Abstract
Melanogenesis is the unique process of producing pigmented biopolymers that are sequestered within melanosomes, which provides color to the skin, hair and eyes of animals and, in the case of human skin, also protects the underlying tissues from UV damage. We review the current understanding of melanogenesis, focusing on factors important to the biochemistry of pigment synthesis, the biogenesis of melanosomes, signaling pathways and factors that regulate melanogenesis, intramelanosomal pH, transport and transfer of melanosomes, and pigmentary disorders related to the dysfunction of melanosome-related proteins. Although it has been known for some time that many of the factors that affect melanogenesis are derived from keratinocytes, fibroblasts, endothelial cells, hormones, inflammatory cells and nerves, a number of new factors that are involved in that regulation have recently been reported, such as factors that regulate melanosome pH and ion transport.
Keywords: eumelanin, pheomelanin, pigmentary disorders, pigmentation, regulation, skin
The color of the skin, hair and eyes of mammals (and other species) is derived from the production and distribution of pigmented biopolymers known as melanins. Melanins are synthesized within specialized membrane-bound organelles termed melanosomes that are exclusively produced in melanocytes and in retinal pigment epithelial cells. The precise migration, distribution, differentiation, proliferation and function of melanoblasts (the melanocyte precursor) and melanocytes determine the visible phenotype of those tissues, and also regulates melanocyte function in a number of minor sites in the body where they also reside, such as the inner ear, brain, heart and so on.
In human skin, melanocytes are localized at the dermal/epidermal border in a characteristic regularly dispersed pattern. Each melanocyte at the basal layer of the epidermis is functionally related to underlying fibroblasts in the dermis and to keratinocytes in the epidermis. Those three types of cells are highly interactive and communicate with each other through secreted factors and their receptors and via cell-to-cell contacts to regulate the function and phenotype of the skin.
Biochemical pathway of eumelanin & pheomelanin synthesis
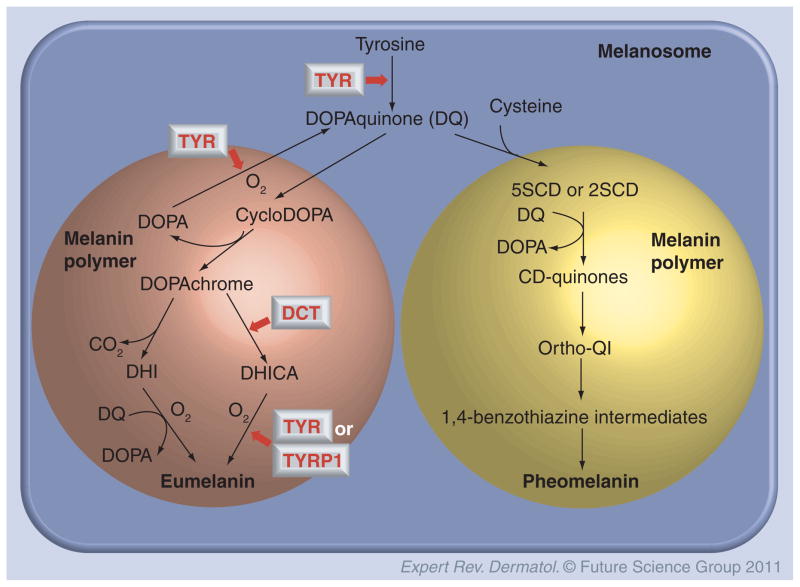
Melanins can be produced in two chemically distinct types, black-to-brown eumelanin and yellow-to-reddish-brown pheomelanin [1]. Eumelanin and pheomelanin are both derived from the common precursor dopaquinone (DQ), which is formed following the oxidation of the common amino acid L-tyrosine by tyrosinase (TYR), which involves a transient intermediate L-3,4-dihydroxyphenylalanine (DOPA) (Figure 1). Eumelanins are dark and highly polymerized while pheomelanins contain sulfur and are lighter and less polymerized.
Figure 1. Biosynthetic pathways of eumelanin and pheomelanin.
The activities of TYR, TYRP1 and DCT are involved in the production of eumelanin, but only TYR and cysteine are required for the production of pheomelanin from DQ.
