obtained by biennial questionnaires. A randomly selected subset of men (634; 26%) completed biennial urologic examinations that included serum PSA measurements. Information on prostate biopsy and prostate cancer was obtained through review of community medical records.
Results
Of 634 statin users, 38 (6%) were diagnosed with prostate cancer compared to 186 (10%) of 1813 non-statin users. Statin use was associated with a decreased risk of receiving a prostate biopsy (HR: 0.31; 95% CI: 0.24, 0.40), prostate cancer diagnosis (HR: 0.36; 95% CI: 0.25, 0.53) and high-grade (Gleason ≥7) prostate cancer diagnosis (HR: 0.25; 95% CI: 0.11, 0.58). Statin use was also associated with a non-significant decreased risk of exceeding a PSA threshold of 4.0 ng/mL (HR: 0.63; 95% CI: 0.35, 1.13). In addition, longer duration of statin use was associated with lower risk of these outcomes (all tests for trend p<0.05).
Conclusions
Statin use is associated with a decreased risk of prostate cancer diagnosis. This association may be explained by decreased detection or cancer prevention.
Keywords: statin, prostate cancer, population-based
INTRODUCTION
Prostate cancer is the second leading cause of cancer mortality in men and is the most commonly diagnosed non-cutaneous malignancy.1 The incidence of prostate cancer in the United States is estimated to exceeded 192,000 cases in 20091 and treatments are expensive and associated with adverse events.2 Given the associated societal burden, interventions that prevent the development or growth of prostate cancer could have a large beneficial impact.
Hydroxymethylglutaryl-CoA reductase inhibitors (statins) are a class of medications that reduce cholesterol levels and prevent cardiovascular events. However, statins may also have anti-neoplastic effects3 and have been shown to induce apoptosis and growth arrest in prostate cancer cell lines.4 Statins may exert these effects through cholesterol mediated5 or non-cholesterol mediated mechanisms, as these drugs lower potentially carcinogenic isoprenoids and have anti-inflammatory effects that may protect cells from neoplastic transformation.3
Observational studies examining statin use and risk of prostate cancer have been contradictory.6–16 Possible explanations for inconsistent findings in previous studies are heterogeneous patient populations, variable durations of statin exposure and short lengths of follow-up. Another potential source of confusion is that statins may reduce serum prostate specific antigen (PSA).17, 18 Since elevated serum PSA is the most common indication for prostate biopsy, statin use may be associated with decreased likelihood to receive biopsy and subsequent underdetection of cancer. The objectives of this study were to determine if statin use was associated with a decreased risk of having an elevated PSA level, receiving a prostate biopsy, and being diagnosed with prostate cancer in a large, population-based cohort study.
MATERIALS AND METHODS
Study Subjects
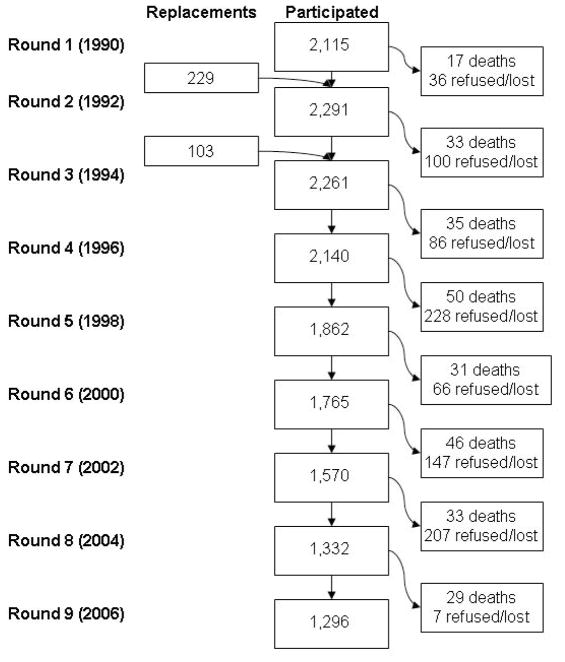
The Olmsted County Study of Urinary Symptoms and Health Status among Men was initiated in 1990 and is comprised of a randomly selected population-based cohort from Olmsted County, Minnesota. A detailed description of this cohort is published elsewhere.19, 20 Briefly, men between 40 and 79 years of age on January 1, 1990 were eligible to be included in a longitudinal cohort designed to study the natural history of benign urologic disease in the community. Using the record linkage system from the Rochester Epidemiology Project,21 men were excluded if they had a history of prostate cancer, prostatectomy, or other urologic conditions (bladder cancer or surgery, urethral surgery, or strictures). Of the eligible men, 2115 (55%) agreed to participate and completed