Abstract
Mesechymal stem cells as pluripotent cells are involved in the differentiation of adipocytes under regulation of genes and transcription factors. The plasticity observed between adipocytes and osteoblasts differentiation is the basis of transdifferentiation, observed in both experimental and clinical level. This review analyzes not only the adipose tissue as an endocrine organ but also the underlying mechanism of trans-differentiation between adipocytes and osteoblasts. Fat and bone tissue interaction is altered by activation or silencing of genes, signaling molecules and transcription factors. Disorders of this interaction include ectopic ossification syndromes and other bone disorders like osteoporosis and multiple myeloma. Further research will reveal the instinct mechanisms of this imbalance in the pathophysiology of many metabolic disorders such as diabetes mellitus, atherogenesis e.t.c.
Keywords: mesenchymal stem cells, adipogenesis, osteoblastogenesis, osteoporosis, ectopic ossification syndromes, multiple myeloma, review
Mesenchymal stem cells (MSCs) are pluripotent, potentially differentiated cells with high mitotic index. The differentiation mechanisms of their precursor forms (progenitors) and their development into mature cells (osteoblasts, adipocytes, etc) are not fully understood. Probably silencing or over-expression of genes in specific stages of differentiation of MSCs is fatal for each cell line. Similarly, the activation of specific signalling pathways involved in differentiation of MSCs seems to play an important role in the process of maturation of cells1.
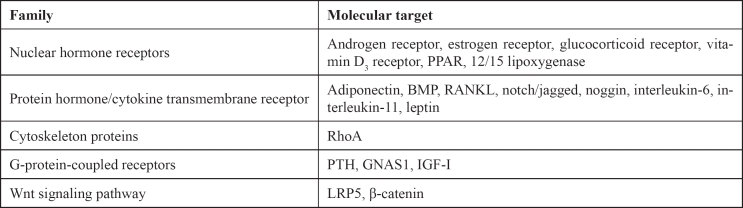
Osteoporosis, defined by the International Osteoporosis Foundation (IOF), is a multifactorial disease characterized by systemic bone loss and deterioration of bone microarchitecture. Clinical studies have shown that bone loss in osteoporotic patients and in people with age-dependent bone loss, is associated with increased adipose tissue in the bone marrow2,3. Experimental models of mammals after ovariectomy, immobility or treatment with glucocorticoids revealed the deposition of fat cells into the bone marrow4–9. Notice that fat cells in bone marrow have common origin with MSCs, potentially differentiated in osteoblasts. It is speculated that the presence of dynamic equilibrium between adipogenesis and extensive formation of bone is the "melting point" in the prevention or therapeutics of diseases characterized by disturbances of the balance (Table 1).
Table 1. Therapeutic targets of balancing osteoblasts and adipocytes on molecular level.
Adipogenesis and hormones of fat tissue: the "new" endocrine organ
Adipogenesis is the process by which undifferentiated precursor cells differentiate into mature adipocytes. At the stages of embryogenesis, but also in the evolution of the human organism, the deposition of fat tissue in the bone marrow is a passive process of storing fat cells within the marrow cavities that are not required to haematopoiesis. The complex process of differentiation of adipocytes is characterized by changes in cellular morphology, hormone sensitivity, gene expression and secretory capacity. In particular, adipocytes secret local factors, such as adepsin, leptin, adiponectin, TNF-α (Tumor Necrosis Factor-α) and angiotensingen. These factors are characterized by significant pleiotropic effect on the local microenvironment of bone marrow cells, including osteoblasts10–12.