CD: CysteinylDOPA; DCT: DOPAchrome tautomerase; DHI: 5,6-dihydroxyindole; DHICA: 5,6-dihydroxyindole-2-carboxylic acid; DOPA: L-3,4-dihydroxyphenylalanine; DQ: Dopaquinone; QI: Ortho-quinonimine; TYR: Tyrosinase; TYRP1: Tyrosinase-related protein-1.
Eumelanogenesis
The first step in eumelanogenesis after the production of DQ involves the spontaneous cyclization of the quinone to produce cyclodopa, which then rapidly undergoes a redox exchange with another DQ molecule to produce one molecule each of DOPAchrome and DOPA [2]. DOPAchrome is then spontaneously decomposed by decarboxylation at neutral pH to form 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) in a 70:1 ratio [3]. However, in the presence of an enzyme specifically produced in melanocytes termed DOPAchrome tautomerase (DCT), also known as TYR-related protein-2 (TYRP2), DOPAchrome undergoes tautomerization to exclusively produce DHICA [4]. The ratio of DHICA to DHI in natural eumelanins is thus determined by the activity of DCT [5]. Finally, DHI and DHICA are further oxidized and polymerized to form eumelanins (Figure 1).
Recently, the redox exchange reaction between DHI and DQ has been studied and elucidated [6]. It was concluded that during eumelanogenesis, DHI oxidation takes place by a redox exchange with DQ, although such a reaction is likely to be less efficient for DHICA. Therefore, DHICA may require oxidation to the quinone form by the direct action of TYR in humans [7] or by TYR-related protein-1 (TYRP1) in mice [8,9]. Interestingly, human TYRP1 is unable to catalyze the DHICA oxidation [10], and mouse TYR is unable to catalyze the DHI oxidation. The activities of these two TYR-related proteins, TYRP1 and DCT, greatly affect the quantity and quality of eumelanins, their ratio of DHI- to DHICA-derived subunits and the degree of their polymerization [11,12]. Fibrillar melanosomes are characteristic of eumelanin production, and result from the expression of a structural matrix protein known as Pmel17 [13], which is an amyloid protein required for the generation of the internal fibrils (those fibrils are typically lacking in melanosomes containing pheomelanin).
Pheomelanogenesis
Pheomelanogenesis proceeds through several distinctive steps at the monomer level. The first step is the reductive addition of cysteine to DQ to produce two major types of cysteinylDOPA (CD) isomers, 5SCD and 2SCD; the second step is the redox exchange between CD and DQ to produce CD-quinones and DOPA; the third step is the cyclization of CD-quinones through dehydration to form the ortho-quinonimine (QI) [14–16], after which QI is rearranged with/without decarboxylation to form the 1,4-benzothiazine intermediates that are finally polymerized to pheomelanin [17,18]. We noted above that DQ plays a pivotal role in promoting eumelanogenesis, and it should be noted here that DQ is also involved in the production of CD in the first step of pheomelanogenesis and the oxidation of CD. In other words, DQ plays pivotal roles not only in eumelanogenesis, but also in pheomelanogenesis (Figure 1). Note that the activities of TYR, TYRP1 and DCT are involved in the production of eumelanin, but by contrast, only TYR and the precursor amino acid cysteine are necessary for the production of pheomelanin.
A three-step pathway for mixed melanogenesis
Melanocytes produce mixtures of eumelanin and pheomelanin, and therefore, melanogenesis should be considered as ‘mixed melanogenesis’. Ito et al. reported a three-step pathway for mixed melanogenesis [19], wherein the total amount of melanin produced is proportional to DQ production, which is in turn proportional to TYR activity. In fact, melanogenesis proceeds in three distinct stages. The first stage is the production of CD isomers, which continues as long as the cysteine concentration is above 0.13 μM. The second stage is the oxidation of CD to produce pheomelanin, which continues as long as CD is present at concentrations above 9 μM. The last stage is the production of eumelanin, which begins only after the majority of CD and cysteine is depleted. Therefore, the ratio of eumelanin to pheomelanin is determined by TYR activity and the availability of tyrosine and cysteine in melanosomes [2].
