Abstract
Background and aim: The loco-regional recurrence of laryngeal carcinoma in patients who underwent total laryngectomy is related to numerous factors. Aim of the present study was to investigate the role of patient's age and tumor size in the recurrence rate of patients. Additional aim of the current study was to investigate the possible associations between the size of the tumor and other characteristics.
Patients and methods: In 1st Department of Otorhinolaryngology of AHEPA University Hospital, from 1992 to 2007, 255 patients with laryngeal carcinoma underwent total laryngectomy. Accurate data regarding the size of the tumor were obtained. Total laryngectomy was the initial treatment in 212 patients, while in the remaining 43 patients was performed as salvage surgery after recurrence.
Results: The median tumor size was 2.74 cm (0.8-5.5 cm). There was no significant difference in the median tumor size between the patients who had recurrence (2.87 cm) and the disease free patients (2.69 cm). The median size of glottic tumors (2.47 cm) was smaller than that of supraglottic (2.95 cm) and of subglottic tumors (3.27 cm) (p<0.05). Among the 255 patients, recurrence of the tumor occurred in 73 (28.7%). Statistical analysis of the data showed that the tumor size was affecting the recurrence rate in a different manner, according the stage of the tumor. The recurrence rate in T3 neoplasms was higher in larger tumors than in smaller (13.2% for tumors<2cm, 62% for tumors>4cm), while T4 carcinomas appeared to have the opposite behavior (66.5% for tumors <2cm, 23% for tumors >4cm). The median tumor size in T4 patients that recurred was smaller than in those with no recurrence (2.8 cm Vs 3.3 cm). This behavior was observed in T4 tumors from all sites. Patients who experienced recurrence and had positive neck lymph nodes at the time of the initial diagnosis appeared to have smaller laryngeal tumors (2.7 cm), compared to with the same group of patients with no recurrence (3.5 cm). Supraglottic location and advanced T stage showed a statistically significant impact on disease free survival, based on Cox regression model.
Conclusions: Smaller sized tumors in patients with locally advanced laryngeal cancer (T4) or regionally (N+) appear to have more aggressive behavior and higher recurrence rate. Thus, the small tumor size could be regarded as an unfavorable prognostic factor for those laryngeal cancer cases.
Keywords: tumor, size, laryngeal, carcinoma, recurrence
Laryngeal carcinoma is the most common cancer of head and neck region, accounting for 30%-40% of the new diagnosed malignancies of this area1. The American Cancer Society estimated that in 2008, about 12,250 people in the United States would have been found to have laryngeal cancer and about 3,670 will die of this disease1. Squamous cell carcinoma is the predominant histologic type, and approximately 40% of patients will have stage III or IV disease when first diagnosed2. Despite the increasing interest in application of larynx preservation procedures, total laryngectomy remains the treatment of choice in most cases, especially in patients with advanced stage disease.
In current clinical practice, the prognosis of laryngeal cancer is typically elaborated on the basis of a number of prognostic factors that include demographic, clinical and histopathological characteristics. However, controversial data have been reported, regarding the value of the above mentioned agents. Numerous studies have imputed prognostic significance to the age and sex of patients, to the status of lymph nodes and to the subsite located, size and other characteristics of the tumor3–6.
The aim of the present study is to investigate the role of patient's age and the tumor size in the recurrence rate of the tumor in patients with laryngeal carcinoma treated with total laryngectomy. Additional aim is to explore the possible associations between the size of the tumor and other characteristics of laryngeal carcinomas located in certain subsites.
Patients and methods
During the period from January 1992 until December 2007, 1042 patients with laryngeal carcinoma were diagnosed and treated in the 1st Otorhinolaryngology Department of AHEPA University Hospital. The clinical staging of these carcinomas was performed according to their location and extension, based on TNM system, using the classification of American Joint Cancer Committee (AJCC) and the International Unit against Cancer (UICC)7,8.
In 449 patients, total laryngectomy was considered essential for their therapeutic strategy, either as a primary therapeutic option or as salvage surgery after failure of radiotherapy or elective surgical procedures. In the present study, the inclusion criteria were: i) all patients who underwent total laryngectomy, excluding those for whom missing data regarding the tumor characteristics and patients postsurgical course, ii) record of measurement of the maximum diameter (in mm) of the tumor, as it was measured macroscopically in the surgical specimen immediately after the procedure of total laryngectomy.