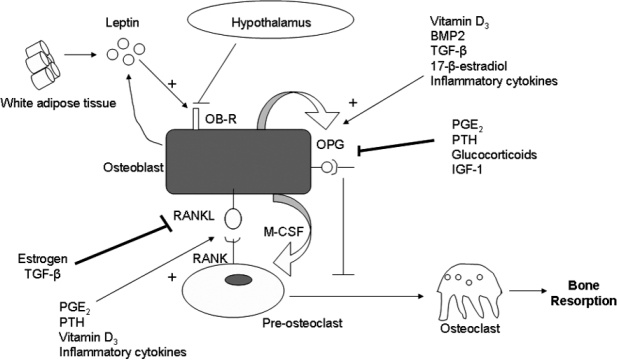
Further studies indicate the trans-differentiation process (diversion during differentiation of a cell line to another of common origin) between the cells of the bone marrow, in the differentiation and maturation mechanisms of adipocytes13,14. One of the main characteristics of osteoblasts and adipocytes is plasticity which is observed in both experimental and clinical level. It seems that the excretion of some or sometimes all of the adipokines, are fatal for the adipocyte which can be converted into osteoblast. Today the adipose tissue is not considered to be a storage tissue but is characterized as an endocrine organ with an important role in the secretion adipokines (leptin, adiponectin) and hormones (estrogen, vitamin D3, androsterone, cortisone), involved in the pathophysiology of different disorders entities, such as diabetes, obesity, inflammation and atherogenesis. In particular, leptin seems to be an indicator of the energy state of human organism. Lack of leptin, leads to obesity with significant involvement in the mechanisms regulating bone metabolism (trans-differentiation). In particular, leptin controls the RANKL/OPG axis (Receptor Activator for Nuclear Factor k B Ligand / Osteoprotegerin) by inhibiting the expression of RANKL and inducing the ORG in pre-osteoblasts and mononuclear cells in circulation (Figure 1). The diversion of an adipocyte into osteoblast is considered to be a multifactorial process regulated by all these factors10,11,15–19.
Figure 1. The interaction of white adipose tissue and other factors in differentiation of osteoblast.
Physiologically, adipogenesis is strictly controlled by hormones, cytokines, nutrients, alterations in the expression and/or activity of transcription factors that directly affect the different mechanisms of adipocyte differentiation. Transcription factors such as CEBP-α,- β,-δ (CCAT/enhancer binding protein-α,-β,-δ), PPARγ (Peroxisome proliferator-activated receptor-γ) and SREBP1 (sterol-regylatory element-binding transcription factor 1) are the "lead players" of adipocyte differentiation. Taking account that C/EBPδ is expressed in early differentiation of pre-adipocyte while in the final stages of maturation C/EBPα is expressed11,12. Alongside FgF10 (Fibroblast growth factor-10) acts synergistically in the final stages of maturation of fat cells, according to studies in knockout mice (Fgf10-/-)20.
Particularly PPAR proteins are investigated and their implication in controlling the balance between osteoblastogenesis and adipogenesis. The PPARs' are transcription factors that appear in three different isoforms: PPAR-α, PPAR-γ and PPAR-β/δ. The transcription factor PPARγ ligands include the long chains and oxidized derivatives of fatty acids and thiazolidinedione compounds. This factor is expressed in two isoforms, RARγ1 and PPARγ2 due to alternative splicing or promoters of primary genes. Synthetic agonists of ARγ such as derivatives thiazolidinedione are used as insulin- sensitive substances in the treatment of diabetes mellitus type II. However, adipogenesis is an unwanted effect of these compounds for the treatment of metabolic diseases in general. Studies have shown that the activation of PPARγ receptor, promotes adipogenesis of stromal cells in bone marrow and inhibits osteoblastogenesis10,11,21. Especially PPARγ2 induces the differentiation process of adipose tissue associated with bone metabolism. It seems that the ligands of PPARγ not only activate this nuclear receptor but also suppress the Cbfa-1 (Core binding factor a1)11,14,21,22. Furthermore studies focus on lipoxygenase inhibitors which reduce the concentration of oxidized fatty acids, by inhibiting the ligands of PPARα and/or PPARγ, thereby promoting adipogenesis and suppressing bone regeneration. Current research must be focus on the genes that encode nuclear receptors, in order to clarify the mechanisms underlying adipogenesis11,14.
Notch/Delta signalling pathway and RhoA gene in adipogenesis
The signalling pathway of Notch/Delta factors and their receptors associated with the EGF (Epidermal Growth Factor) are under current research. Overexpression of notch in vitro inhibits osteogenesis, but promotes adipogenesis in cell models, through downregulation of the Wnt receptor function and the switch of β-catenin (necessary factors for the process of adipogenesis). The Wnt peptides and their receptors, interact with LRPs (Low-density Receptor-related proteins)10,11,23,24. Thus mutations of LRP5 are associated with loss of bone mass. Substances, such as lithium, alter Wnt signalling pathway by inhibiting adipogenesis. Undifferentiated stromal cells and pre-adipocytes involved in Wnt / LRP signalling pathway, express Pref-1 (Preadipocyte factor-1), a factor which inhibits adipogenesis, causing skeletal abnormalities11,25. In addition dlk protein, a member of EGF family of proteins, contributes to the inhibition of adipogenesis hematopoiesis, glandular and neuroendocrine cell differentiation through intervention of Notch1 signalling pathway, with induced bone regeneration11.