Signaling pathways involved in regulating melanogenesis
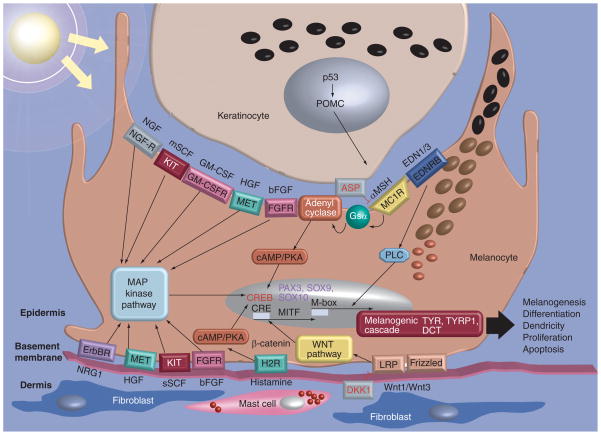
Microphthalmia-associated transcription factor (MITF) is a key regulator of mammalian pigmentation that is regulated by environmental factors, including UV, and by factors secreted from keratinocytes, fibroblasts and other cells. MITF controls not only melanogenesis, but also differentiation, dendricity, proliferation and apoptosis through various pathways and mechanisms (Figure 2) [20]. All three of the melanogenic enzymes (TYR, TYRP1 and DCT) that play key roles in melanogenesis have been demonstrated to be transcriptional targets of MITF, as are many other melanocyte-specific proteins. Promoter-reporter studies revealed that promoters of TYR [21,22], TYRP1 [23,24] and DCT [23] are activated by cotransfected MITF. The human TYR promoter contains an M-box (an extended E-box, AGTCATGTGCT), located approximately 100 bp upstream of the transcription start site, and also an E-box (ACATGTGA) at the initiator. Interestingly, the E-box is more important for the promoter function of MITF than is the M-box [21]. The p53 tumor suppressor protein was also demonstrated to participate in the increased melanogenesis that occurs after UV irradiation and acts via two mechanisms:
Figure 2. Selected factors and signaling pathway regulating melanocyte function.
Various factors that regulate melanocyte function in the skin are shown. Antagonists of receptor binding are shown in red.
Adapted from [20].
It stimulates expression of proopiomelanocortin (POMC) in epidermal keratinocytes, which in turn activates neighboring melanocytes via the melanocortin 1 receptor (MC1R);
It directly stimulates the expression of the genes encoding TYR and TYRP1 (shown in reporter assays) in melanocytes, and potential binding sites for p53 have been identified in the TYRP1 promoter [25].
Tyrosinase mRNA levels are increased via a p53-dependent mechanism upon UV irradiation of melanoma cells in culture, and p53 is required for the thymidine dinucleotide-induced increase of TYR function in mouse epidermis [26].
Other transcription factors, such as dimerization cofactor of hepatocyte nuclear factor 1 (DcoH)/hepatocyte nuclear factor 1 (HNF1) α, have also been shown to be involved in regulating TYR transcription in skin melanocytes [27]. According to Hou and coworkers, mouse embryonic melanocytes require the coordinated action of MITF and the transcription factor Sox10 for TYR induction, because both pigmentation and TYR expression in Sox10-deficient neural tube explant cultures can only be rescued by exogenous Sox10, which acts upstream of MITF, but not by exogenous MITF alone [28]. The promoter of the human TYRP1 gene also possesses an M-box (AATCATGTGCT) which is localized approximately 210 bp upstream of the start of transcription and, unlike the mouse promoter, the human promoter harbors the TATA sequence [29]. While the M-box is necessary for promoter upregulation by MITF [23], the TYRP1 promoter also binds and is activated by the paired box 3 (Pax3) transcription factor [30].
