SUMMARY
Hypothalamic neuropeptide Y (NPY) has been implicated in control of energy balance, but the physiological importance of NPY in the dorsomedial hypothalamus (DMH) remains unclear. Here we report that knockdown of NPY expression in the DMH by adeno-associated virus-mediated RNAi reduced fat depots in rats fed regular chow and ameliorated high-fat diet-induced hyperphagia and obesity. DMH NPY knockdown resulted in development of brown adipocytes in inguinal white adipose tissue through the sympathetic nervous system. This knockdown increased uncoupling protein 1 expression in both inguinal fat and interscapular brown adipose tissue (BAT). Consistent with the activation of BAT, DMH NPY knockdown increased energy expenditure and enhanced the thermogenic response to a cold environment. This knockdown also increased locomotor activity, improved glucose homeostasis and enhanced insulin sensitivity. Together, these results demonstrate critical roles of DMH NPY in body weight regulation through affecting food intake, body adiposity, thermogenesis, energy expenditure and physical activity.
INTRODUCTION
The hypothalamus plays a central role in maintaining energy homeostasis (Cone, 2006; Elmquist et al., 1999; Schwartz et al., 2000; Spiegelman and Flier, 2001). Various hypothalamic nuclei, including the arcuate nucleus (ARC), dorsomedial hypothalamus (DMH), paraventricular nucleus (PVN), lateral hypothalamic area (LH), and ventromedial hypothalamus (VMH) have been demonstrated to have specific functions in the regulation of energy balance. Although the observations that DMH lesions result in hypophagia and decreased body weight indicate the importance of the DMH in maintaining energy homeostasis (Bellinger and Bernardis, 2002), the neural mechanisms through which the DMH acts to affect energy balance remain unclear.
Neuropeptide Y (NPY) is an important hypothalamic orexigenic peptide (Clark et al., 1984; Levine and Morley, 1984; Stanley and Leibowitz, 1984). Numerous reports have demonstrated the actions of NPY in the ARC in the control of energy balance. The ARC contains two distinct populations of neurons: orexigenic neuropeptide NPY/agouti-related protein (AgRP) neurons and anorexigenic proopiomelanocortin (POMC) neurons. These two neural systems integrate hormonal (such as leptin and insulin) and nutrient signals to modulate food intake and body weight (Cone, 2006; Elmquist et al., 1999; Schwartz et al., 2000; Spiegelman and Flier, 2001). Modulation of ARC NPY signaling in adult animals significantly impacts energy balance. Genetic ablation of neurons expressing NPY/AgRP in adult mice results in a lean and hypophagic phenotype (Bewick et al., 2005; Gropp et al., 2005). Adeno-associated virus (AAV)-mediated expression of antisense Npy cRNA in the ARC of adult rats decreased NPY expression and resulted in decreased food intake and body weight (Gardiner et al., 2005). We have shown that knockdown of NPY expression in the ARC via AAV-mediated RNA interference (RNAi) attenuated the feeding response to food deprivation (Yang et al., 2009).
NPY is also expressed by neurons within the DMH, but the function of NPY in this nucleus has yet to be fully determined. Recent reports imply that NPY in the DMH may serve as an important neuromodulator to influence energy balance. We and other investigators have reported that Npy gene expression was elevated in the DMH in specific animal models with increased energy demands (Bi et al., 2003; Kawaguchi et al., 2005; Smith, 1993). Induction or overexpression of Npy in the DMH has also been found in several rodent models of obesity (Bi et al., 2001; Guan et al., 1998; Kesterson et al., 1997; Tritos et al., 1998). Moreover, whereas NPY in the ARC is under the control of leptin, its regulation in the DMH is leptin-independent (Bi et al., 2003). Overall, these observations suggest that NPY in the DMH is important for maintaining energy homeostasis, but the mechanism of action of DMH NPY in controlling energy balance differs from that of ARC NPY.
We have manipulated DMH NPY signaling using viral-mediated alterations of NPY expression in the DMH for examination of the role of DMH NPY in controlling energy balance. We found that AAV-mediated overexpression of NPY in the DMH of lean rats increased food intake and body weight, and exacerbated diet-induced obesity (Yang et al., 2009). Knockdown of NPY expression in the DMH via AAV-mediated RNAi ameliorated the hyperphagia, obesity and diabetes of Otsuka Long-Evans Tokushima Fatty (OLETF) rats (Yang et al., 2009) in which DMH NPY overexpression has been proposed to play an etiological role (Bi et al., 2001). Thus, these results implicate DMH NPY in modulating food intake and energy balance. It is unclear, however, whether NPY in the DMH is physiologically important in maintaining energy homeostasis and/or whether DMH NPY affects other aspects of energy balance including energy expenditure, thermoregulation, adipogenesis and physical activity. Here, we sought to ascertain such roles of DMH NPY in body weight regulation by using AAV-mediated RNAi for specifically knocking down NPY expression in the DMH of rats.
RESULTS
AAV-mediated knockdown of NPY expression in the DMH
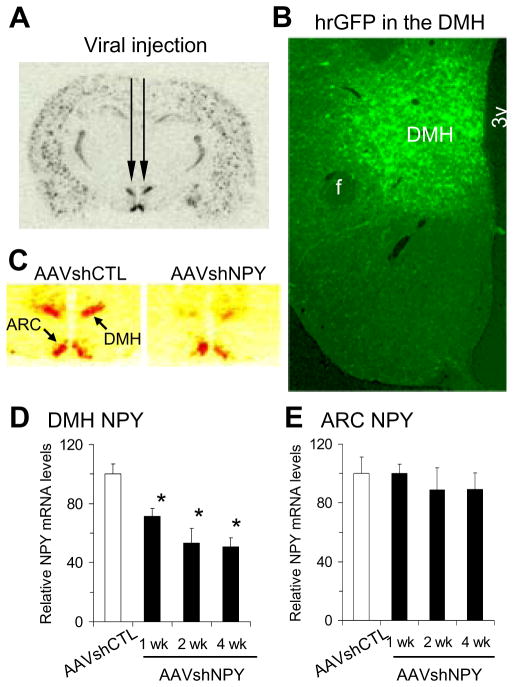
We generated a recombinant vector of AAV-mediated RNAi with NPY-specific short hairpin RNA (AAVshNPY) containing humanized Renilla green fluorescent protein (hrGFP) marker as previously reported (Yang et al., 2009). To test the idea that DMH NPY may be an important neuromodulator of energy balance under normal conditions, we first determined the effect of AAV-mediated RNAi on Npy gene expression in Sprague-Dawley rats by injecting this vector bilaterally into the DMH (Fig. 1A). We established that the viral vectors infected neurons within the DMH as early as 1 week after viral injection, led to a robust infection within 2 weeks (Fig. 1B, hrGFP-positive neurons), and produced significant knockdown of Npy mRNA expression in the DMH (Fig. 1C) by 28%, 47% and 49% at 1, 2 and 4 weeks post-viral injections, respectively, compared to rats receiving control vector injections (AAVshCTL, Fig. 1D). This knockdown effect was site-specific since no hrGFP-positive neurons were detected in the ARC (Fig. 1B) and Npy mRNA levels were unaltered in the ARC (Fig. 1E). Consistent with our previous report (Yang et al., 2009), the effects of AAV-mediated RNAi on Npy mRNA expression in the DMH were long lasting; 16 weeks post-viral injection, Npy mRNA levels remained reduced by 36% (data not shown).