In human MSCs culture, the insertion of the RhoA gene has been shown to induce the bone cell differentiation with stretched form. The shape of the cell, the tendency of cytoskeleton and the particular gene expression, significantly influence the differentiation of MSCs into bone cells. Exercise and particularly mechanical stress seem to strengthen the bone11. Therefore mechanical stress and collagenous matrix somehow triggers the MSCs in the bone marrow in the process of differentiation into bone cells11,26.
Hereditary disorders associated with ectopic ossification and other bone disorders
POH (Progressive Osseous Heteroplasia) is a rare disease characterized by ectopic ossification, starting in childhood in adipocytes of hypodermic layer. In the years this process is changing at the lower connective tissue. Genetic studies in patients with POH demonstrated important mutations of GNAS1 gene (GNAS1 - Guanine Nucleotide-Binding α-polypeptide 1), that are characterized by reduced levels of the α-functional subunit of Gproteins27.
Similar mutations in the GNAS1, are observed in patients with AHO (Albright's hereditary osteodystrophy). AHO was first described in 1942 and is characterized by morphological and endocrine abnormalities. Shortness of the metatarsal bones and round facies are the major malformations described in patients with AHO. In addition these patients have a significant resistance to parathyroid hormone with hypocalcemia, hyperphosphatemia. There may also have resistance to other hormones (ovarian, thyroid, neuro-pituitary gland). Surprisingly the inheritance of the mutant gene from the father leads to POH, while from the mother to AHO (location in the 20th chromosome, 20q12-q13.2), as a result of genomic imprinting. Studies in knockout mice demonstrated the different expression of GNAS1 in tissue level. Although we know that this gene is expressed in various tissues, what it is not known yet is the exact mechanism by which the mutation is transmitted to GNAS1. However the mutation leads to ectopic ossification of adipocytes' origin that transforms fat cells into osteoblasts27–29.
A disease that its entity has some common phenotypic features with POH is FOP (fibrodysplasia ossificans progressive). FOP is an autosomal dominant disease that is characterized by skeletal abnormalities and progressive development of bone segments in tendons and muscles. It is observed that fibroblasts convert into osteocytes by endochondral ossification. Studies in Drosophila melanogaster revealed mutations in the genetic locus ddp, with a similar phenotype of FOP. Ddp genetic locus encodes a protein that belongs to TGF-β superfamily of proteins (transforming growth factor-β), called BMP2 (Bone morphogenetic protein-2). However, in human peripheral monocytes of patients with FOP, there was an overexpression of BMP4, mRNA a morphogenetic protein responsible for any endochondral heterotopic ossification27,30. This morphogenetic protein seems to be a key factor for involving not only bone regeneration process but also the transition between fat and bone cell.
A great example of this imbalance is Multiple Myeloma (MM). MM cells suppress osteoblast formation and differentiation and thereby inhibit bone formation. Under physiological conditions the osteogenic differentiation of mesenchymal cells is tightly regulated either by system hormones, such as parathyroid hormone (PTH), estrogens and glucocorticoids or by local growth factors, including the BMP family, interleukins, TGF-β, insulin growth factor (IGF) and fibroblast growth factor 2. Moreover these factors activate specific intracellular signal pathways that modify the expression and activity of several transcription factors in mesenchymal and osteoprogenitor cells, which result in osteoblastic differentiation. Further studies also are necessary to clarify a possible connection between adipogenesis, osteoblastogenesis and the pathophysiology of MM31–36.
Several mechanisms are potentially involved in MM - induced inhibition of osteoblast formation and differentiation. MM cells inhibit osteoblastogenesis by blocking Runx2 activity in mesenchymal and osteoprogenitor cells through direct cell-to-cell contact with the involvement of very late antigen a (VLA-4) / vascular cell adhesion molecule 1 (VCAM-1). Soluble factors as interleukin (IL-7) may contribute to the suppression of Runx 2 activity by MM cells. Direct production of the Wnt inhibitor Dickkopf-1 (Dkk-1), secreted frizzled related protein (sFRP)-3 and rarely sFRP-2 by MM cells as well as the overproduction of hepatocyte growth factor could inhibit osteoblast formation. Finally, IL-3 overproduction in the MM microenviroment is involved in the inhibition of osteoblast formation and differentiation36.