a self-administered questionnaire biennially. Surveys included questions on life-style factors such as smoking, alcohol use, medication use, and demographic characteristics, as well as questions on urologic function. A randomly selected subset (476 of 537 men [89%]) from this group participated in a detailed biennial clinical examination including measurement of PSA. In 1992 and 1994, men who did not participate in this active follow-up were replaced by randomly selected men from the Olmsted County population (332 total replacements, 158 clinic subset participants). The recruitment and attrition of study participants at each round of follow-up is shown in Figure 1. All men were also passively followed through their community medical records at each round of follow-up.
Figure 1.
Study participation in each round of follow-up
Statin Exposure
At baseline, each study participant was asked to report all prescribed and over-the-counter medications that were taken on a daily basis. Medications were subsequently grouped into classes by the research team on receipt of the questionnaires. This information, along with the starting date, dosage, unit of administration, and directions for use, when such information was available, were used in these analyses. Current medication use and starting date were ascertained again by questionnaires in round 4 (1996), round 6 (2000) and biennially thereafter.
Outcome Variables
Serum PSA levels were measured in the subset of men who participated in the detailed biennial clinical exam. Serum samples were obtained prior to any prostatic manipulations, including digital rectal exam and transrectal ultrasound.22 Several PSA thresholds were evaluated including 2.5 ng/ml, 4.0 ng/ml and age-specific thresholds.20 Prostate biopsy, prostate cancer, tumour grade (Gleason sum ≥7 considered high- grade), and pathologic stage were determined from detailed medical record and pathology report review. The 2002 American Joint Committee on Cancer pathologic staging system was used for men treated with radical prostatectomy.23
Potential Confounders
Variables that might confound or modify associations between statin use and elevated PSA level, or risk of biopsy or prostate cancer were considered. Presence of diabetes was determined by self-report of a diabetes diagnosis or anti-hyperglycemic medication use. Presence of hypertension was determined by self-report of high blood pressure or anti-hypertensive medication use (angiotensin-converting enzyme inhibitors, angiotensin II receptor inhibitors, beta-blockers, calcium channel blockers, or diuretics; with the exception of loop diuretics, as these were expected to be prescribed to treat hypertension or edema following heart or renal failure rather than primary hypertension). Presence of coronary heart disease (CHD) was determined by ongoing surveillance of CHD in the Olmsted County community (RO1 HL59205; PI Veronique Roger).24, 25 CHD was defined as sudden cardiac death, myocardial infarction, and angiographically-diagnosed coronary disease.25–27 Smoking was assessed through the question “Have you smoked at least 100 cigarettes in your entire life?” and subjects were classified as “ever smoker” or “never smoker” accordingly. Use of non-steroidal anti-inflammatory medications (NSAIDs) and use of lower urinary tract symptoms/benign prostatic enlargement (LUTS/BPE) medications (5α-reductase inhibitors, α-adrenergic receptor inhibitors (α-blockers), and herbal medications) were ascertained via self-report on the biennial questionnaires. Finally, anthropometric measures were obtained at baseline by trained study nurses using a standardized protocol. Body mass index (BMI) was calculated as weight (kg) divided by height (m2), and individuals with a BMI≥30 kg/m2 were classified as obese.