Regarding to treatment modality used, we included in this study only patients who received the same therapeutic plan, total laryngectomy with the same neck treatment, without preoperative or postoperative radiotherapy with the aim to retrospectively identify the prognostic contribution of tumor size in the disease-free survival of these patients. Thus, we ultimately included in this study 255 laryngeal cancer patients who received total laryngectomy as the treatment of choice for their carcinoma.
Statistical analysis
Statistical analysis was performed using the SPSS 15.0 (SPSS, Inc, Chicago, IL) statistical package. The x2 test, independent samples t-test and the Pearson correlation coefficient were used for the comparison of the results of our study. Survival curves for disease-free survival were estimated by the Kaplan-Meier analysis. Cox regression model was selected in order to identify the variables with the greatest prognostic value. In all cases p values less than 0.05 were considered significant.
Results
The mean age of the 1042 patients with laryngeal carcinoma was 62 years. Regarding the recurrence rate, in patients with age below the mean of 62 years the rate was 34.8%, while in patients with age above of the mean the rate reached the 36.8%. Considering the recurrence rates in different age groups, 31% of patients under the 40 years, 32% of patients between 41 and 50 years old, 36% of patients between 51 and 60 years, 38% of patients between 61 and 70 years, 33.5% of patients between 71 and 80 years and 47 % of patients over the 81 years had a recurrence of their cancer after their initial treatment. The comparison of the above rates did not conclude to any statistical significant differences.
Of the 255 patients who met the inclusion criteria of this study, total laryngectomy was the initial treatment in 212 patients, while in the remaining 43 patients the operation was performed as salvage surgery after recurrence. The age of these patients varied between 36 and 78 years, mean 61.8 ± 8.5 years. 248 of the 255 patients were male, and only 7 (2.7%) were female. Postoperatively, the median follow up time for the patients was 49 months.
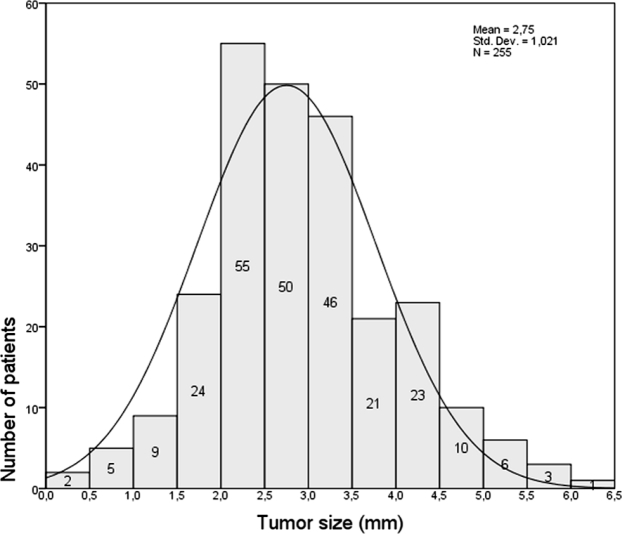
The tumor size in these 255 patients ranged from 0.5 to 5.8 cm, mean 2.74 cm (SD: 1.02 cm) (figure 1).
Figure 1. Range of tumor size in patients of present study.
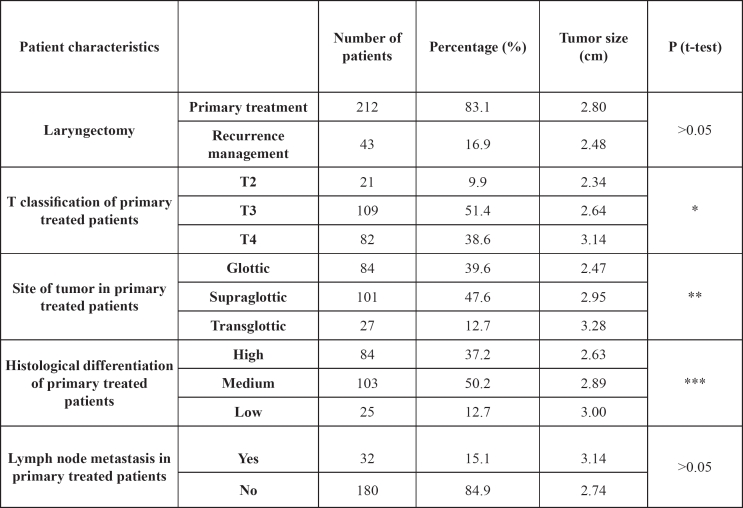
The clinical and histological characteristics of patients are presented in Table 1. The mean tumor size of 212 patients that received total laryngectomy as initial treatment modality (primary treated patients) was 2.8 cm, while the tumor size of 43 patients who had salvage total laryngectomy was 2.48 cm. The above difference was not statistical significant (p> 0.05).
