Abstract
Background and aim: Significant bone loss develops in the first months and continues years after spinal cord injury. A cross – sectional comparative study was performed to evaluate factors influencing bone loss in spinal cord injured men with paraplegia.
Patients and Methods: We studied 31 paraplegic men in chronic stage (>1.5 years) in comparison with 30 able-bodied men of similar age, height, and weight. The paraplegic men were allocated into 2 subgroups based on the neurological level of injury; high paraplegics (n=16, T4-T7 neurological level of injury) and low paraplegics (n=15, T8-T12 neurological level of injury). The influence of positive and negative factors (spasticity, standing-therapeutic walking, and duration of paralysis) on bone structures was evaluated by pQCT measurement of the total, trabecular and cortical bone mineral density (BMDtot, BMDtrab, BMDcort, respectively) and cortical thickness (THIcort) at the distal tibial epiphysis and the tibial diaphysis at 4% and 38% proximal to the distal end of the tibia. The stress strain index (SSI) was measured at 14% (SSI2) and at 38% (SSI3) of the tibial diaphysis, and the difference SSI3 - SSI2 (δSSI3-2) was calculated.
Results: In all paraplegics, bone mineral density parameters were significantly reduced compared to the control group (BMDtot: p<0.0005, BMDtrab: p<0.0005, BMDcort: p=0.029, THIcort: p=0.019, SSI2: p=0.009, SSI3: p=0.003, respectively). Paraplegics who used standing frames or long brace orthoses had statistically significant higher bone mass and geometric parameters (BMDtrab: p=0.03, BMDtot: p=0.01, THIcort: p=0.013, respectively), while spasticity did not protect bone. The duration of paralysis was significantly related to trabecular bone loss (r=-0.5, p=0.05) and cortical thickness (r=-0.6, p=0.006) in high paraplegics and to δSSI3-2 in low paraplegics (r=0.534, p=0.03).
Conclusions: The neurological level of injury adversely affects bone strength in paralyzed lower extremities such as the distal tibia. Standing or therapeutic walking could possibly have a positive effect in cortical and trabecular bone in paraplegia.
Keywords: spinal cord injury, paraplegia, bone loss, pQCT, men
Spinal cord injury (SCI) associated paraplegia causes an extreme and sudden immobilization that leads to an altered pattern of loading of the lower extremities and alteration in skeletal structure. These result in bone loss and increased risk of fractures. Bone loss in spinal cord injury patients has already been documented1–4 using dual energy X-ray absorptiometry (DXA)5,6 and recently peripheral quantitative computed tomography (pQCT) 7,8 that allows for non-invasive measurement of true volumetric densities, assessment of cortical and trabecular bone density separately, and evaluation of the geometrical properties of the long bones.
Although there are a few studies investigating the effect on bone in paraplegia according to the neurological level of injury7–10, no significant difference between paraplegics and tetraplegics was found for cortical or trabecular bone mineral density (BMD) of the tibia9. In addition, many factors influence bone loss in SCI paraplegics, including age and sex, level of injury, duration of paralysis, neurological complete injuries, hormonal status, ambulatory status, spasticity, and rehabilitation interventions1,2,7,8,10,11.
The aim of the present pQCT study was to evaluate the influence of positive and negative factors in spinal cord injured men. The rationale was that the neurological level of injury, the duration of paralysis, standing-therapeutic walking and spasticity affect bone loss at the lower extremities in SCI paraplegic men.
Patients and Methods
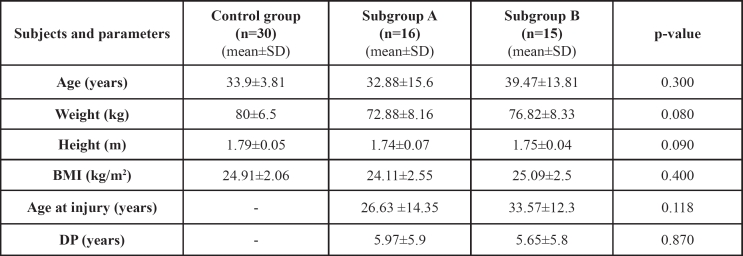
Sixty one men were included in this study. The study group included 31 complete paraplegic men (AIS A) in chronic stage (>1.5 years) that means stabilization of the neurological status and absence of spinal shock. The control group was recruited from 30 volunteers. All subjects of both groups were matched to age, height and weight. Each subject of the control group was interviewed and clinically examined by the first author (YD) according to a baseline personal data questionnaire based on anthropometric and clinical information. The protocol was designed according to the Declaration of Helsinki and approved by the Ethics Committee of Athens University. All subjects gave written informed consent to be included in this study.