Figure 1.
Adeno-associated virus (AAV)-mediated knockdown of NPY expression in the dorsomedial hypothalamus (DMH). (A) Bilateral injections of AAV vectors into the DMH. (B) Representative micrograph shows hrGFP (humanized Renilla green fluorescent protein) expression in the DMH post-viral DMH injection as examined under fluorescence microscopy. (C) 35S-labeled in situ hybridization histochemistry shows suppressed Npy expression in the DMH (pseudo red) in rats receiving bilateral DMH injections of AAVshNPY compared with rats receiving bilateral DMH injections of AAVshCTL. (D–E) Mean ± SEM Npy mRNA levels significantly decreased in the DMH of AAVshNPY rats at 1, 2 and 4 weeks after viral injection compared with AAVshCTL rats (D), but Npy mRNA levels in the arcuate nucleus (ARC) did not differ between AAVshCTL and AAVshNPY rats at any time points (E). n = 5 per group. *P<0.05 compared with AAVshCTL rats. f: fornix; 3v: the third ventricle.
Effects of DMH NPY knockdown on regulation of body weight
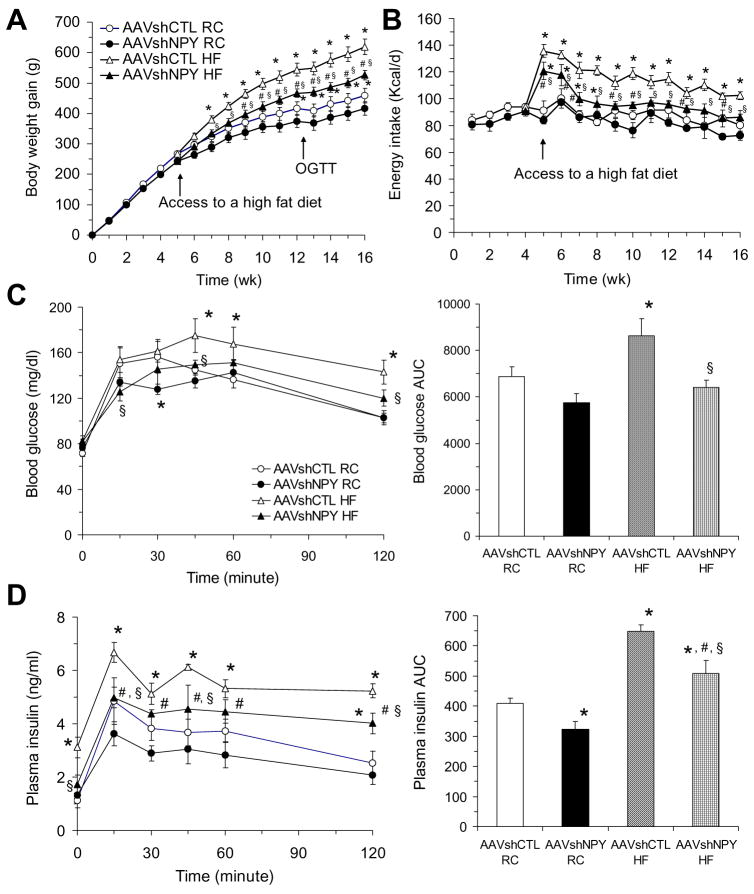
Following determination of viral-mediated knockdown of NPY expression in the DMH, we examined whether this knockdown affects body weight regulation. We found that DMH NPY knockdown resulted in a small but significant decrease in body weight gain over the first 5 weeks post-viral injection when rats were maintained on regular chow (RC, P =0.035, Fig. 2A). The weight gain of NPY knockdown rats was reduced by about 9%. Since high-fat diet (HF) increases body weight and induces obesity, we next assessed the effect of DMH NPY knockdown on HF-induced weight gain. Half the NPY knockdown and control rats were challenged with HF at 5 weeks post-viral injection. We found that NPY knockdown significantly reduced HF-induced increases in weight gain (P=0.023). Control rats fed HF gained significantly more weight by 2 week (P =0.026) and had gained 35% more weight by 11 weeks compared to control rats on RC. In contrast, NPY knockdown rats fed HF gained body weight more slowly, only achieving significantly increased body weight by 4 weeks (P =0.021) and having only 26% more weight by 11 weeks compared to those on RC (Fig. 2A). As a result, the body weight gain of NPY knockdown rats remained relatively normal until 7 weeks on HF compared to control rats on RC and was significantly less than control rats in 11 weeks on HF (Fig. 2A).
Figure 2.
Effects of DMH NPY knockdown on food intake, body weight, and glucose tolerance. (A) Body weight gain in AAVshCTL and AAVshNPY rats with access to a regular chow (RC) or high fat (HF) diet. OGTT, time of oral glucose tolerance test. (B) Daily food intake in the four groups of rats. (C) Blood glucose response to oral glucose administration. AUC indicates the area under the curve. (D) Plasma insulin response to oral glucose administration. Values are means ± SEM. n = 6 rats per group. *P<0.05 compared with AAVshCTL RC rats, #P<0.05 compared with AAVshNPY RC rats and §P<0.05 compared with AAVshCTL HF rats.
Since we have demonstrated the effects of DMH NPY on food intake and meal patterns in both OLETF and intact rats (Yang et al., 2009), we examined whether DMH NPY knockdown altered daily food intake in the present study. Although daily energy intake did not differ between the two groups of rats over 16 weeks on RC, DMH NPY knockdown significantly reduced HF-induced hyperphagia (Fig. 2B). Both groups of rats increased daily intake dramatically upon initial access to HF, but the degree of increase and its duration were significantly reduced in NPY knockdown rats (Fig. 2B). While control rats on HF remained hyperphagic, NPY knockdown rats normalized energy intake in 4 weeks on HF (Fig. 2B).