Table 1. Clinical and histological characteristics of patients who underwent total laryngectomy as primary treatment option.
* <0.05 comparing tumor size of T4 with T2 and T3 but not between T2 and T3.
** <0.05 comparing tumor size of glottic with supraglottic and transglottic, but not between supraglottic and transglottic.
*** >0.05 comparing tumor size with 3 histological differentiation tumor types.
Regarding the tumor size in different laryngeal subsites, the mean size of glottic carcinomas (2.47 cm) was smaller than the supraglottic (2.95 cm) and transglottic (3.27 cm) carcinomas and that difference was statistically significant (p: 0.001 and 0.0003 respectively). The difference in the mean size of supraglottic and transglottic tumors was not statistical significant (p>0.05).
In present study, a positive correlation between the tumor size and the clinical stage of patient, based on TNM system, was recorded (p: 0.001). The mean tumor size of T4 carcinomas (3.14 cm) was greater, when compared with the mean tumor size of T2 (2.34 cm) and T3 (2.64 cm) carcinomas and that differentiation was statistical significant (p: 0.02 and 0.01 respectively). No statistical significant difference in mean tumor size was proved between T2 and T3 tumors (t-test, p: 0.19). Regarding the laryngeal subsites of tumors, the above important difference between T3 and T4 carcinomas was detected only for glottic and not for supraglottic tumors.
The histological grade of tumors differentiation was correlated negatively with its size, indicating that the lower the grade was, the higher the mean tumor size was (p: 0.049). When grouping the tumors according to the grade of their differentiation, the mean tumor size was not significantly different between the three groups (p>0.05).
Local recurrence of the tumor occurred in 73 OF the 255 patients (28.7%). There was no statistically significant difference found in the mean size of the tumor between the group of patients who had a recurrence (2.87 cm) and those who remained disease free (2.69 cm) (p<0.05).
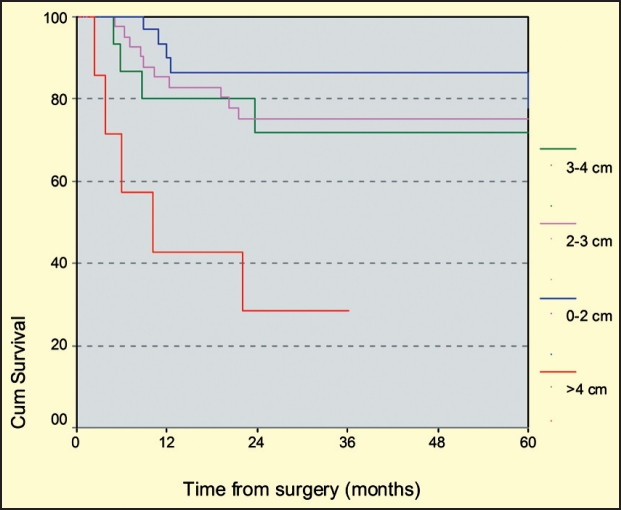
The total of 255 patients was divided in T3 and T4 subgroup and the mean tumor size was calculated for the patients who experienced recurrence and for those who remained disease free. The recurrence rate for patients with T3 tumors was 22% (24 of 109 patients). The mean size of the tumors in the T3 subgroup of patients was greater in those had a recurrence (mean size 3 cm), when compared to the disease free T3 patients (mean size 2.54 cm) and this difference was statistically significant (p: 0.04). In addition, recurrence rate for tumors measuring less than 2cm was 13.2%, while for tumors measuring more than 4cm was 62% (p<0.05, log rank test) (figure 2).
Figure 2. Kaplan-Meier survival curve of T3 tumors according to tumor size (log rank test, p<0.05), demonstrating the negative prognostic impact of tumor size greater than 4cm.