DOPAchrome tautomerase is expressed very early during melanoblast differentiation in the developing embryo, approximately when MITF begins to be expressed. The 5′-regulatory region of the DCT gene contains an M-box (GGTCATGTGCT) positioned approximately 135 nucleotides upstream of the transcription start site [31] and the promoter responds to MITF coexpression [23]. Another important transcription factor specifically involved in regulating the expression of DCT is SOX10, which also regulates the expression of MITF. A human DCT promoter–reporter construct was demonstrated to be activated by SOX10 [32,33] and SOX10 and MITF act in a synergistic manner to activate that promoter [34,35]. These data are in accordance with the observation that mouse heterozygous embryos carrying the Sox10dom mutation transiently lack DCT expression (around days 11–12) in the melanoblast lineage, and MITF alone is incapable of triggering DCT transcription in these early MITF-positive cells [33]. In a recent report, another member of the Sox family, Sox5, inhibited the Sox10-stimulated activity of the DCT promoter in melanocytes [36]. A conserved cAMP-response element (CRE)-like element in the DCT promoter might also contribute to gene expression through direct regulation by CRE-binding (CREB) protein [21]. Distal enhancers have been described for the TYR and TYRP1 genes in mice [37]. An upstream regulatory region for human TYR is reportedly located approximately −9 kb upstream from the start of transcription [38,39]. This sequence, which shows a homology with a similar distal locus found in the mouse and functions as an enhancer in transfection assays, may prove to be important for the pigment cell-specific expression of human TYR.
Hoek et al. reported a DNA microarray study using SK-Mel28 human melanoma cells that overexpress MITF, which confirmed the regulation of the above genes and identified other possible MITF target genes involved in melanosome biogenesis and function [40]. Among them, the lysosomal trafficking regulator (LYST) gene is known as a pathogenic gene involved with Chediak–Higashi syndrome (CHS), an autosomal recessive disease with immunodeficiency [41], and Hermansky–Pudlak syndrome 4 (HPS4) mutations cause Hermansky–Pudlak syndrome (HPS) [42]. Recently, Levy et al. demonstrated that expression of DICER, a central regulator of miRNA maturation, is strongly induced during melanocyte differentiation via direct transcriptional regulation by MITF. DICER plays a crucial role in melanocyte survival involving, in part, the post-transcriptional processing of miRNA-17, which leads to downregulation of the proapoptotic protein Bcl-2-interacting mediator of cell death (BIM) [43].
Melanogenesis is regulated hormonally by α-melanocyte stimulating hormone (α-MSH) and its receptor MC1R through the cAMP/PKA signaling cascade (Figure 2). The POMC gene encodes a precursor that is processed to form α-MSH, adrenocorticotropic hormone (ACTH) and β-endorphin. The POMC gene is active mainly in the pituitary, but POMC-derived peptides are also produced in keratinocytes and in melanocytes [44,45]. Although α-MSH is a potent inductor of pigmentation in mammalian skin, β-MSH and ACTH also possess melanogenic activities [44,46,47]. The hormonal stimulation by α-MSH mediated through MC1R results in increased intracellular cAMP levels and leads to the activation of PKA. This hormonal signaling pathway has specific consequences in melanocytes as the MITF promoter possesses a CRE element that is bound by CREB proteins that are phosphorylated by PKA and that then activate the transcription of MITF. Interestingly, α-MSH is able to control melanogenesis independently of MC1R, possibly by acting directly in melanosomes [48].
Another receptor that is upregulated by MITF is the endothelin receptor B (EDNRB), which serves as a receptor for endothelins, and is also regulated by MITF. Moreover, signaling by endothelins 1 and 3 activates MAPK, which then subsequently phosphorylates MITF and also stimulates MITF expression [49].