Paraplegics were allocated into two subgroups according to the neurological level of injury (NLI); subgroup A included 16 men with high paraplegia (T4-T7 level), and subgroup B included 15 men with low paraplegia (T8-T12 level). Anthropometric factors, including age, height, weight and bone mass index (BMI) were recorded in both subgroups of the study group and the control group; clinical parameters such as duration of paralysis and neurological level of injury were recorded in the study group using a questionnaire and complete physical examination (Table 1). Spasticity was assessed using the Ashworth scale12. None of the subjects involved in this study were given any bone acting drugs. All subjects of both groups were examined by peripheral quantitative computed tomography system (pQCT XCT-3000, Stratec Medizintechnik, Germany) in distal epiphyses and midshafts of the tibia. The distal end of the tibia was used as an anatomical point to measure the bone parameters at 4%, 14%, and 38% of the tibial length proximal to this point. From the epiphyseal scans, trabecular (BMDtrab) and total (BMDtot) mineral density were measured; from the shaft scans, cortical BMD (BMDcort) and cortical thickness (THIcort) were determined. Bone strength was estimated using the stress-strain index (SSI) that was derived from the section modulus and the volumetric density of the cortical area at 14% (SSI2) and 38% (SSI3) of the tibia diaphysis proximal to the distal end of the tibia; the difference SSI3-SSI2 (δSSI3-2) was calculated.
Table 1. The anthropometric data and clinical parameters of the control group and the study subgroups.
BMI: Body mass index; DP: Duration of paralysis
The influence of positive and negative factors on bone structure was evaluated. Spasticity and standing frames or long brace orthoses for therapeutic walking were considered as positive factors, while neurological injury and the duration of paralysis (DP) were considered as negative for the preservation of normal bone structure
Statistical analysis of the variables between the study and the control groups was done using the one factor analysis of variance with no repeated measurements model (one way ANOVA) and Bonferroni test for pair wise comparisons. Statistical analysis of the variables between the study subgroups was done using analysis of covariance model (ANCOVA) controlling for duration of paralysis. Analysis of the data was done using the Statistical Package for Social Sciences (version 10.0) software (SPSS Inc., Chicago, IL).
Results
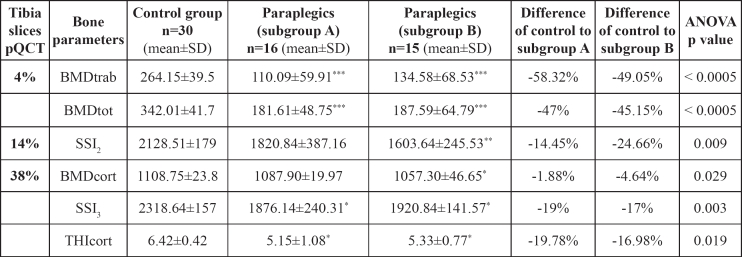
In all paraplegics, bone parameters were significantly reduced in both subgroups compared to the control group; BMDtot, BMDtrab of the distal tibial epiphyses and BMDcort of the tibial diaphysis were significantly reduced by 47% and 45% (p< 0.0005), 58% and 49% (p<0.0005), and 1.9% and 4.6% (p=0.029) in subgroups A and B, respectively, compared to the control group; THIcort decreased by 19.8% and 17.6% (p=0.019), SSI2 decreased by 14.45% and 24.66% (p=0.009), and SSI3 decreased by 19 % and 17% (p=0.003) in subgroups A and B, respectively, compared to the control group (Table 2).
Table 2. BMD parameters as measured by pQCT, and statistical significance of the control and the study subgroups.