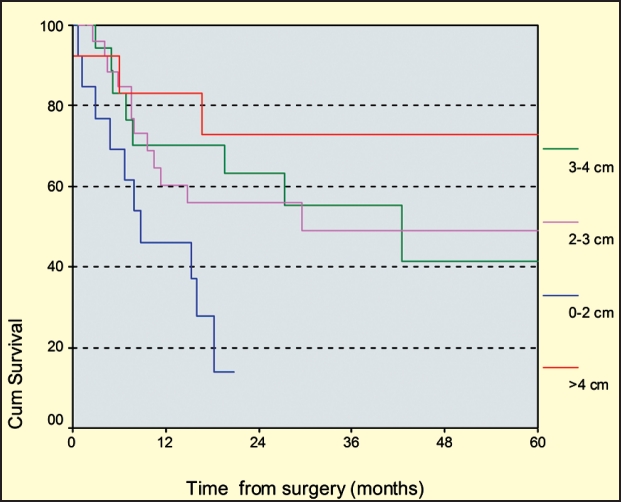
Tumor size had the exactly opposite effect on the recurrence rate of patients with T4 tumors. The recurrence rate of the T4 subgroup was 40.25% (33 of the 82 patients). Interestingly, the mean tumor size in T4 patients who experienced recurrence was smaller than in those with no recurrence (2.8 cm vs 3.3 cm, p>0.05). Investigating the edges of the T4 subgroup of patients, we found that T4 tumors measuring less than 2cm had a recurrence rate of 66.5%, while those measuring more than 4cm recurred only in 23%% (p<0.05, log rank test) (figure 3). This behavior was consistent in all T4 tumors, without reference to their initial location.
Figure 3. Kaplan-Meier survival curve of T4 tumors according to tumor size (log rank test, p<0.05), demonstrating the aggressive character of tumors with size greater than 4cm.
Regarding the lymph node status, 32 of 212 patients (15%) had cervical clinical lymphadenopathy at the initial diagnosis of laryngeal carcinoma. The mean tumor size of patients with positive lymph status (3.14 cm) was statistical higher, when compared with patients without neck lymphadenopathy (2.74 cm) that received total laryngectomy as initial treatment (p: 0,04). Patients who had recurrence and at the time of the initial diagnosis had positive neck lymph nodes, appeared to have smaller laryngeal tumors (2.7 cm), when compared with the patients positive neck lymph nodes and at the time of initial diagnosis had no recurrence (3.5 cm).
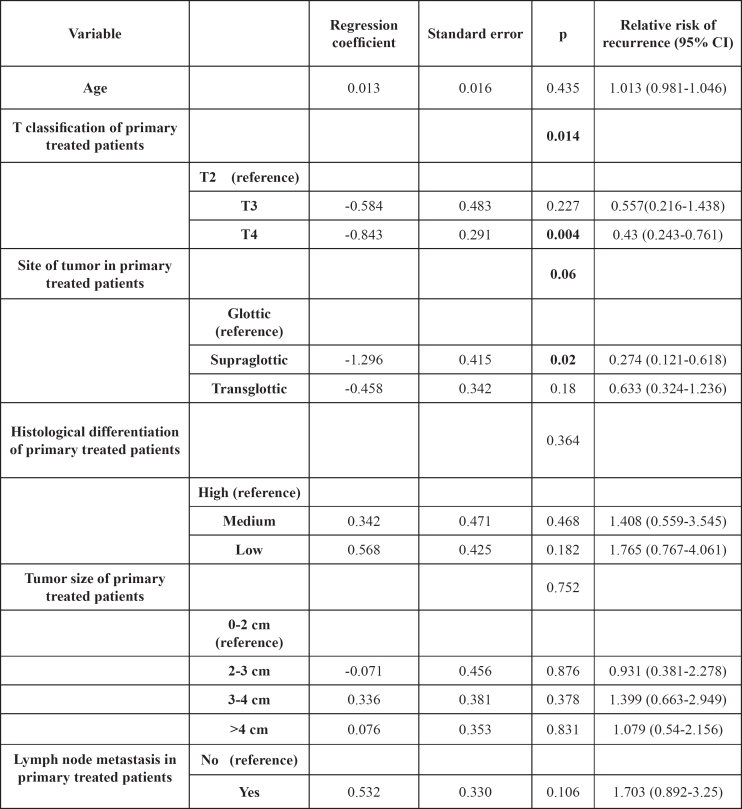
Finally, six variables (age, tumor size, T stage, N status, tumor site and histologic grade) were included in the multivariate analysis for patients that underwent total laryngectomy as primary therapeutic option, in order to estimate the prognostic significance of each of them (table 2). Supraglottic location and advanced T stage showed a statistically significant impact on disease free survival.