Hormonal signaling is also involved in skin responses to UV irradiation. In keratinocytes, UVB exposure induces the expression of POMC via p53, which leads to the secretion of α-MSH and the upregulation of melanogenesis via the MC1R [46,50]. In addition, the expression of corticotropin-releasing hormone (CRH) is also stimulated by UVB in melanocytes, which is mediated by the CREB–PKA signaling with consequent stimulation of POMC expression through the CRH-R1 receptor [50]. As noted above, the POMC gene has been reported to be p53-responsive following UV irradiation, and the POMC promoter contains a p53 binding site that is necessary for its highest activity [51]. The in vivo tanning response and induction of POMC mRNA are dependent on p53, further suggesting that the p53 protein is an important mediator of UV-induced melanogenesis via increased transcription of POMC [51]. However, POMC-knockout mice display normal melanin pigmentation, and some POMC mutations occur in humans who do not show any pigmentary change, although patients with several severe POMC mutations show pigmentary changes ranging from brown hair to red hair, which implies a more complex regulation of UV-induced pigmentation via induction of POMC transcription [52,53]. Hormonal regulation also underlies the switch of pigment type formation in the skin. α-MSH signaling of the MC1R receptor is inhibited by the agouti signaling protein (ASP, ASIP in humans) that can compete with α-MSH in binding to MC1R. High expression of ASP is associated with yellow pigmented bands in mouse hair. Thus, MC1R and its ligands, α-MSH and ASIP, regulate the switch between eumelanin and pheomelanin synthesis in melanocytes [44,54]. ASP was demonstrated to downregulate MITF gene expression, and hence its targets, by antagonizing the effect of α-MSH, thus favoring pheomelanogenesis by reduced production of eumelanin [55]. Interestingly, a similar profile of genes was inversely regulated by ASP and α-MSH in a microarray analysis [56]. Recently, it has been demonstrated that the receptor-binding domain of ASIP efficiently antagonizes the MSH–MC1R signaling by reducing cAMP levels, while it induces no changes in pigmentation, demonstrating that the negative regulation of differentiation by ASP signaling is independent of the cAMP–CREB pathway [54]. Other hormones, such as steroids, can also influence pigmentation [44,46], and a recent study reported that even cholesterol is capable of increasing the expression of MITF and its target genes in melanocytes, possibly through the upregulation of the CREB protein [57].
We recently reported two fibroblast-derived paracrine factors, dickkopf-related protein 1 (DKK1) and neuregulin-1 (NRG1), that regulate melanogenesis. DKK1, which is secreted at high levels by fibroblasts in the dermis of the palms/soles, suppresses melanocyte growth and function by inhibiting the Wnt/β-catenin signaling pathway [58,59]. NRG1, which is highly expressed by fibroblasts derived from darker skin, significantly increases pigmentation in a reconstructed skin model and in cultured human melanocytes. It is considered that NRG1, acting through the ErbB3 or ErbB4 receptor, leads to the activation of intracellular signaling that include the PI3K and the MAPK pathways to regulate melanogenesis [60].
Finally, the post-transcriptional regulation of melanogenesis independent of MITF should be noted, since MITF is required, but is not sufficient, to induce the expression of melanogenic enzymes [61]. In fact, Newton et al. demonstrated that the inhibition of TYR activity by resveratrol is not due to changes in MITF, but instead is explained both by direct TYR inhibition and by a post-transcriptional effect that reduces the amount of fully processed TYR. They also reported that the intracellular elevation of cAMP increases protein levels for MITF, TYR and DCT, but there is no concomitant increase in TYR or DCT mRNAs [62]. Recently, Bellei et al. demonstrated that downregulation of p38 expression leads to an increase in the levels of differentiation-associated markers such as melanin synthesis and the expression of TYR and TYR-related proteins. They also demonstrated that the mechanism involved in the p38-mediated regulation of melanogenesis is the ubiquitin–proteasome pathway, where melanogenic enzymes are degraded [63].
Regulation of melanogenesis by pH
Fuller et al. reported that melanosomes of melanocytes derived from light human skin have low levels of TYR activity and are more acidic than the melanosomes of melanocytes derived from dark human skin, which have high TYR activity [64]. Since then, the importance of melanosomal pH to skin pigmentation has been widely accepted, although the mechanisms and molecular players involved in the regulation of melanosome pH remain to be elucidated. One of the main factors involved in this pH regulation is vacuolar (V)-ATPase; its presence in melanosomes has been demonstrated by proteomic analysis [65]. Recently, Cheli et al. reported that activation of the cAMP pathway by α-MSH or forskolin increases the pH of melanosomes and regulates the expression of several V-ATPases and ion transporters [66]. Notably, cAMP upregulates the expression of V-ATPase subunits, which should result in the acidification of melanosomes. V-ATPases are a key component in the regulation of organellar pH and, more specifically, of melanosome acidification.