Data Analysis
Separate analyses were conducted for PSA thresholds, prostate biopsy, prostate cancer and high-grade prostate cancer. Men with a history of a prostate biopsy or elevated PSA level at baseline were excluded from the analyses of these outcomes. For PSA outcomes (the clinic subset) follow-up was from the start of the study until the outcome event in the analysis or the last study visit. PSA measurements after the diagnosis of prostate cancer were censored. For prostate biopsy and prostate cancer outcomes, follow-up was from the start of the study until the outcome event or last follow-up. In each analysis, participants who reported statin use prior to the development of the outcome were considered exposed. Duration of medication use was defined as the time from the reported start of the medication to the event date or date of last follow-up (no event).
Cox proportional hazard models were used to estimate associations between statin use or duration of statin use and each of the outcomes and are presented as hazard ratios (HR) and their associated 95% confidence intervals (CI). Time was calculated from start of the study to the date of event or date of last follow-up. Figures are plotted by age at event for clarity of presentation. Proportional hazards assumptions were assessed using Schoenfeld residuals. As statin users frequently differ in health status from non-statin users, such conditions (such as diabetes or heart disease) could confound observed associations. Differences between the two groups were examined and are reported in Table 1. Multivariable models were then used to adjust for these potential confounders including age, comorbidities (diabetes, hypertension, CHD), use of NSAIDs, 5-α reductase inhibitors, and α-blockers. Additional multivariable-adjusted models which included a 4-level term representing non-users and tertile of duration were used to assess the linear trend in duration of statin use.
Table 1.
Characteristics of statin users and non-statin users
| Characteristic | Statin Use* N (%) | No Statin Use N (%) | Chi-Square p Value | Age- Adjusted Chi-Square p Value |
|---|---|---|---|---|
| Age (at 1/1/1990) | ||||
| 40–49 | 245 (38.6) | 898 (49.5) | <0.0001 | |
| 50–59 | 212 (33.4) | 388 (21.4) | ||
| 60–69 | 140 (22.1) | 308 (17.0) | ||
| 70+ | 37 (5.8) | 219 (12.1) | ||
| Diabetes | 143 (22.6) | 142 (7.8) | <0.0001 | <0.0001 |
| Hypertension | 479 (75.6) | 744 (41.0) | <0.0001 | <0.0001 |
| Coronary Heart Disease | 257 (40.5) | 285 (15.7) | <0.0001 | <0.0001 |
| Use of non-steroidal anti-inflammatory drugs (NSAIDs) | 552 (87.1) | 1023 (56.4) | <0.0001 | <0.0001 |
| Ever smoked | 413 (65.1) | 1132 (62.4) | 0.19 | 0.24 |
| Alcohol use once a week or more | 276 (43.5) | 701 (38.7) | 0.43 | 0.46 |
| Obese | 187 (29.5) | 457 (25.2) | 0.51 | 0.47 |
| 5-α reductase inhibitor use | 78 (12.3) | 126 (7.0) | <0.0001 | <0.0001 |
| α-blocker use | 182 (28.7) | 328 (18.1) | <0.0001 | <0.0001 |
| PSA level>age-specific reference range† | 12 (8.1) | 51 (11.2) | 0.29 | 0.30 |
| PSA≥2.5 ng/ml† | 19 (15.2) | 104 (24.5) | 0.03 | 0.049 |
| PSA≥4.0 ng/ml† | 19 (12.8) | 57 (12.8) | 0.99 | 0.74 |
| Prostate biopsy | 79 (13.7) | 433 (23.6) | <0.0001 | <0.0001 |
| Prostate cancer | 38 (6.0) | 186 (10.3) | 0.001 | 0.002 |
| High-grade prostate cancer | 7 (1.1) | 49 (2.8) | 0.01 | 0.02 |
Number of statin users varies with the outcome examined: PSA level >age-specific ref range: n=148 (24·50%); PSA≥2.5 ng/ml: n=125 (22.77%); PSA≥4.0 ng/ml: n=149 (25.04%); prostate biopsy: n=577 (23.90%); prostate cancer: n=634 (25.91%); high-grade prostate cancer: n=662 27.05%).