*p-value< 0.05,** p-value< 0.005,*** p-value< 0.0005 of Bonferroni-tests for control group vs. subgroup A and subgroup B. BMDtrab: BMD trabecular; BMDtot: BMD total; BMDcort: BMD cortical; THIcort: Cortical thickness; SSI2: Stress Strain Index at 14% of the tibial diaphysis; SSI3: Stress Strain Index at 38% of the tibial diaphysis.
Paraplegics of both subgroups who used standing frames or long brace orthoses for standing-therapeutic walking had statistically significant higher BMDtrab (141.60±59.71 mg/cm3 vs. 87.41±47.23 mg/cm3, p=0.03), BMDtot (796±54 mg/cm3 vs. 700±132 mg/cm3, p=0.01) and THIcort (4.98±0.52 mm vs. 4.12±1.16 mm, p=0.013) compared to wheelchair bound paraplegics.
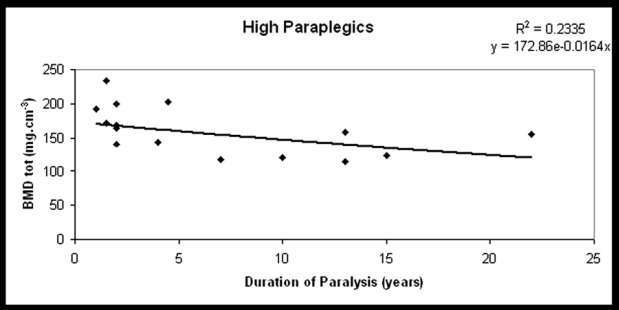
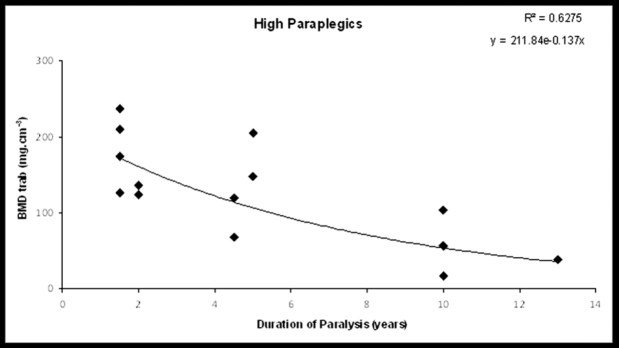
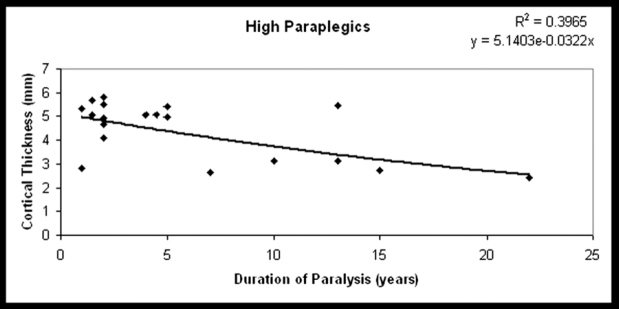
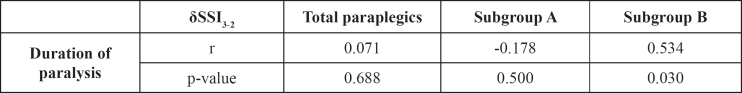
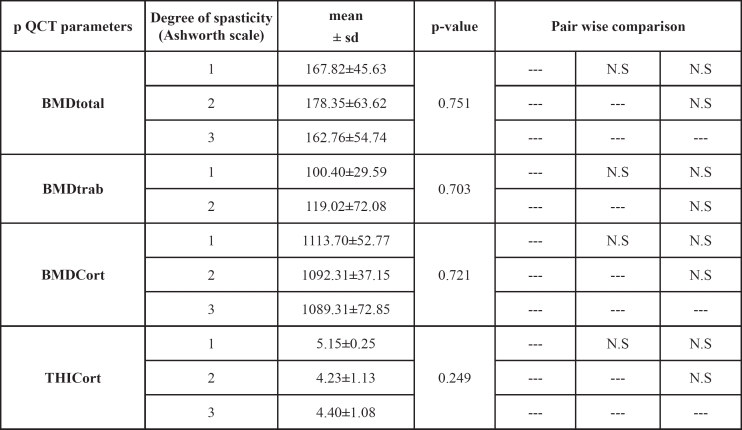
The DP was not significantly related to BMDtrab, BMDtot, or BMDcort in the study group (r=-0.203, p=0.23, r=-0.27, p=0.1, r=-0.1, p=0.6, respectively), except for subgroup A in which a significant reduction of BMDtrab and cortical thickness was observed (r=-0.5, p=0.05 and r=-0.6, p=0.006, respectively) (Figures 1 to 4). SSI2 was significantly related to DP in total paraplegics (r=-0.423, p=0.008), subgroup A (r=-0.419, p=0.074), and subgroup B (r=-0.473, p=0.041). SSI3 was significantly related to DP only in subgroup A (r=-0.475, p=0.04). However, δSSI3-2 was significantly related to DP only in subgroup B (r=0.534, p=0.03) (Table 3). None significant relationship was found between the intensity of bone loss and spasticity (Table 4).