Conclusion
Understanding the process of differentiation of adipocytes and trans-differentiation into osteoblasts is crucial in order to identify genes and other factors that may contribute to the pathophysiology of hereditary ectopic ossificans and other bone or joint disorders37. It is known that disturbances of the balance between osteoblastogenesis and adipogenesis lead to metabolic diseases such as obesity, diabetes e.t.c. Therefore therapeutic interventions must focus on manipulating the "thin line" between osteoblastogenesis and adipogenesis.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesechymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Meunier P, Aaaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Burkhhardt R, Ketner G, Bohm W, Schmidmeir M, Schlag R, Frisch B, et al. Changes in trabecular bone, haematopoiesis and bon marrow vessels in aplastic anaemia, primary osteoporosis and old age: a comparative histomorphometric study. Bone. 1987;8:157–164. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 4.Wronski TJ, Walsch CC, Ignaszewski LA. Histological evidence for osteopenia and increased bone turnover in ovariectomized rats. Bone. 1986;7:119–123. doi: 10.1016/8756-3282(86)90683-6. [DOI] [PubMed] [Google Scholar]
- 5.Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head. A histochemical and electron microscopi study. J Bone Joint Surg. 1985;67A:755–763. [PubMed] [Google Scholar]
- 6.Martin RB, Zissimos SL. Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone. 1991;12:123–131. doi: 10.1016/8756-3282(91)90011-7. [DOI] [PubMed] [Google Scholar]
- 7.Dokos C, Myronidou-Tzouveleki M. SERMs: the lovely multipotential drugs targeting osteoporosis? Review of Clinical Pharmacology and Pharmacokinetics. 2007;21:75–82. [Google Scholar]
- 8.Miniare P, Meunier PJ, Edouard C, Bernard J, Coupron J, Bourret J. Quantitative histological data on disuse osteoporosis. Calcif Tissue Res. 1974;17:57–73. doi: 10.1007/BF02547214. [DOI] [PubMed] [Google Scholar]
- 9.Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;58:1674–1686. doi: 10.1002/art.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packard CJ, Rader DJ. In: Lipids and Atherosclerosis. Hatzitolios AI, Savopoulos CI, editors. Athens: Mendor Publications; 2008. Greek Edition. [Google Scholar]
- 11.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Perera RJ, Marcusson EG, Koo S, Kang X, Kim Y, White N, et al. Identification of novel PPARγ target genes in primary human adipocytes. Gene. 2006;369:90–99. doi: 10.1016/j.gene.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Park SR, Oreffo RO, Triffitt JT. Interconversion potential of cloned human marrow adipocytes in vitro. Bone. 1999;24:549–554. doi: 10.1016/s8756-3282(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 14.Akune T, Ohba S, Kamekua S, Yamaguchi M, Chung U-il, Kubota N, et al. PPAR γ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas T, Martin A. Bone metabolism and energy balance: role for leptin. Joint Bone Spine. 2005;72:471–473. doi: 10.1016/j.jbspin.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Burgera B, Hofbauer L, Thomas T, Gori F, Lassam J, Laasko K, et al. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–3553. doi: 10.1210/endo.142.8.8346. [DOI] [PubMed] [Google Scholar]
- 17.Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge J, Malakellis M, et al. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 18.Martin A, de Vittoris R, David V, Moraes R, Begeot M, Lafage-Prous MH, et al. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology. 2005;146:3652–3659. doi: 10.1210/en.2004-1509. [DOI] [PubMed] [Google Scholar]