PSA outcomes only for the 634 men who participated in the in-clinic examinations.
RESULTS
Of the 2447 patients at study inception, 634 used statins prior to prostate cancer diagnosis or last follow-up (TABLE 1). Statin users were older, more likely to have a comorbid disease and more commonly used NSAIDs, 5-α reductase inhibitors, and α-blockers (TABLE 1). Median follow-up was similar for both statin users (median=15.7 years; Q1, Q3=15.1, 16.5) and non-statin users (median=15.2 years; Q1, Q3=13.1, 16.2). Of the entire cohort, 51 patients (2%) were lost to passive follow-up (did not have medical records available to review for biopsy or prostate cancer information) over 18 years.
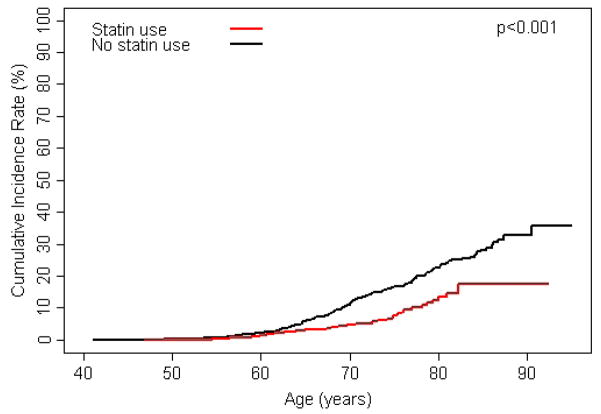
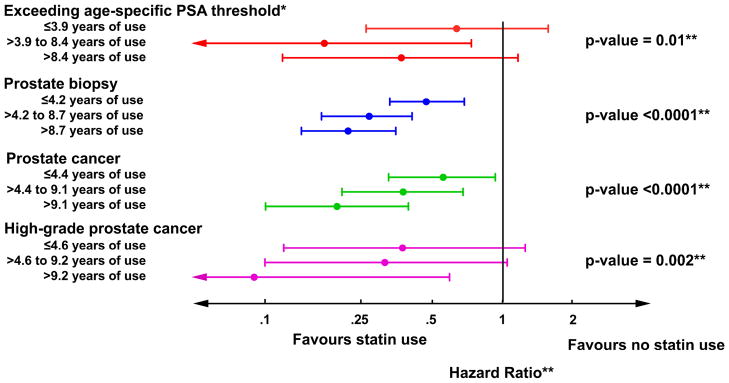
Statin use was associated with decreased risk of developing elevated PSA levels, receiving a prostate biopsy, overall risk of prostate cancer, and risk of high-grade prostate cancer (FIGURES 2A–D). Additionally, after adjusting for age, diabetes, hypertension, coronary heart disease, NSAID use, 5-α reductase inhibitor use, and α-blocker use, associations strengthened slightly (TABLE 2). Many study subjects had been exposed to statin medications for prolonged periods and those who received statins longest had the lowest risk of each outcome (all tests for trend p<0.05; FIGURE 3). Of those diagnosed with prostate cancer, 18 (47%) statin users and 103 (55%) non-statin users were treated with radical prostatectomy. Tumour stage was available for 106 (88%) of these men and locally advanced disease (pT3) was present in 4 (22%) statin users and 32 (31%) non-statin users. No statin user was diagnosed with lymph node metastases compared to three in the non-statin group.
Figure 2.