Figure 1. BMDtot at the distal tibial epiphyses plotted against DP in high paraplegics (the best fit line for the exponential time course of decline).
Figure 2. BMDtrab at the distal tibial epiphyses plotted against DP in high paraplegics (the best fit line for the exponential time course of decline).
Figure 3. THIcort of the tibial diaphysis plotted against DP in high paraplegics (the best fit line for the exponential time course of decline).
Figure 4. BMDcort of all paraplegic men involved in this study in relation to DP. None difference is observed.
Table 3. p-value and δSSI3-2 related to the duration of paralysis.
Table 4. BMD parameters as measured by pQCT in various spasticity degrees in all paraplegics.
BMDtrab: BMD trabecular; BMDtot: BMD total; BMDcort: BMD cortical; THIcort: Cortical thickness; NS: non significant
Discussion
The rationale of the present pQCT study was to evaluate possible changes of trabecular and cortical bone, geometric and mechanical properties of the tibia regarding the neurological level of injury, the duration of paralysis, the spasticity and the use of supportive measures on bone parameters in the lower extremities of SCI paraplegic men compared to able-bodied controls. This will be useful for the management of osteoporosis and the rehabilitation of osteoporotic fractures in these patients.
The effects of long-term immobilization and paraplegia on bone are well documented3,7,13. An ongoing demineralization at the proximal tibia 3 years after SCI has been reported13. Also, a decrease in bone mineral content of approximately 4% per month in trabecular bone rich areas and 2% per month in cortical bone rich areas during the first year post injury has been observed after spinal cord section3. Others, using pQCT as in the present study found that bone mass loss is related to BMD decrease, while in the shaft it is related to reduced cortical wall thickness by the process of endosteal resorption7. In line with the literature, in both paraplegic subgroups of the present study, bone parameters were significantly reduced compared to the control group.
The reduction of cortical thickness in paraplegics is related to alterations of endosteal and periosteal circumference2,8. Since periosteal circumference is comparable to able-bodied individuals, a significant increase of endosteal circumference in paraplegics leads to reduction of cortical thickness8. The increase of endosteal bone in these patients suggests endosteal remodeling many years after the injury.2,8. In the present study, a higher loss of BMDtrab at the distal tibial epiphysis of high paraplegics was observed. On the contrary, in low paraplegics, the central and the peripheral cross-sectional area of bone were similarly affected. The similar reduction of BMD-tot in both paraplegic subgroups suggests that the cortical shell is more affected in low paraplegics.
There are few data on the effect of the neurological level of injury, duration of paraplegia, spasticity, and the use supportive measures and therapeutic walking on the mechanical strength in paralyzed legs3,4,7,11,14–18. In a study, using DXA to measure areal BMD the authors reported inverse relationships between duration after injury and leg percentage-matched BMD14. Others showed an exponential decrease in BMDtrab and BMDtot and no decrease in BMDcort of the diaphyses at 2 months to 50 years after the injury in tetraplegic and paraplegic men7. However, in the present study, although there was a statistical significant decrease of BMDcort of the tibia this was not related to the duration of paralysis suggesting a trend for recovery of cortical bone during the years of paralysis. An exponential decrease was found only for BMDtrab, BMDtot and THIcort in high paraplegics. This was a puzzling result that could be partially explained by population's differences and our small number of paraplegic study group; thus we believe that a similar large-scale study could increase the power of our results.