Table 2. Cox regression model for the prognostic impact on disease free survival of patients, having total laryngectomy as primary therapeutic option.
CI: confidence interval
Discussion
Management of laryngeal cancer has traditionally relied on radiation and surgery, but, currently, is increasingly focused on organ-conservation strategies for tumors of advanced stage. Laryngeal preservation has emerged as a viable alternative to radical surgery. Despite this attempt to maintain a functional larynx and the growing interest in chemoradiation and larynx-sparing operations, many patients will inevitably require total laryngectomy. Moreover, total laryngectomy remains the standard of care for surgical salvage of radiation failure in laryngeal cancer. It should be noted that patients with recurrence after failed radiation therapy present with advanced tumors and total laryngectomy is the treatment of choice.
In clinical oncology, an issue of great importance in dealing with treatment selection in patients with laryngeal cancer is the identification of unfavorable prognostic determinants. The prognosis for patients with laryngeal cancer is determined by patient factors, tumor factors, and treatment factors.
It should be noted that the current TNM classification of laryngeal cancer based on the criterion of the tumor extent in the laryngeal sites and subsites and on the criterion of vocal cord mobility makes no objective references to the dimensions of the tumors and to the degree of tumor cell differentiation. Despite the fact that the present study indicates the positive correlation of tumor size and clinical stage, an advanced stage carcinoma, based on TNM system, is not evidential of a sizable tumor9. Tumor size is recognized as prognostic factor and ideally might contribute to an early identification of the different subgroups of patients with different prognosis3,11–12.
According to the present study, tumor size has prognostic significance for patients that underwent total laryngectomy. In the group of patients with advanced laryngeal cancer, large T3 tumors (>4 cm) and small T4 tumors (<2 cm) seem to have the greater recurrence rate. To best of our knowledge, this the first report of the aggressive character of small T4 laryngeal tumors. Reviewing the literature, numerous studies indicate that large tumor size of laryngeal carcinomas is associated with unfavorable disease free survival3,10–12.
Among the patient factors, age, gender, tobacco and alcohol use habits and performance status have already been reported, with contrasting results 13–15. Results of the present study indicate that the disease free survival of patients seems to be unrelated to their age. Regarding the grade of histological differentiation of the carcinoma, its prognostic significance is unsettled. Our study does not confirm previous reports, supporting that low grade tumors (G3) which have higher proliferation than G2 and G1 tumors, are responsible for tumor relapse, either locally or locoregionally11,16,17.
Tumor characteristics such as site of the primary tumor, T stage, N status and TNM stage have been established to have prognostic significance in a number of previous studies3,5,12,16. The findings of present study certify the prognostic significance of supraglottic location and advanced T stage.
The supraglottic locations of the tumor, the transglottic site and the subglottic extension have been implicated with poor prognosis3,16. Moreover, poor prognosis has been associated with the advanced T stage of the tumor3,4,6,12,18. It should be noted that, according to the results of our study, the significant different tumor size between T3 and T4 carcinomas applied to all patients with glottic tumors, but for not for those with supraglottic tumors. The above finding supports the easier modification of supraglottic tumors from T3 stage to T4, due to the absence of distinct anatomic boundaries to the supraglottic region of larynx.
Conclusions
Our study results confirm the existence of several prognostic factors representing clinical characteristics and tumor-related conditions that, if assessed at diagnosis, can be useful in estimating levels of risk expectancy for optimizing treatment strategies. The smaller sized tumors in patients with advanced laryngeal cancer, either locally (T4) or regionally (N+) appear to have more aggressive behavior and higher recurrence rate. Thus, the small tumor size could be regarded as an unfavorable prognostic factor for those laryngeal cancer cases.
References
- 1.American Cancer Society Cancer facts & figures 2007. 2007.