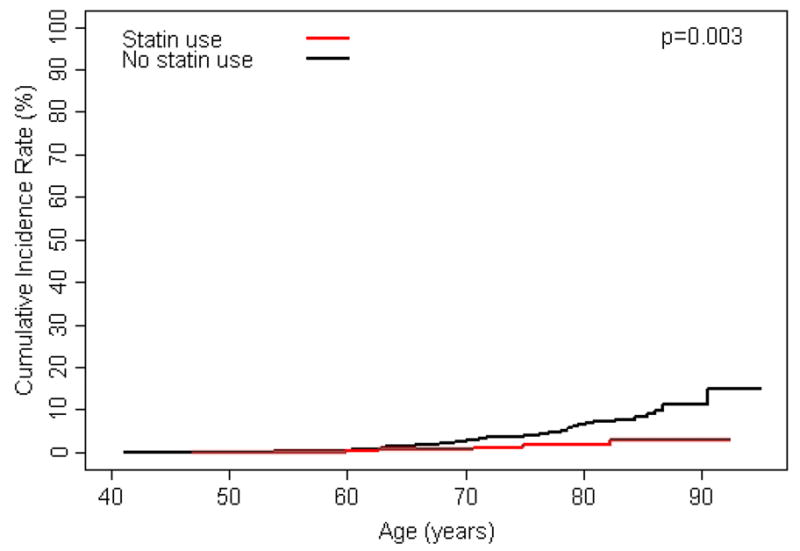
A) cumulative incidence of exceeding age-specific PSA reference range by statin use; B) cumulative incidence of prostate biopsy by statin use; C) cumulative incidence of prostate cancer by statin use; and D) cumulative incidence of high-grade prostate cancer (Gleason ≥ 7) by statin use.
Table 2.
Risk of elevated PSA level, prostate biopsy, prostate cancer, and high-grade prostate cancer in statin users compared to non-statin users
| Outcome | Unadjusted | Multivariable-Adjusted* |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| PSA level>age-specific reference range† | 0.47 (0.25–0.87) | 0.39 (0.19–0.81) |
| PSA≥2.5 ng/ml† | 0.39 (0.24–0.63) | 0.31 (0.18–0.52) |
| PSA≥4.0 ng/ml† | 0.65 (0.39–1.09) | 0.63 (0.35–1.13) |
| Prostate biopsy | 0.47 (0.37–0.60) | 0.31 (0.24–0.40) |
| Prostate cancer | 0.48 (0.34–0.69) | 0.36 (0.25–0.53) |
| High-grade prostate cancer | 0.32 (0.15–0.71) | 0.25 (0.11–0.58) |
Adjusted for age, diabetes, hypertension, coronary heart disease, NSAID use, 5-α reductase inhibitor use, and α-blocker use.
PSA outcomes only for the 634 men who participated in the in-clinic exams
Figure 3.
Associations between duration of statin use (stratified by tertiles) and outcome using non-statin users as the referent group. P-values represent tests for trend. *PSA outcomes only for the 634 men who participated in the in-clinic exams. **Adjusted for age, diabetes, hypertension, coronary heart disease, NSAID use, 5-α reductase inhibitor use, and α-blocker use.
DISCUSSION
In this large population-based longitudinal cohort, statin use was associated with decreased risk of exceeding PSA thresholds, receiving a prostate biopsy, and receiving a diagnosis of prostate cancer or high-grade prostate cancer. Additionally, for all outcomes, longer duration of use was associated with lower risk. The association between statin use and prostate cancer diagnosis may be a consequence of less frequent prostate biopsies in statin users due to PSA suppression. Alternatively, statins may have anti-neoplastic effects beyond their established cardiovascular functions.
The observed association between statin use and decreased risk of exceeding PSA thresholds is consistent with other data.17, 18 In a recent single-arm observational study, statin users experienced a 4.1% median decline in serum PSA within one year of starting therapy and 39% of patients with pre-statin PSA concentrations greater than 4 ng/ml fell below this threshold within one year of initiating statin therapy.17 Since elevated serum PSA is a common indication for proceeding to prostate biopsy, suppression of PSA could explain the lower risk of biopsy among statin users. However, if statins only suppressed PSA and had no effect on prostate cancer, we would expect biopsies of statin users to be delayed, resulting in more advanced-stage disease at diagnosis. In our cohort, statin users had decreased risk of high-grade tumors and did not have an increased risk of advanced-stage (pT3) disease. Others have also found a decreased risk of advanced-stage prostate cancer among statin users.8, 11, 13–15 Thus, indirectly, it seems statin use does not simply delay or prevent diagnosis by suppressing PSA.