DMH NPY knockdown improves glucose homeostasis
We next tested the effects of DMH NPY knockdown on glucose homeostasis. Although oral glucose administration resulted in similar patterns of glucose clearance in NPY knockdown and control rats on RC (Fig. 2C), NPY knockdown rats required less insulin secretion to clear the glucose as indicated by a reduction in the area under the response curve of insulin in NPY knockdown rats (Fig. 2D), suggesting that down-regulation of DMH NPY expression enhances insulin sensitivity. HF access caused hyperinsulinemia and impaired glucose clearance in control rats as determined by high fasting insulin levels and elevated blood glucose and plasma insulin levels in response to oral glucose (Fig. 2C and D). DMH NPY knockdown significantly ameliorated these changes. NPY knockdown rats on HF had normal glucose response to an oral glucose load (Fig. 2C) and normal fasting insulin levels (Fig. 2D) relative to control rats on RC. Although the area under the response curve of insulin in NPY knockdown rats on HF was higher than that of control rats on RC, the levels were significantly reduced compared to control rats on HF (Fig. 2D).
DMH NPY knockdown promotes development of brown adipocytes in white adipose tissue
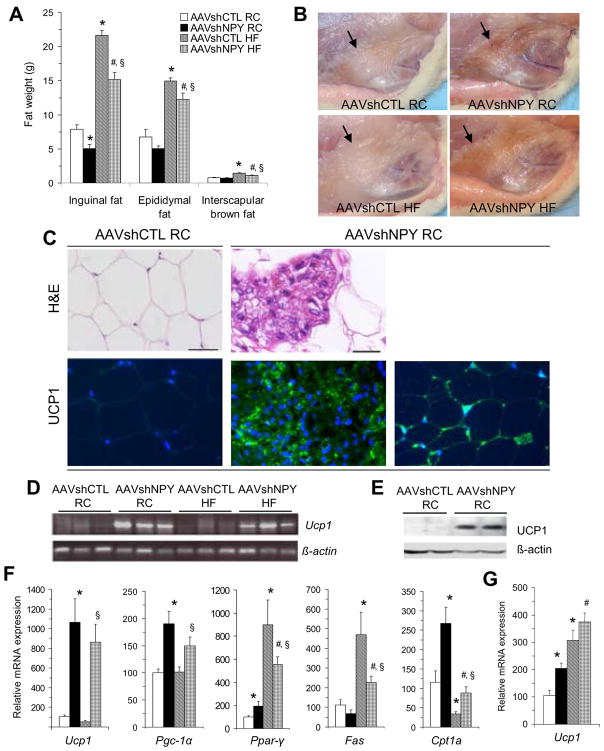
Examination of body fat mass revealed a site-specific effect of DMH NPY knockdown on adiposity. We found that subcutaneous inguinal fat mass was significantly decreased in NPY knockdown rats on RC compared to control rats (Fig. 3A) and observed that the color of the inguinal fat appeared significantly darker (brownish) in NPY knockdown rats than that of control rats (Fig. 3B). This color change was also found in the subcutaneous axillary white fat areas, but not in other subcutaneous, epididymal and visceral white fat depots (including mesenteric, retroperitoneal and perirenal fat) in NPY knockdown rats (data not shown). Moreover, while high-fat diet resulted in significant increases in fat accumulation in inguinal and epididymal white and interscapular brown fat in control rats, all these increases were significantly decreased in NPY knockdown rats on HF (Fig. 3A). Although NPY knockdown rats on HF accumulated more inguinal fat than those on RC (Fig. 3A), the fat still appeared more brown (Fig. 3B).
Figure 3.
DMH NPY knockdown promotes development of brown adipocytes in inguinal white adipose tissue (WAT). (A) Fat weights at three different sites in the four groups of rats. (B) The color of inguinal WAT became dark (brownish) in AAVshNPY rats compared to that of AAVshCTL rats as indicated by black arrows. (C) Representative H&E (hematoxylin and eosin) stain shows unilocular adipocytes in inguinal WAT of AAVshCTL rats (upper left) and multilocular adipocytes (brown-like adipocytes) in inguinal WAT of AAVshNPY rats (upper middle); Mitochondrial uncoupling protein 1 (UCP1) was detected in inguinal adipocytes in AAVshNPY rats (green, lower middle) by using immunostaining with anti-UCP1 antibody and nuclei (blue) were counterstained by DAPI (4′,6-Diamidino-2-phenylindole); UCP1 immunoreactive (green) unilocular adipocytes were also detected in inguinal WAT of AAVshNPY rats (lower right), but undetectable in inguinal WAT of AAVshCTL rats (lower left). Scale bar indicates 20μm. (D) Ucp1 mRNA was expressed in the inguinal fat of AAVshNPY rats as determined by RT-PCR. (E) UCP1 protein was produced in the inguinal fat of AAVshNPY rats as determined by Western blot. (F) mRNA expression levels in the inguinal adipose tissue including Ucp1, peroxisome proliferator-activated receptor-γ (PPARγ)-coactivator-1α (Pgc-1 α), Ppar-γ, fatty acid synthesis (Fas) and carnitine palmitoyltransferase 1α (Cpt1α). (G) Ucp1 mRNA expression in the interscapular brown adipose tissue. Values are means ± SEM. n = 6 rats per group. *P<0.05 compared with AAVshCTL RC rats, #P<0.05 compared with AA VshNPY RC rats and §P<0.05 compared with AAVshCTL HF rats.
We next characterized inguinal adipose tissue in NPY knockdown rats. Hematoxylin and eosin (H&E) staining revealed that inguinal adipocytes in control rats contained unilocular adipocytes, i.e., showing typical white adipocytes (Fig. 3C, upper left), whereas both the size and number of white adipocytes were reduced in inguinal adipose tissue of NPY knockdown rats. In addition, the cells formed new large clusters that contained multilocular adipocytes (brown-like adipocytes) and were surrounded by white adipocytes (Fig. 3C, upper middle). In support of brown adipocyte formation, these cells showed robust immunostaining (green) for mitochondrial uncoupling protein 1 (UCP1, a marker of brown adipose tissue, BAT, Fig. 3C, lower middle). UCP1 immunostaining (green) was also detected in a number of unilocular adipocytes in inguinal adipose tissue of NPY knockdown rats (Fig. 3C, lower right), but undetectable in those of control rats under basal conditions (Fig. 3C, lower left). Quantitative real-time RT-PCR (reverse transcriptase-polymerase chain reaction) and Western blot analyses further confirmed UCP1 expression in the inguinal fat of NPY knockdown rats (Fig. 3D and E). Levels of Ucp1 mRNA expression were significantly elevated in NPY knockdown rats relative to their controls (Fig. 3F). By contrast, Ucp1 mRNA expression was undetectable (or unchanged) in other white fat depots including epididymal, mesenteric, retroperitoneal and perirenal fat in NPY knockdown rats (data not shown). We also examined another BAT-select gene, peroxisome proliferator-activated receptor-γ (PPAR-γ) coactivator-1a (PGC-1a, Handschin and Spiegelman, 2006) and found that Pgc-la was also highly expressed in the inguinal fat of NPY knockdown rats (Fig. 3F). Together, these results provide clear evidence that DMH NPY knockdown promotes development of brown adipocytes in inguinal white adipose tissue (WAT), or causes inguinal WAT into BAT transformation.