The effect of passive mechanical loading of bones, standing and therapeutic ambulation on BMD of the lower extremities in paraplegics has been controversial 11,17. Paraplegics unable to bear weight on their limbs (sitting in the wheelchair) are not exposed to forces to stimulate bone formation. Immobility leads to a changing pattern of loading in the paralysed areas, which respond by alteration in skeletal structure8. A beneficial effect on bone mass using passive mechanical loading has been shown at the femoral shaft, but not at the hip joint17. Others reported no correlation for passive standing-training to bone status11. Most patients with low level paraplegia can become ambulant with Knee Ankle Foot Orthoses (KAFOs). In the present study the subject's level of lesion were T4 to T7 vs. T8 to T12 (AIS-A), making the groups comparable in their physical abilities, the potential to ambulate with KAFOs and elbow crutches. Subjects above T7 are not able (mostly) to walk with KAFOs because of inability to use trunk muscles, which is necessary for walking with KAFOs. Subjects with T7 (and above) had more instability than their counterparts with T8 and T12 levels of lesion and only performed therapeutic standing in frames. Furthermore, factors (associated medical complications, amount and nature of rehabilitation and individual factors like age, activity level) that might influence the functional stability were controlled by the inclusion criteria. Paraplegics who used standing frames or long braces orthoses had statistically significant higher bone mass (BMD trab, BMD tot) and geometric parameters (cortical thickness) independently of the functional level, meaning that standing or therapeutic ambulation could possibly have a positive effect in cortical and trabecular bone in paraplegia. Goemaere et al found that passive mechanical loading can have a beneficial effect on the preservation of bone mass in the region of the femoral shaft, but not at the proximal hip17. Alekna et al found after a long follow – up period that standing in particular after two years gave significant higher BMD in legs, pelvis and the total body18. On the contrary, Eser et al found no correlation for passive standing-training to bone status11.
In the present study, the neurological level of injury (NLI) has been evaluated in relation to the DP and BMD factors. The δSSI3-2 was increased in low paraplegics compared to high paraplegics. However, the δSSI3-2 was found statistically significant to the duration of paralysis only in low paraplegics. This finding suggests that not only the mechanical loading but also the neurological factor seems to be a significant regulator in osteoporosis during the years of paralysis. This may possibly result from the increased capacity of standing and direct effect of mechanical loading of the distal tibia in low paraplegic patients.
There is clinical evidence of sympathetic regulation of bone metabolism in humans that has a clinically important role in diseases characterized by excessive sympathetic activity15. Changes in the autonomic nervous system are proposed to cause attrition of bone in SCI patients via changes in vascular tone and blood flow16.
Because of the non significant duration of paralysis between paraplegic groups, and despite the similar paralytic effect on bone in paraplegics, we suggest that the two paraplegic groups act differently in mechanical loading of the tibia.
Controversial results have also been reported regarding the effect of spasticity on BMD in SCI paraplegics3,4,10,17. A cross-sectional study of 41 SCI paraplegics reported less reduction of BMD in the spastic paraplegics SCI patients compared to the flaccid paraplegic SCI patients4. Löfvenmark et al in 18 motor complete SCI men matched for time since injury, gender, and age (9 had severe spasticity, and 9 had spasticity that was either mild or not present) found no difference in BMD depending on level of spasticity19. Others, in line with the present study, reported that spasticity may be protective against bone loss in SCI patients, however, without any preserving effect in the tibia11. A possible explanation for that could lie in the fact that in the present study all paraplegics were above T12 level with various degrees of spasticity according to the Ashworth scale. In addition, muscle spasms affecting the lower leg would mainly be extension spasms resulting in plantar flexion thus creating little resistance to the contracting muscles. Furthermore, the measuring sites of the tibia did not include any muscle insertions of either the knee or the ankle extensor muscles. Other investigators also have not been able to establish a correlation between BMD and muscle spasticity3,20.
By employing pQCT as a volumetric, quantitative, non-invasive regional measurement methodology we were able to assess separately cortical, trabecular and various geometric properties in fracture sites of the paralyzed lower extremities such as the tibia. The small number of paraplegic men in this study may be considered a limitation; we believe that a similar large-scale study could increase the statistical power of our results. Another limitation is that in the present study the neurological level of injury was at the T12 level or higher in all paraplegics, with various degrees of spasticity according to the Ashworth scale. Our results may have been biased of the effect of spasticity on bone parameters of the lower extremities.