The decreased risk of overall prostate cancer observed in our cohort is not consistent with all previous studies. Clinical trials of statins for cardiovascular outcomes found no association between statin use and prostate cancer incidence (examined as a secondary outcome).28 Additionally, several cohort studies, with designs similar to ours, have found no overall association between statin use and risk of prostate cancer.8, 9, 11,14 These differences may be explained by duration of statin use. If statins prevent development of prostate cancer, long periods of exposure may be necessary. In randomized trials, duration of follow-up prior to open label use of statins was limited, ranging from 3.9 to 6 years.29–34 Duration of statin use was also limited in previous cohort studies, but there was some suggestion that longer use might be associated with a decreased risk of prostate cancer. For example, in the California Men’s Health Study, 18% had taken a statin medication for 5 or more years.8 In that group, there was a significant decreased overall risk of prostate cancer among statin users (relative risk [RR], 0.72; 95% CI, 0.53–0.99). A similar, but non-significant association was observed in the Health Professionals Follow-up Study among men who used statins for 5 or more years (RR, 0.85; 95% CI, 0.71–1.03).14 In our study, over half of the statin users used these medications for more than 5 years, and 33% for more than 9 years, with the strongest protective associations observed in men who had used statins for the longest period of time. Therefore, if statins are protective against developing prostate cancer, long durations of use or exposure to statins at a young age may be necessary for a protective association to emerge.
The external validity of our findings may be compromised if patients who participated in the study were different from patients who did not participate. We could not directly measure the impact of volunteer bias; however, at baseline, participants and non-participants had similar rates of receiving general medical exams and had comparable prevalence of diabetes, cardiovascular events and non-urologic malignancies.35 In addition, the incidence of prostate cancer in the cohort was similar to what we would expect based on the United States age-interval incidence.1 Thus, it appears that findings from this study would be applicable to a broader population. However, it is important to note that these men were Caucasian and results may not be generalizable to men of other racial or ethnic populations.
Several other potential limitations should be considered when interpreting our study results. First, in a recent editorial, it was suggested that statin users may be differentially screened for prostate cancer compared to non-statin users.36 Differential screening would likely result in detection of more cancers among the group that is more frequently screened. While we do not have detailed screening information on all patients, screening exposure of this cohort was assessed through 1998 and statin users were more likely to have received frequent PSA screenings (>3 PSA measurements) (34% vs 21%, chi-square p value <0.001) and frequent digital rectal exams (>4 digital rectal exams) compared to non-statin users (29% vs 20%, chi-square p value <0.001).37 Furthermore, a subset of the men in the study received routine PSA screening as part of the longitudinal cohort study, regardless of statin use. In a secondary analysis only examining the men who received the routine PSA screening, the associations between statin use and risk of prostate biopsy were similar to the entire cohort (multivariable-adjusted HR, 0.35 for the clinic subgroup compared to 0.31 for the entire cohort). These data suggest that the observed association between statin use and risk of prostate cancer was not due to decreased screening among statin users.
Additionally, it is possible that differences in baseline levels of PSA may have confounded the association between statin use and the outcomes of interest if statin users differed from non-statin users in their initial PSA levels. However, we conducted a secondary analysis, adjusting our study results for baseline PSA level in participants in the in-clinic portion of the study (who had such measures available). The association between statin use and prostate biopsy changed from a HR=0.47 (95% CI: 0.37, 0.60) to a HR=0.51 (95% CI: 0.34, 0.77). The association between statin use and prostate cancer changed from a HR=0.48 (95% CI: 0.34, 0.69) to a HR=0.53 (95% CI: 0.28, 1.02). As results did not change significantly after adjusting for baseline PSA level, it is unlikely that baseline differences in PSA levels confounded observed associations.