To test the possiblity of effects of DMH NPY knockdown on adipogenesis and fat metabolism in the inguinal adipose tissue, we examined gene expression for PPAR-γ, fatty acid synthase (FAS) and carnitine palmitoyltransferase la (CPTla). PPAR-γ is an important transcription factor in the development of both white and brown fat cells (Rosen et al., 1999). Compared to control rats, Ppar-γ mRNA levels were significantly increased in NPY knockdown rats, and while high-fat diet increased Ppar-γ mRNA levels in control rats, this increase was significantly reduced in NPY knockdown rats (Fig. 3F), suggesting that DMH NPY knockdown may contribute to brown adipogenesis in the inguinal fat and also limits HF-induced white fat adipogenesis. DMH NPY knockdown also affected metabolism in the inguinal fat. FAS plays a key role in fatty acid synthesis, whereas CPTla is the rate-limiting enzyme controlling fatty acid oxidation. Compared to control rats, Cpt1a gene expression was significantly increased in the inguinal fat of NPY knockdown rats with a trend toward a decrease in Fas gene expression, indicating a shift from lipogenesis to fatty acid oxidation in this tissue (Fig. 3F). High-fat diet induced more fatty acid synthesis in control rats, with increased Fas gene expression and decreased Cptla gene expression, whereas DMH NPY knockdown reversed these alterations (Fig. 3F).
DMH NPY knockdown increases BAT activation
Brown fat is mainly deposited in the interscapular area of rats where it plays a primary role in nonshivering thermogenesis through activation of UCPl (Cannon and Nedergaard, 2004). We next examined whether DMH NPY knockdown affected activity of interscapular BAT. We found that Ucpl gene expression was significantly increased in interscapular BAT of NPY knockdown rats on RC compared to control rats (Fig. 3G), suggesting that DMH NPY knockdown results in increased BAT activity. When rats were fed HF, Ucpl gene expression was significantly elevated in interscapular BAT in both groups (Fig. 3G), implying that HF induces thermogenesis in these two groups. Although Ucpl gene expression was slightly higher in NPY knockdown rats than in control rats on HF, the difference was not statistically significant (Fig. 3G).
Sympathetic mediation of development of brown adipocytes in white adipose tissue
To test whether the sympathetic nervous system (SNS) mediates the development of brown adipocytes in inguinal WAT, we examined whether sympathetic denervation altered brown adipocyte formation by injecting the neurotoxin 6-hydroxydopamine (6-OHDA) unilaterally into the inguinal fat area two weeks prior to bilateral DMH injections of the vector AAVshNPY (Fig. 4A). At sacrifice, examination of the inguinal fat pads revealed that while the inguinal adipose tissue became dark brown on the side of saline injection, the fat tissue remained relatively white (or significantly less brown) in the side of 6-OHDA injection (Fig. 4B). DMH NPY knockdown resulted in significant increases in the level of norepinephrine (NE) within the saline-treated inguinal fat pads as compared to control rats (Fig. 4C). 6-OHDA treatment prevented this increase (Fig. 4C). Fat NE levels were significantly decreased in the side of 6-OHDA treatment relative to the control side in both groups of rats and the levels of NE within the 6-OHDA-treated inguinal fat pads did not differ between the two groups of rats (Fig. 4C). Consistent with the change of fat color, numerous clusters of brown-like adipocytes (multilocular adipocytes) were found in the side of saline-treated inguinal adipose tissue of NPY knockdown rats, whereas brown-like adipocytes were dramatically reduced by 6-OHDA treatment (Fig. 4D). Determination of UCPl expression confirmed that 6-OHDA treatment prevented UCPl expression at both the protein and mRNA levels (Fig. 4E and F). Thus, sympathetic denervation prevented development of brown adipocytes in inguinal WAT.
Figure 4.
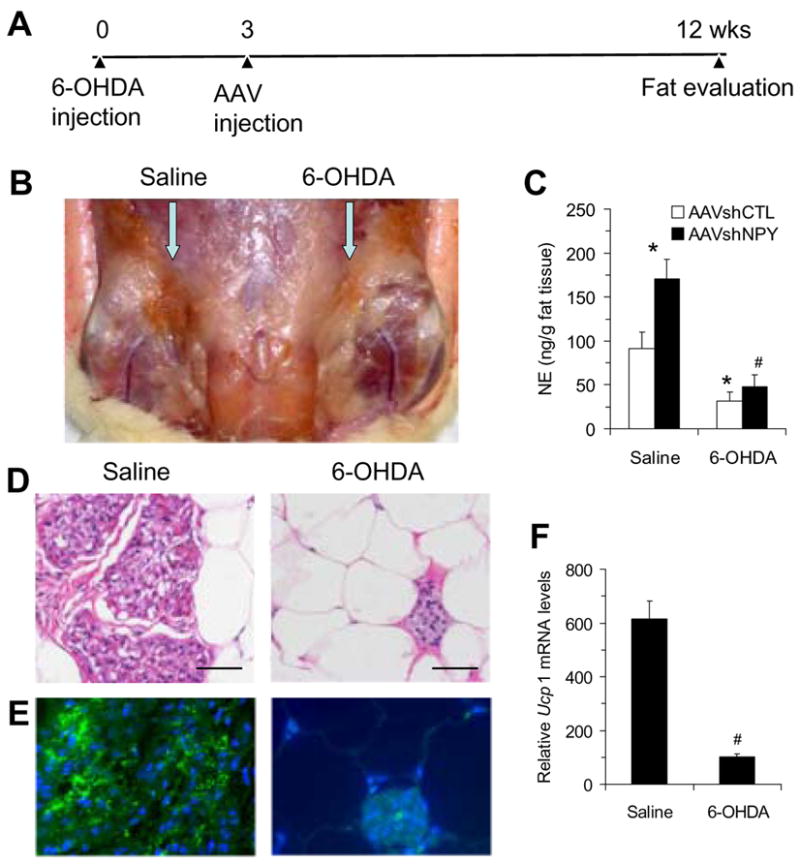
Sympathetic denervation in the inguinal adipose tissue of AAVshNPY rats. (A) Schedule of sympathetic denervation experiment. (B) Development of brown adipocytes in the inguinal WAT was prevented by the local injection of 6-hydroxydopamine (6-OHDA) compared to the contralateral injection of saline in AAVshNPY rats. (C) Norepinephrine (NE) concentration in inguinal fat pads. (D) H&E staining shows clusters of multilocular adipocytes (brown-like adipocytes) in the side of saline-treated inguinal fat pad, but barely in the 6-OHDA-treated side. Scale bar indicates 50μm. (E) UCP1 immunostaining was highly detected in the side of saline-treated inguinal fat pad, but not in the 6-OHDA-treated side. (F) 6-OHDA treatment prevented Ucp1 mRNA expression in the inguinal adipose tissue. Values are means ± SEM. n =5 rats for control group and 10 rats for NPY knockdown group. *P<0.05 compared to the saline-treated inguinal fat pad of AAVshCTL rats and #P<0.05 compared to the saline-treated inguinal fat pad of AAVshNPY rats.