However, the pH of organelles is also greatly influenced by their internal ionic equilibrium, which is mainly regulated by ion pumps, such as Na+/K+-ATPase, and by ion-specific channels, such as chloride, potassium and sodium channels [67]. The role of these ion pumps and channels in melanosomes still needs further investigation. Furthermore, ion exchange through the vast family of solute carriers (SLCs) is also predicted to play a pivotal role in the regulation of melanosomal pH. In fact, a potassium- dependent sodium/calcium exchanger, the NCKX5 protein encoded by SLC24A5, has been detected in melanosomes [68], and has been demonstrated to control melanogenesis in both human and mouse melanocytes [69]. In addition, SLC45A2, formerly termed MATP or AIM1, is also localized in melanosomes and is the pathogenic gene for oculocutaneous albinism (OCA) type 4 [70]. The P protein, encoded by the OCA2 gene, is mutated in OCA type 2; this protein is related to the sodium/sulfate transporter of the SLC13 family. The P protein has also been implicated in regulating melanosomal pH. Indeed, melanosomes in melanocytes derived from pink-eyed dilution mice were reported to have a less acidic pH that did not favor melanin synthesis [71,72]. However, these observations are not in agreement with a number of other studies demonstrating that melanogenesis is stimulated by agents that increase melanosomal pH [64,73–75], or that cAMP increases melanogenesis and melanosomal pH. The exact role of these pumps, channels and exchangers in the regulation of melanosome pH and in modulating skin pigmentation needs to be further investigated.
Genome-wide association studies have revealed several new candidate genes that regulate melanogenesis, such as SLC24A4, interferon regulatory factor 4 (IRF4) and two-pore segment channel 2 (TPCN2) [76]. Among them, it has recently been revealed that human two-pore channel 2 protein (TPC2), encoded by TPCN2, localized on lysosomal membranes, induces nicotinic acid adenine dinucleotide phosphate (NAADP)-dependent calcium ion transport from acidic organelles [77]. This protein is possibly also involved in the pH regulation of melanogenesis.
Transport of melanosomes from melanocytes to keratinocytes & between keratinocytes
Melanosomes mature in a well-characterized series of steps, divided into stages I, II, III and IV, according to their structures and content of pigment. ‘Early’ melanosomes refers to stages I and II (little or no pigment), while ‘late’ melanosome refers to stages III and IV (some to complete pigment). In melanocytes, the ‘late’ melanosomes bind to microtubules and undergo actin-dependent transport toward the cell periphery, following which they are transferred to keratinocytes [78]. The sum of these transport and transfer processes are critical to the eventual distribution of melanin pigments in the skin. Thus, the intracellular transport of melanosomes is directed by microtubules (composed of α:β-tubulin dimers) and actin filaments (composed of actin monomers). Microtubules act as tracks for the transport of several intra-cellular organelles, including lysosomes and sorting vesicles [79,80]. The trafficking of sorting vesicles to their target organelles is controlled by two classes of microtubule-associated motor proteins – kinesins and cytoplasmic dyneins. Kinesins power the plus-end-directed microtubule-based motility, while cytoplasmic dyneins drive the minus end motility [81,82]. Dyneins and kinesins also have well-established roles in retrograde and in anterograde transport of melanosomes [83–86]. We have demonstrated that the movement of early melanosomes is dominated by dyneins and spectrin [87].
It has also been accepted that the Rab27a–melanophilin (Mlph)–myosin Va (Myo5a) tripartite complex is related to the capture of melanosomes within the distal, actin-rich regions of the dendrites [88–92]. Moreover, Rab27a links synaptotagmin-like protein2-a (Slp2-a) with phosphatidylserine, thereby docking melanosomes at the plasma membrane, which suggests the role of Slp2-a as a regulator of melanosome exocytosis [93].
Despite the fact that it has been known for decades that the transfer of melanosomes from melanocytes to their neighboring keratinocytes and their subsequent distribution throughout the epidermis of the skin and hair is critical to pigmentation of those tissues, the factors involved in those processes have remained stubbornly enigmatic [94,95]. Several hypotheses have been proposed to explain that transfer, including the cytophagocytosis of melanocyte dendrite tips [96], the exocytosis of melanosomes into the extracellular space and their subsequent uptake by phagocytosis into keratinocytes [97,98], and filopodia-mediated melanosome transfer [99–101]. Recently, yet another hypothesis to explain this was proposed by Singh et al., the filopodial-phagocytosis model, which would explain melanosome transfer from melanocytes to keratinocytes and even between keratinocytes under both constitutive and facultative (i.e., post-UVR) conditions [102].