It is also possible that participation in the study may have influenced participant behavior. For example, participants may have been generally more aware of prostate health issues after enrollment in the study, and thus, may have been more likely to be screened for prostate cancer with subsequent detection of prevalent cancer. Given that statin use increased during the study period, early detection of prevalent prostate cancer would make statins artificially seem protective. However, the proportion of new cancers diagnosed in men between 60 and 69 years of age did not differ significantly based on the year of diagnosis (5.4% from 1990 to 1994 versus 6.6% from 2000 to 2004) suggesting that this was not occurring in our population.
Statins might also appear protective for prostate cancer if statin users were more likely to succumb to competing causes of death compared to non-statin users. However, in our population, statin users were less likely to die during follow-up compared to non-users (age-adjusted HR: 0.32; 95% CI: 0.24, 0.43), suggesting that this explanation of our findings is also unlikely.
Data on diabetes, hypertension, and all medications were self-reported, and we lacked information on diet and exercise in this population. Discrepancies between self-report and actual health conditions could bias observed associations. Additionally, while comorbid conditions did not appear to confound the association between statins and prostate cancer, other unmeasured factors such as diet and exercise could account for the observed associations. In the absence of randomized trials with systematic end-of-study biopsy, the effect of unknown confounders and the potential for detection bias remain significant limitations to this and any other observational study evaluating prostate cancer prevention.
We had little information on statin dose and start and stop dates for medication used. For the reported analyses, we assumed that once a man reported use of a statin, he continued to take the statin for the duration of follow-up. A recent review article suggests that up to 60% of patients who are prescribed statins stop use within the first 6 months of prescription.38 However, in this cohort, long-term medication adherence appeared to be more common. Of the 350 men who reported statin use at baseline (1990) and who had data available in 2000, 290 (83%) also reported statin use in 2000. Additionally, of the 251 men who reported new use of statins in 2000, and who also had information in 2006, 208 (83%) again reported statin use. It was not possible, however, for us to assess interim changes in statin use, nor whether men who reported statin use actually took the medications.
CONCLUSION
In conclusion, anti-neoplastic effects of statins on prostate cancer are supported by a growing number of observational studies. Whilst most have observed a decrease in advanced-stage disease, in this longitudinal cohort study with extended follow-up, statin use was associated with a large reduction in risk of overall prostate cancer, particularly among men who used statins for a long period of time. These data suggest statins may have a preventive effect against prostate cancer and provide compelling evidence for further study.
Acknowledgments
The authors thank the men who participate in the Olmsted County Study and all study personnel. The authors would also like to thank Dr. Veronique L. Roger for her support and collaboration. Funded by U.S. Public Health Service, National Institutes of Health (DK58859, AR30582, and 1 UL1 RR024150-01) and Merck Research Laboratory.