- 19.ang DC, Tsay HJ, Lin SY, Chiou SH, Li MJ, Chang TJ, et al. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One. 2008;3:e1540. doi: 10.1371/journal.pone.0001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konishi M, Asaki T, Koike N, Miwa H, Miyake A, Itoh N. Role of Fgf10 in cell proliferation in white adipose tissue. Mol Cell Endocrinol. 2004;218:119–128. doi: 10.1016/j.mce.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Lecka-Czernik B, Moerman EJ, Grant DF, Lehman JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-γ2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Mishima H, Sakai S, Liu YK, Ohyabu Y, Uemura T. Gene expression analysis of major lineage-defining factors in human bone marrow cells: effect of aging, gender, and age-related disorders. J Orthop Res. 2008;26:910–917. doi: 10.1002/jor.20623. [DOI] [PubMed] [Google Scholar]
- 23.ennell JA, MacDougald OA. Wnt signaling inhibits adipogenesis through β-catenin-dependent and independent mechanisms. J Biol Chem. 2005;280:24004–24010. doi: 10.1074/jbc.M501080200. [DOI] [PubMed] [Google Scholar]
- 24.Day TF, Guo Z, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Developmental Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes H, Mentink A, Bank R, Stoop R, van Blitterswijk C, de Boer J. Endogenous collagen influences differentiation of human multipotent mesechymal stromal cells. Tissue Eng Part A. 2010;16:1693–1702. doi: 10.1089/ten.TEA.2009.0341. [DOI] [PubMed] [Google Scholar]
- 27.Job-Deslandre C. Inherited ossifying diseases. Joint Bone Spine. 2004;71:98–101. doi: 10.1016/s1297-319x(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein LS, Yu S. The role of genomic imprinting of Gsα in the pathogenesis of Albright hereditary osteodystrophy. TEM. 1999;10:81–85. doi: 10.1016/s1043-2760(98)00124-6. [DOI] [PubMed] [Google Scholar]
- 29.Gopal RaoVVN, Schnittger S, Hansmann I. G protein Gsα (GNAS1), the probable candidate gene for Albright hereditary osteodystrophy, is assigned to human chromosome 20q12-q13.2. Genomics. 1991;10:257–261. doi: 10.1016/0888-7543(91)90508-c. [DOI] [PubMed] [Google Scholar]
- 30.Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressive. Am J Pathol. 2004;165:1107–1115. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Raeve HR, Vanderkerken K. The role of the bone marrow microenvironment in multiple myeloma. Histol Histopathol. 2005;20:1227–1250. doi: 10.14670/HH-20.1227. [DOI] [PubMed] [Google Scholar]
- 32.Georgiou KR, Foster BK, Xian CJ. Damage and recovery of the bone marrow microenvironment induced by cancer chemotherapy - potential regulatory role of chemokine CXCL12/receptor CXCR4 signalling. Curr Mol Med. 2010;10:440–453. doi: 10.2174/156652410791608243. [DOI] [PubMed] [Google Scholar]
- 33.akeuchi Y, Watanabe S, Ishii G, Takeda S, Nakayama K, Fukumoto S, et al. Interleukin-11 as a stimulatory factor for bone formation prevents bone loss with advancing age in mice. J Biol Chem. 2002;277:49011–49018. doi: 10.1074/jbc.M207804200. [DOI] [PubMed] [Google Scholar]
- 34.Kodama Y, Takeuchi Y, Suzawa M, Fukumoto S, Murayama H, Yamato H, et al. Reduced expression of interleukin-11 in bone marrow stromal cells of senescene-accelerated mice (SAMP6): relationship to osteopenia with enhanced adipogeneseis. J Bone Miner Res. 1998;9:1370–1377. doi: 10.1359/jbmr.1998.13.9.1370. [DOI] [PubMed] [Google Scholar]
- 35.Fritton JC, Kwashima Y, Mejia W, Courtland HW, Elis S, Sun H, et al. The insulin-like growth factor-1 binding protein acidlabile subunit alters mesenchymal stromal cell fate. J Biol Chem. 2010;285:4709–4714. doi: 10.1074/jbc.M109.041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giuliani N, Mangoni M, Rizzoli V. Osteogenic differentiation of mesenchymal stem cells in multiple myeloma: Identification of potential therapeutic targets. Exp Hematol. 2009;37:879–886. doi: 10.1016/j.exphem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Mohanty ST, Kottam L, Gambardella A, Nicklin MJ, Coulton L, Hughes D, et al. Alterations in the self-renewal and differentiation ability of bone marrow mesenchymal stem cells in a mouse model of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R149. doi: 10.1186/ar3098. [DOI] [PMC free article] [PubMed] [Google Scholar]