DMH NPY knockdown increases energy expenditure
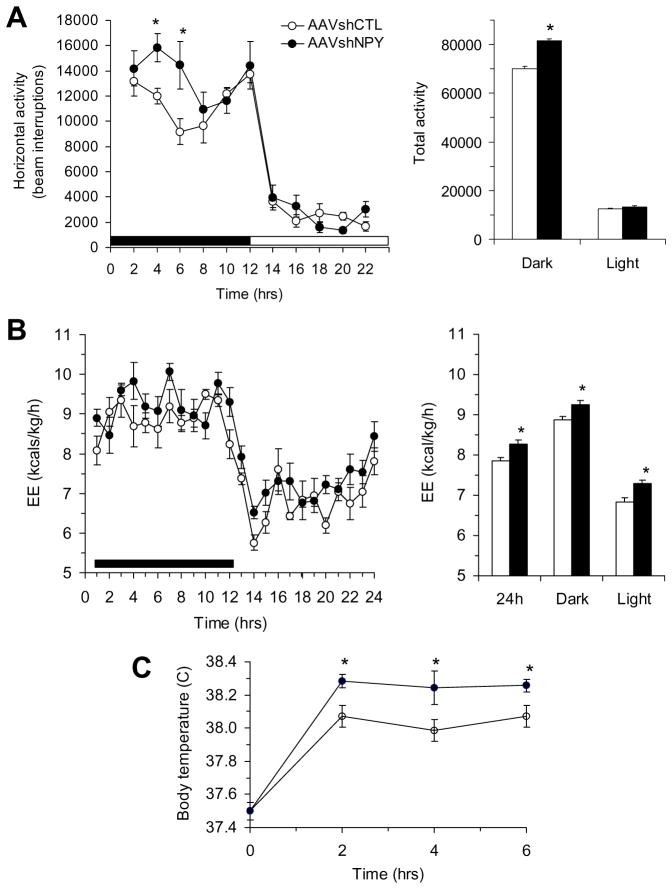
We next examined whether DMH NPY knockdown affected energy expenditure in another cohort of NPY knockdown and control rats receiving bilateral DMH viral injections. We found that NPY knockdown rats increased locomotor activity, particularly during the dark period (Fig. 5A). Moreover, indirect calorimetry revealed that energy expenditure was significantly increased during both dark and light phases of the circadian cycle in NPY knockdown rats (Fig. 5B). Since NPY knockdown rats showed brown adipocytes in inguinal fat and increased UCPl expression in this inguinal and the interscapular BAT, we tested whether DMH NPY knockdown affected thermogenesis. Although core body temperature did not differ between NPY knockdown and control rats at room temperature (24°C), NPY knockdown rats had a greater increase in thermogenic response to 6 h of cold exposure (6°C) compared to their control counterparts (Fig. 5C).
Figure 5.
Effects of DMH NPY knockdown on locomotor activity, energy expenditure and body temperature response to cold environment. (A) Locomotor activity during the 22-h period. (B) Energy expenditure during the 24 h period. (C) Body temperature during the 6-h cold exposure (6°C). Values are means ± SEM. n = 7–8 rats per group. * P<0.05 compared to AAVshCTL rats.
DISCUSSION
The DMH plays an important role in maintaining energy homeostasis. Lesions of the DMH resulted in hypophagia and reduced body weight (Bellinger and Bernardis, 2002). Disinhibition of neurons in the DMH provoked nonshivering thermogenesis and elevated core body temperature (Zaretskaia et al., 2002). Despite these observations, the neural mechanisms underlying these actions of the DMH remain undetermined. Here we establish a critical role for NPY in the DMH in regulating energy homeostasis by using AAV-mediated RNAi to knock down NPY expression in the DMH of intact rats.
We first assessed the effect of DMH NPY knockdown on regulation of body weight. Consistent with the orexigenic effect of DMH NPY (Yang et al., 2009), we found that DMH NPY knockdown significantly decreased diet-induced hyperphagia, resulted in slower weight gain on both RC and HF diets and reduced body fat mass. In addition, we noted selective effects of DMH NPY on inguinal adiposity and BAT thermogenesis. DMH NPY knockdown resulted in development of brown adipocytes in inguinal WAT, increased UCP1 expression in the inguinal and interscapular BAT and increased energy expenditure and cold-induced thermogenesis. DMH NPY knockdown promoted inguinal lipid mobilization and decreased diet-induced fat accumulation. DMH NPY knockdown also resulted in increased locomotor activity. Together, our results demonstrated that DMH NPY affects multiple aspects of energy homeostasis including food intake, body adiposity, thermogenesis, energy expenditure and physical activity.