Conclusions
Longitudinal studies are sparse and long term longitudinal chronic studies are unavailable. The present study, using pQCT successfully established a significant relation between the use of standing frames and brace orthoses in cortical and trabecular bone of the tibia in SCI paraplegic men. However, spasticity has not been shown to effect bone parameters. These findings are probably useful for clinical rehabilitation of patients with paraplegia.
Acknowledgments
We thank K. Kalogera and C. Kapsis, for their significant contribution and technical assistance in this study.
Footnotes
Disclosure
The authors have no conflict of interest.
References
- 1.Mamoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal Cord. 2006;44:203–210. doi: 10.1038/sj.sc.3101832. [DOI] [PubMed] [Google Scholar]
- 2.Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 3.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- 4.Demirel G, Yilmaz H, Paker N, Onel S. Osteoporosis after spinal cord injury. Spinal Cord. 1998;36:822–825. doi: 10.1038/sj.sc.3100704. [DOI] [PubMed] [Google Scholar]
- 5.Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–214. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- 6.Szollar SM, Martin EM, Sartoris DJ, Parthemore JG, Deftos LJ. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil. 1998;77:28–35. doi: 10.1097/00002060-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, et al. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Dionyssiotis Y, Trovas G, Galanos A, Raptou P, Papaioannou N, Papagelopoulos P, et al. Bone loss and mechanical properties of tibia in spinal cord injured men. J Musculoskelet Neuronal Interact. 2007;7:62–68. [PubMed] [Google Scholar]
- 9.Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86:498–504. doi: 10.1016/j.apmr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Dauty M, Perrouin-Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–309. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 11.Eser P, Frotzler A, Zehnder Y, Schiessl H, Denoth J. Assessment of anthropometric, systemic, and lifestyle factors influencing bone status in the legs of spinal cord injured individuals. Osteoporos Int. 2005;16:26–34. doi: 10.1007/s00198-004-1638-x. [DOI] [PubMed] [Google Scholar]
- 12.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- 13.Biering-Sorensen F, Bohr H, Schaadt O. Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia. 1988;26:293–301. doi: 10.1038/sc.1988.44. [DOI] [PubMed] [Google Scholar]
- 14.Clasey JL, Janowiak AL, Gater DR. Relationship between regional bone density measurements and the time since injury in adults with spinal cord injuries. Arch Phys Med Rehabil. 2004;85:59–64. doi: 10.1016/s0003-9993(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzman RJ. New treatments for reflex sympathetic dystrophy. N Engl J Med. 2000;343:654–656. doi: 10.1056/NEJM200008313430911. [DOI] [PubMed] [Google Scholar]
- 16.Chantraine A, van Ouwenaller C, Hachen HJ, Schinas P. Intramedullary pressure and intra-osseous phlebography in paraplegia. Paraplegia. 1979;17:391–399. doi: 10.1038/sc.1979.75. [DOI] [PubMed] [Google Scholar]
- 17.Goemaere S, Van Laere M, De Neve P, Kaufman JM. Bone mineral status in paraplegic patients who do or do not perform standing. Osteoporos Int. 1994;4:138–143. doi: 10.1007/BF01623058. [DOI] [PubMed] [Google Scholar]
- 18.Alekna V, Tamulaitiene M, Sinevicius T, Juocevicius A. Effect of weight-bearing activities on bone mineral density in spinal cord injured patients during the period of the first two years. Spinal Cord. 2008;46:727–732. doi: 10.1038/sc.2008.36. [DOI] [PubMed] [Google Scholar]
- 19.Löfvenmark I, Werhagen L, Norrbrink C. Spasticity and bone density after a spinal cord injury. J Rehabil Med. 2009;41:1080–1084. doi: 10.2340/16501977-0469. [DOI] [PubMed] [Google Scholar]
- 20.Gross M, Roberts JG, Foster J, Shankardass K, Webber CE. Calcaneal bone density reduction in patients with restricted mobility. Arch Phys Med Rehabil. 1987;68:158–161. [PubMed] [Google Scholar]