- 2.Myers EN, Smith MR, Myers J, Hanna E. Cancer of the Head and Neck. Philadelphia: Saunders; 2003. [Google Scholar]
- 3.Danic D, Maruic M, Uzarevic B, Milicic D. Prognostic factors in squamous cell carcinoma of the larynx. ORL J Otorhinolaryngol Relat Spec. 2000;62:143–148. doi: 10.1159/000027735. [DOI] [PubMed] [Google Scholar]
- 4.Eiband JD, Elias EG, Suter CM, Gray WC, Didolkar MS. Prognostic factors in squamous cell carcinoma of the larynx. Am J Surg. 1989;158:314–317. doi: 10.1016/0002-9610(89)90123-2. [DOI] [PubMed] [Google Scholar]
- 5.Spector JG, Sessions DG, Haughey BH. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope. 2001;111:1079–1087. doi: 10.1097/00005537-200106000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Stankiewicz C. Prognostic significance of lymph node reactivity in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol. 1994;251:418–422. doi: 10.1007/BF00181968. [DOI] [PubMed] [Google Scholar]
- 7.American Joint Committee on Cancer (AJCC) Manual for staging of cancer. Philadelphia: JB Lippincott; 1997. pp. 127–137. [Google Scholar]
- 8.International Union Against Cancer . TNM classification of malignant tumours. New York: Wiley-Liss; 1997. pp. 93–100. [Google Scholar]
- 9.Lukits J, Timar J, Juhasz A, Dome B, Paku S, Repassy G. Progression difference between cancers of the larynx and hypopharynx is not due to tumor size and vascularization. Otolaryngol Head Neck Surg. 2001;125:18–22. doi: 10.1067/mhn.2001.116187. [DOI] [PubMed] [Google Scholar]
- 10.Gallo O, Sarno A, Baroncelli R, Bruschini L, Boddi V. Multivariate analysis of prognostic factors in T3 N0 laryngeal carcinoma treated with total laryngectomy. Otolaryngol Head Neck Surg. 2003;128:654–662. doi: 10.1016/S0194-59980300228-6. [DOI] [PubMed] [Google Scholar]
- 11.Pulkkinen JO, Klemi P, Martikainen P, Grenman R. Apoptosis in situ, p53, bcl-2 and AgNOR counts as prognostic factors in laryngeal carcinoma. Anticancer Res. 1999;19:703–707. [PubMed] [Google Scholar]
- 12.Jin YT, Kayser S, Kemp BL. The prognostic significance of the biomarkers p21WAF1/CIP1, p53, and bcl-2 in laryngeal squamous cell carcinoma. Cancer. 1998;82:2159–2165. [PubMed] [Google Scholar]
- 13.Gavilan J, Gavilan C, Manos-Pujol M, Herranz J. Discriminant analysis in predicting survival of patients with cancer of the larynx or hypopharynx. Clin Otolaryngol Allied Sci. 1987;12:331–335. doi: 10.1111/j.1365-2273.1987.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 14.Stell PM. Prognosis in laryngeal carcinoma: host factors. Clin Otolaryngol Allied Sci. 1990;15:111–119. doi: 10.1111/j.1365-2273.1990.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 15.Manni JJ, Terhaard CH, de Boer MF. Prognostic factors for survival in patients with T3 laryngeal carcinoma. Am J Surg. 1992;164:682–687. doi: 10.1016/s0002-9610(05)80734-2. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski LP, Franco EL, de Andrade Sobrinho J, Oliveira BV, Pontes PL. Prognostic factors in laryngeal cancer patients submitted to surgical treatment. J Surg Oncol. 1991;48:87–95. doi: 10.1002/jso.2930480204. [DOI] [PubMed] [Google Scholar]
- 17.Morales-Angulo C, Val-Bernal F, Buelta L, Fernandez F, Garcia-Castrillo L, Rama J. Prognostic factors in supraglottic laryngeal carcinoma. Otolaryngol Head Neck Surg. 1998;119:548–553. doi: 10.1016/S0194-5998(98)70123-8. [DOI] [PubMed] [Google Scholar]
- 18.Barra S, Barzan L, Maione A. Blood transfusion and other prognostic variables in the survival of patients with cancer of the head and neck. Laryngoscope. 1994;104:95–98. doi: 10.1288/00005537-199401000-00017. [DOI] [PubMed] [Google Scholar]