Two types of fat, WAT and BAT, exist in mammals including in adult humans (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). While WAT stores excess calories, BAT burns fat to produce heat via nonshivering thermogenesis as a defense against cold. Both types of fat are innervated by the SNS (Bartness and Bamshad, 1998; Cannon and Nedergaard, 2004). Activation of the sympathetic innervation induces lipolysis in WAT (Fredholm and Karlsson, 1970; Weiss and Maickel, 1968), and produces thermogenesis through mitochondrial UCP1 in BAT (Cannon and Nedergaard, 2004). Sympathetic activation via treatment of β-adrenergic agonist or cold stress has also been demonstrated to cause development of brown adipocytes in white fat pads (Himms-Hagen et al., 1994; Jimenez et al., 2003; Nagase et al., 1996). In contrast, intracerebroventricular administration of NPY increases WAT lipoprotein lipase activity (suggesting increased lipid storage) and decreases BAT GDP binding activity (indicating decreased thermogenic activity) in addition to its orexigenic effect (Billington et al., 1991) and central administration of NPY also suppresses sympathetic activity in interscapular BAT in rats (Egawa et al., 1991). These observations imply that central NPY may serve as a neuromodulator of the SNS controlling both WAT lipogenesis and BAT thermogenesis. Our current findings provide support for this view and further identify DMH NPY as an important contributing factor to these effects. We found that DMH NPY knockdown resulted in development of brown adipocytes (or white into brown adipocyte transformation) in inguinal WAT and reduced inguinal fat accumulation and that sympathetic denervation prevented this brown adipocyte formation. DMH NPY knockdown also resulted in increased UCP1 expression in the interscapular BAT. These results indicate that DMH NPY normally modulates SNS signaling to influence adiposity and energy homeostasis and that knockdown of NPY expression in the DMH results in increases in peripheral sympathetic tone selectively in the inguinal fat and interscapular brown fat. As a result, Ucp1 gene expression was up-regulated in the inguinal fat and interscapular BAT of NPY knockdown rats, leading to increased thermogenesis and overall increased energy expenditure; and increases in Cpt1a gene expression with a trend for a decrease in Fas gene expression in the inguinal fat of NPY knockdown rats appear to cause increased fatty acid oxidation in adipose tissue (increased lipid mobilization), and overall reduce body adiposity.
Bamshad and colleagues (1998) have investigated the central nervous system origins of SNS outflow to WAT. By using viral transsynaptic retrograde tracer, they found that viral tracer was less detected in the DMH in animals receiving epididymal viral injection than those receiving inguinal injection (Bamshad et al., 1998), implying that the central nervous control of inguinal WAT is more DMH-related than that of epididymal WAT. In support of this view, we found that DMH NPY knockdown specifically affected lipid mobilization and brown adipocyte formation in inguinal WAT through the SNS. This suggests that DMH NPY is an important factor influencing sympathetic innervation in inguinal WAT, but not epididymal WAT. Overall, in combination with the evidence that the DMH is involved in thermoregulation (Dimicco and Zaretsky, 2007), our results suggest that NPY in the DMH may serve to modulate actions of both inguinal WAT and interscapular BAT in maintaining energy homeostasis.
WAT contains mature adipocytes for storage of lipids and other types of cells including preadipocytes, fibroblasts, pericytes, endothelial cells, and various blood cells in the stromal-vascular fraction (SVF) (Ailhaud et al., 1992). Although white fat progenitor cells have been demonstrated to reside in the adipose SVF (Tang et al., 2008), types of brown fat precursor cells in WAT or whether precursor cells in the SVF of WAT possess the ability to develop into both white and brown adipocytes is unclear. Reversible physiological transdifferentiation between WAT and BAT implies that white and brown adipocytes are mixed in most fat dopets in rodents (including inguinal WAT, Cinti, 2009). Barbatelli et al. (2010) further reported that the emergence of cold-induced brown adipocytes in mouse white fat depots (including inguinal WAT) is determined predominantly by white to brown adipocyte transdifferentiation. This trandifferentiation is thought to be directly derived from mature white adipocytes as determined by adipocytes with intermediate features between white and brown adipocytes (referred as transdifferentiating paucilocular adipocytes, Barbatelli et al., 2010). The present study did not find clear UCP1 immunoreactive paucilocular cells in inguinal fat of NPY knockdown rats as proposed above. In fact, we found numerous clusters of brown-like adipocytes surround by white adipocytes as well as various UCP1 immunoreactive unilocular adipocytes in inguinal adipose tissue of NPY knockdown rats. We further found a significant elevation of Ppar-γ expression, an essential factor for adipogenesis, in this inguinal fat tissue. Therefore, although there is still the possibility of white into brown adipocyte transdifferentiation in this rat model, our results imply that development of brown adipocytes in inguinal WAT resulting from DMH NPY knockdown may be directly derived from brown fat-like precursor cells in the SVF. To this end, the identification of such brown fat-like precursor cells or determination of this develomental origin merits further investigation.
In addition, we demonstrated a role for DMH NPY in regulation of spontaneous physical activity. We found that knockdown of NPY expression in the DMH resulted in increased locomotor activity. Based on the evidence that nonexercise activity thermogenesis from spontaneous physical activity may play a pivotal role in protection against fat gain (Levine et al., 1999), our results suggest that the effect of DMH NPY on physical activity may also contribute to its influence on body weight control. Moreover, consistent with the previous report of the nocturnal effect of DMH NPY on feeding behavior (Yang et al., 2009), DMH NPY knockdown produced a dark phase-specific effect on locomotor activity. These results provide additional evidence indicating a potential role for DMH NPY in regulation of day-night rhythms. Previous studies have suggested a role for the DMH in food-entrainable circadian behavior. Although there is some controversy over the effect of DMH lesions on food anticipatory activity (Gooley et al., 2006; Landry et al., 2006), a robust oscillation of Per1 and Per2 expression has been found in the DMH under restricted feeding (Mieda et al., 2006). Whether NPY in the DMH is involved in this circadian regulation remains to be determined.
The finding of a role for DMH NPY in glucose homeostasis is also intriguing. Previous studies have shown that the DMH contains both glucoreceptive and glucose-sensitive neurons and lesions of the DMH alter feeding response to exogenous glucose and insulin (Bellinger and Bernardis, 2002), implicating this region in the regulation of glucose homeostasis. We found that DMH NPY knockdown enhanced insulin sensitivity, improved glucose tolerance and prevented diet-induced hyperglycemia and hyperinsulinemia. These results indicate an important role of NPY in the DMH in the regulation of glucose homeostasis. Whether this is a direct result of reduced NPY expression in the DMH or a consequence of the demonstrated brown adipocytes in inguinal fat, activation of interscapular BAT, and resulting in increased thermogenesis and subsequent lean phenotypes is unclear. Nevertheless, we demonstrate that alterations in DMH NPY signaling influence insulin sensitivity and glucose homeostasis, but the mechanisms through which DMH NPY acts to affect insulin action and regulate glucose levels merit further investigation.
In summary, we demonstrate the physiological importance of DMH NPY in energy homeostasis. DMH NPY affects food intake, body adiposity, thermogenesis, energy expenditure and physical activity to regulate body weight. These results indicate that orexigenic NPY in the DMH normally serves as a key factor in maintaining energy homeostasis and also point to the DMH as a potential target site for therapies aimed at combating obesity and/or diabetes.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats were purchased from Charles River Laboratories, Inc., and were individually housed on a 12:12 h light-dark cycle (lights on at 0600h) in a temperature-controlled colony room (22–24°C) with ad libitum access to tap water and standard laboratory rodent chow, except where noted. All procedures were approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University.