Footnotes
Conflicts of interest: Dr. Nehra is a consultant with Pfizer, Glaxo-Smith-Kline and Sanofi and Dr. Jacobsen is an employee of Kaiser Permanente
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Gomella LG, Johannes J, Trabulsi EJ. Current prostate cancer treatments: effect on quality of life. Urology. 2009;73:S28. doi: 10.1016/j.urology.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 4.Hoque A, Chen H, Xu XC. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2008;17:88. doi: 10.1158/1055-9965.EPI-07-0531. [DOI] [PubMed] [Google Scholar]
- 5.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 6.Agalliu I, Salinas CA, Hansten PD, et al. Statin use and risk of prostate cancer: results from a population-based epidemiologic study. Am J Epidemiol. 2008;168:250. doi: 10.1093/aje/kwn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coogan PF, Rosenberg L, Palmer JR, et al. Statin use and the risk of breast and prostate cancer. Epidemiology. 2002;13:262. doi: 10.1097/00001648-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Flick ED, Habel LA, Chan KA, et al. Statin use and risk of prostate cancer in the California Men’s Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2218. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 9.Friis S, Poulsen AH, Johnsen SP, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 10.Graaf MR, Beiderbeck AB, Egberts AC, et al. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs EJ, Rodriguez C, Bain EB, et al. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 12.Kaye JA, Jick H. Statin use and cancer risk in the General Practice Research Database. Br J Cancer. 2004;90:635. doi: 10.1038/sj.bjc.6601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murtola TJ, Tammela TL, Lahtela J, et al. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 14.Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 15.Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162:318. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 16.Blais L, Desgagne A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med. 2000;160:2363. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton RJ, Goldberg KC, Platz EA, et al. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100:1511. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 18.Cyrus-David MS, Weinberg A, Thompson T, et al. The effect of statins on serum prostate specific antigen levels in a cohort of airline pilots: a preliminary report. J Urol. 2005;173:1923. doi: 10.1097/01.ju.0000158044.94188.88. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen SJ, Guess HA, Panser L, et al. A population-based study of health care-seeking behavior for treatment of urinary symptoms. The Olmsted County Study of Urinary Symptoms and Health Status Among Men. Arch Fam Med. 1993;2:729. doi: 10.1001/archfami.2.7.729. [DOI] [PubMed] [Google Scholar]
- 20.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860. [PubMed] [Google Scholar]
- 21.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 22.Roberts RO, Jacobson DJ, Girman CJ, et al. Insulin-like growth factor I, insulin-like growth factor binding protein 3, and urologic measures of benign prostatic hyperplasia. Am J Epidemiol. 2003;157:784. doi: 10.1093/aje/kwf054. [DOI] [PubMed] [Google Scholar]
- 23.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6. New York: Springer-Verlag; 2002. Prostate; pp. 309–316. [Google Scholar]
- 24.Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 25.Roger VL, Killian J, Henkel M, et al. Coronary disease surveillance in Olmsted County objectives and methodology. J Clin Epidemiol. 2002;55:593. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- 26.Arciero TJ, Jacobsen SJ, Reeder GS, et al. Temporal trends in the incidence of coronary disease. Am J Med. 2004;117:228. doi: 10.1016/j.amjmed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Goraya TY, Jacobsen SJ, Belau PG, et al. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted County, Minnesota. Mayo Clin Proc. 2000;75:681. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- 28.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. 2008;123:899. doi: 10.1002/ijc.23550. [DOI] [PubMed] [Google Scholar]
- 29.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. Jama. 1998;279:1615. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 30.The Lipid Study Group. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. 2002;359:1379. doi: 10.1016/S0140-6736(02)08351-4. [DOI] [PubMed] [Google Scholar]
- 31.Heart Protection Study Collaborative Group. The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomised placebo-controlled trial [ISRCTN48489393] BMC Med. 2005;3:6. doi: 10.1186/1741-7015-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287:3215. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 33.Ford I, Murray H, Packard CJ, et al. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 34.Strandberg TE, Pyorala K, Cook TJ, et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364:771. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 35.Gades NM, Jacobson DJ, McGree ME, et al. Dropout in a longitudinal, cohort study of urologic disease in community men. BMC Med Res Methodol. 2006;6:58. doi: 10.1186/1471-2288-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platz EA. Epidemiologic musing on statin drugs in the prevention of advanced prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2175. doi: 10.1158/1055-9965.EPI-07-0777. [DOI] [PubMed] [Google Scholar]
- 37.Wallner LP, Sarma AV, Lieber MM, et al. Psychosocial factors associated with an increased frequency of prostate cancer screening in men ages 40 to 79 years: the Olmsted County study. Cancer Epidemiol Biomarkers Prev. 2008;17:3588. doi: 10.1158/1055-9965.EPI-08-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liberopoulos EN, Florentin M, Mikhailidis DP, et al. Compliance with lipid-lowering therapy and its impact on cardiovascular morbidity and mortality. Expert Opin Drug Saf. 2008;7:717. doi: 10.1517/14740330802396984. [DOI] [PubMed] [Google Scholar]