AAV-mediated RNAi vector
As described previously (Yang et al., 2009), the plasmids (pAAVshNPY or pAAVshCTL) containing the two cassettes of CMV (cytomegalovirus) promoter-driven hrGFP marker and mouse U6 promoter- driven shRNA (shNPY or shCTL), flanked by AAV2 inverted terminal repeats (ITR), were constructed using the pAAV-hrGFP plasmid (Stratagene). AAV-293 cells (Stratagene) cultured in DMEM growth medium (containing 4.5 g/L glucose, 110 mg/L sodium pyruvate, and 4 mM L-glutamine, Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum were used for viral packaging. Three plasmids of pAAVshNPY (or pAAVshCTL), pHelper (carrying adenovirus-derived genes) and pAAV-RC (carrying AAV-2 replication and capsid genes) were co-transfected into AAV-293 cells according to the manufacturer’s protocol (Stratagene). Three days after transfection, cells were harvested, and the recombinant viral vector AAVshNPY (or AAVshCTL) was purified using the AAV purification kit (Virapur, LLC) and concentrated using Centricon YM-100 (Millipore) according to the manufacturers’ protocols. Virus titers were determined using quantitative PCR and ~1 × 109 particles/site were used for each virus injection.
AAV-mediated knockdown of NPY expression in the DMH
For determining the effects of the vector AAVshNPY on Npy gene expression in the DMH, 15 rats weighing 270–300 g received bilateral DMH injections of AAVshNPY and were euthanized (n =5) at 1, 2 and 4 weeks post-viral injection. Five control rats received control vector AAVshCTL injections and were euthanized at 4 weeks post-viral injection. DMH viral injection was made as previously described (Yang et al., 2009). Briefly, 0.5 μl/site (~1 ×109 particles/site) of recombinant AAV vectors were injected into the DMH with coordinates: 3.1 mm caudal to bregma, 0.4 mm lateral to midline and 8.6 mm ventral to skull surface at a rate of 0.1 μl/min for 5 min and the injector remained in place for additional 5 min before removal. After euthanization, coronal sections (14 μm) through the hypothalamus were prepared, and the sections containing hrGFP expression were examined on a Zeiss Axio Imager (Carl Zeiss MicroImaging, Inc.). Levels of Npy mRNA expression at areas of the DMH and the ARC (3.0–3.5 mm posterior to bregma (Paxinos and Watson, 2005)) were examined using in situ hybridization with 35S-labeled antisense riboprobes of NPY as previously described (Yang et al., 2009).
Effects of DMH NPY knockdown on food intake and body weight
Following determination of the effects of AAVshNPY on NPY expression, 24 rats weighing 130–150 g were randomly assigned to either bilateral DMH injections of AAVshNPY or AAVshCTL (n =12/group) as described above with coordinates: 2.3 mm caudal to bregma, 0.4 mm lateral to midline and 7.6 mm ventral to skull surface. Rats had ad libitum access to regular chow (15.8% fat, 65.6% carbohydrate, and 18.6% protein in kcal%; 3.37 kcal/g; PMI Nutrition International, LLC). Five weeks post-viral injection, half the rats from each group were switched to ad libitum access to a high-fat diet (60% fat, 20% carbohydrate, and 20% protein in kcal%; 5.2 kcal/g; Research Diets). Food intake was measured weekly and body weight was determined daily. Glucose tolerance tests were conducted 12 weeks post-viral injection. At 16 weeks post-viral injection, rats were euthanized and the adipose tissues were collected and analyzed.
Glucose tolerance test
Following an overnight fast, rats were administered oral glucose (2 g/kg) by gavage. Tail blood was sampled before and 15, 30, 45, 60 and 120 min after giving glucose for measurements of blood glucose and plasma insulin concentrations. Blood glucose levels were determined with a FreeStyle glucometer (TheraSense). Plasma insulin concentrations were determined by a rat insulin radioimmunoassay kit (Linco Research).
H&E stain and immunostaining
Following 4% paraformaldehyde fixation and paraffin embedding, 5μm sections of inguinal adipose tissue were cut via a cryostat. The sections were stained with H&E, and examined on Zeiss Axio Imager. For UCP1 immunostaining, the sections were incubated with goat anti-UCP1 antibody (Santa Cruz Biotechnology, Inc.) at 4°C overnight. After three washes, UCP1 signals were stained with Cy2-conjugated donkey anti-goat secondary antibody (Jackson ImmunoResearch) at room temperature for 60 min. After final washes, the sections were counterstained with DAPI (4′6 diamidi- no-2-phenylindole, for nuclei staining), coverslipped, and examined on Zeiss Axio Imager.
Quantitative real-time RT-PCR
Total RNA was extracted from each sample by using Trizol reagent (Invitrogen) and the remaining organic phase was saved for subsequent protein extraction according to the manufacturer’s protocols. Two-step quantitative real time RT-PCR was performed for gene expression determination. 1 μg of total RNA was reverse-transcribed into first strand cDNA using the RevertAid™ First Strand cDNA Synthesis Kits (FERMENTAS INC.), and the resulting cDNA product was then quantified using iQ SYBR Green Supermix kit (Bio-Rad Laboratories) on iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories). β-actin was used as an internal control for quantification of individual mRNA. A list of primer sets included: UCP1, Forward Primer: 5′-cgttccaggatccgagtcgcaga -3′ and Reverse Primer: 5′-tcagctcttgtcgccgggttttg -3′; PGC1a, Forward Primer: 5′-aatgcagcggtcttagcact -3′ and Reverse Primer: 5′-gtgtgaggagggtcatcgtt -3′; PPAR, Forward Primer: 5′-gcctgcggaagccctttggtgac -3′ and Reverse Primer: 5′-ttggcgaacagctgggaggactc -3′; FAS, Forward Primer: 5′-tcgagacacatcgtttgagc -3′ and Reverse Primer: 5′-tcaaaaagtgcatccagcag -3′; CPT1a, Forward Primer: 5′-ggcagaagagatggcggtcgatg -3′ and Reverse Primer: 5′-ccccaagtcaacggcagagcaga -3′.
Western blot
Proteins were separated by using 4–12% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), and transferred to an Immun-Blot PVDF membrane. The membrane was then incubated with goat anti-UCP1 antibody (1:200 dilution, Santa Cruz Biotechnology), followed by incubation with horseradish peroxidase-labeled donkey anti-goat antibody (Santa Cruz Biotechnology), and detected by using Super Signal West Pico Chemiluminescent Substrate Kit (Thermo Scientific).
Sympathetic denervation
As previously described (Rooks et al., 2005), fifteen rats weighing 100–120 g received 20 microinjections of 6-hydroxydopamine (6-OHDA, 1 μl per injection, 9 mg/ml in 0.15 M NaCl containing 1% ascorbic acid, Sigma Chemical) throughout the left inguinal fat pads. Right pads received an equal volume of vehicle injections and served as within-animal controls. Two weeks after 6-OHDA injections, 10 rats received bilateral DMH injections of AAVshNPY and 5 rats received bilateral DMH injections of AAVshCTL as described above. Twelve weeks after 6-OHDA injections, inguinal adipose tissue was evaluated.
Norepinephrine (NE) measurements
NE concentrations in the inguinal fat were determined using HPLC with electrochemical detection as previously described (Pletnikov et al., 2000; Rooks et al., 2005) with some modifications. Briefly, fat tissue was homogenized on ice by sonication in 0.1 M perchloric acid solution containing dihydroxybenzylamine as an internal standard. The extracts were centrifuged at 7,000 rpm for 15 min at 4°C and filtrated. After filtration, 15 μl of the clear homogenate were injected into the chromatographic column, the peak of NE in chromatograms of samples was identified by its retention time, and NE content was calculated and expressed as nanograms of NE per fat depot.
Locomotor activity
Fifteen rats weighing 130–150 g received bilateral DMH injections of AAVshNPY (n=8) or AAVshCTL (n=7) for examining locomotor activity, energy expenditure and thermogenic response to cold environment. Four weeks post-viral injection, locomotor activity was examined in 40×40×30 cm Plexiglas test chambers with a row of infrared monitoring sensors and a Digiscan computer for data collection and analysis (Accuscan Instruments) as previously described (Aja et al., 2006). Animals were placed into individual chambers 2 h prior to lights out and activity was monitored in 2-h intervals for 24 h with access to food and water ad libitum. The first 2-h period was considered a habituation period. Data on horizontal activity (the number of beam interruptions in 2-h intervals) during the next 22-h period were collected and analyzed.
Indirect calorimetry
Five weeks post-viral injection, rats were placed into individual Oxymax chambers attached to an Oxymax Equal Flow indirect calorimetric system (Columbus Instruments). After 5–7 days of habituation, calorimetric oxygen consumption and carbon dioxide production, daily body weight and food intake were measured on 3 consecutive days, and daily energy expenditure was analyzed.
Cold exposure
Eight weeks post-viral injection, rats were initially habituated by measurement of core body temperature using a rodent rectal probe (OAKTON Instruments) for three to five days. After habituation, rats were exposed to cold environment (6°C) for 6 h during the light period (0900–1500h). Core body temperature was measured at 0, 2, 4 and 6 h of cold exposure using a rodent rectal probe.
Statistical analysis
All values are presented as means ± SEM. Data were analyzed by StatSoft Statistica-7 software. Data for Npy mRNA expression were analyzed using one-way ANOVA. Data for body weight and food intake were analyzed using three-way ANOVA with one repeated factor. Data for blood glucose, plasma insulin, fat mass, mRNA levels and fat NE concentrations were analyzed using two-way ANOVA. Data for UCP1 mRNA levels from sympathetic denervation experiment, locomotor activity and energy expenditure were analyzed using Student’s t test (two-tailed). Data for body temperature were analyzed using two-way repeated measures ANOVA. All ANOVA’s were followed by pairwise multiple Fisher’s LSD comparisons. P < 0.05 was considered as a statistically significant difference.
Acknowledgments
This work was supported by US National Institute of Diabetes and Digestive and Kidney Diseases Grant DK074269 to S.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- Aja S, Bi S, Knipp SB, McFadden JM, Ronnett GV, Kuhajda FP, Moran TH. Intracerebroventricular C75 decreases meal frequency and reduces AgRP gene expression in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R148–154. doi: 10.1152/ajpregu.00041.2006. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol. 1998;275:R1399–1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. Faseb J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R254–260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991;260:R321–327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cinti S. Transdifferentiation properties of adipocytes in the Adipose Organ. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am J Physiol. 1991;260:R328–334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Karlsson J. Metabolic effects of prolonged sympathetic nerve stimulation in canine subcutaneous adipose tissue. Acta Physiol Scand. 1970;80:567–576. doi: 10.1111/j.1748-1716.1970.tb04824.x. [DOI] [PubMed] [Google Scholar]
- Gardiner JV, Kong WM, Ward H, Murphy KG, Dhillo WS, Bloom SR. AAV mediated expression of anti-sense neuropeptide Y cRNA in the arcuate nucleus of rats results in decreased weight gain and food intake. Biochem Biophys Res Commun. 2005;327:1088–1093. doi: 10.1016/j.bbrc.2004.12.113. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Van der Ploeg LH. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Brain Res Mol Brain Res. 1998;59:273–279. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Barbatelli G, Allevi R, Cinti S, Seydoux J, Giacobino JP, Muzzin P, Preitner F. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. Eur J Biochem. 2003;270:699–705. doi: 10.1046/j.1432-1033.2003.03422.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Scott KA, Moran TH, Bi S. Dorsomedial hypothalamic corticotropin-releasing factor mediation of exercise-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1800–1805. doi: 10.1152/ajpregu.00805.2004. [DOI] [PubMed] [Google Scholar]
- Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol Endocrinol. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase I, Yoshida T, Kumamoto K, Umekawa T, Sakane N, Nikami H, Kawada T, Saito M. Expression of uncoupling protein in skeletal muscle and white fat of obese mice treated with thermogenic beta 3-adrenergic agonist. J Clin Invest. 1996;97:2898–2904. doi: 10.1172/JCI118748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Elsevier Academic Press; San Diego, California: 2005. [Google Scholar]
- Pletnikov MV, Rubin SA, Schwartz GJ, Carbone KM, Moran TH. Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of the brain monoaminergic systems. Brain Res Dev Brain Res. 2000;119:179–185. doi: 10.1016/s0165-3806(99)00168-6. [DOI] [PubMed] [Google Scholar]
- Rooks CR, Penn DM, Kelso E, Bowers RR, Bartness TJ, Harris RB. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am J Physiol Regul Integr Comp Physiol. 2005;289:R92–102. doi: 10.1152/ajpregu.00858.2004. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Smith MS. Lactation alters neuropeptide-Y and proopiomelanocortin gene expression in the arcuate nucleus of the rat. Endocrinology. 1993;133:1258–1265. doi: 10.1210/endo.133.3.8365368. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritos NA, Elmquist JK, Mastaitis JW, Flier JS, Maratos-Flier E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology. 1998;139:4634–4641. doi: 10.1210/endo.139.11.6308. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Weiss B, Maickel RP. Sympathetic nervous control of adipose tissue lipolysis. Int J Neuropharmacol. 1968;7:395–403. doi: 10.1016/0028-3908(68)90023-3. [DOI] [PubMed] [Google Scholar]
- Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]