Pigmentary disorders related to dysfunction of melanosome proteins
Most forms of congenital hypopigmentation derived from defects in melanin synthesis are forms of oculocutaneous albinism (OCA), which can be divided into nonsyndromic OCA and syndromic OCA. Nonsyndromic OCA arises from the absence or severe dysfunction of TYR and other key pigment factors (including P, TYRP1 and SLC45A2), which result in OCA types 1–4. Syndromic OCA, HPS, CHS and Griscelli syndrome (GS) have a number of additional features, including a bleeding diathesis, immunodeficiency and neurological dysfunction [103].
Oculocutaneous albinism
Oculocutaneous albinism is an inherited autosomal recessive disorder, which is characterized by hypomelanosis in most normally pigmented tissues, including the skin, hair and eyes, accompanied by reduced visual acuity with nystagmus and photophobia. The defects in melanin biosynthesis or transport result in a deficiency or complete absence of melanin in affected patients. OCA can be categorized into six types – OCA1A, OCA1B, OCA1-temperature-sensitive (OCA1-TS), OCA2, OCA3 and OCA4 [103].
In patients with OCA1A (TYR-negative OCA; Mendelian Inheritance in Man [MIM] 203100), TYR activity is completely lacking owing to a mutation of its encoding gene (TYR) [104]. Melanin formation does not occur throughout the patient’s life, because the first step of melanin synthesis is blocked. Therefore, the OCA1A phenotype is characterized by completely white hair, pinkish skin and red pupils [105].
Oculocutaneous albinism type 1B (yellow-mutant OCA, MIM 606952) patients completely lack detectable pigment at birth and are initially indistinguishable from patients with TYR-negative OCA (OCA1A). However, such patients rapidly develop a yellowish hair pigment in the first few years of life and then continue to slowly accumulate pigment in the hair, eyes and skin over their lifetime. In these patients, TYR activity is greatly decreased, but is not completely absent. A point mutation in the TYR gene causes a small change in TYR conformation, or causes the formation of a new splicing site associated with decreased enzyme activity [106,107].
Oculocutaneous albinism type 1-temperature-sensitive patients have white hair and skin and blue eyes at birth. At puberty, they develop progressively darker hair at cooler areas of the body (extremities) but retain white hair in the warmer areas (scalp, axilla) [108]. A missense mutation in the TYR gene of those patients causes an amino acid substitution that makes the enzyme temperature-sensitive, with very low activity at 35°C and a complete loss of activity above 37°C. The lack of correct enzyme folding results in the retention of TYR in the endoplasmic reticulum and its eventual degradation by proteasomes [109,110].
Oculocutaneous albinism type 2 (MIM 203200) patients have mutations of the P gene [104]. The P gene encodes a melanosomal membrane protein that may regulate the processing and/or transport of TYR [111]. To date, the P protein has an undefined function. The phenotypes of OCA2 have much variety. Patients with complete loss of melanin are indistinguishable from patients with OCA1A, while those with brown hair resemble OCA1B patients [105]. In addition, albinos with a P gene mutation, who would be expected to have yellow hair, may have red hair if MC1R mutations coexist [112]. It should be noted that OCA2 function may be independent of its activity on TYR folding or transport [113].
Oculocutaneous albinism type 3 (MIM 203290), also known as TYRP1 gene-related OCA or Rufous OCA, was first described in South African black individuals and is caused by mutations in the TYRP1 gene [104,111]. OCA3 patients have now also been reported in Caucasian and in Asian Indian populations [114,115]. As noted above, human TYRP1 is unable to catalyze the oxidation of DHICA in contrast to murine TYRP1 which is able to oxidize DHICA [10]. TYRP1 also serves as a type of chaperone to TYR, enhancing its stability so that decreased function of TYRP1 impacts melanin synthesis in several ways [116].
Oculocutaneous albinism type 4 (MIM 606574) results from mutations of the SLC45A2 gene and is considered one of the most common and severe types of OCA in Japan. The clinical phenotype of OCA4 reported in Japanese patients is variable and is similar to OCA2. OCA4 albinism is rare in Caucasian patients [117].
Hermansky–Pudlak syndrome
Hermansky–Pudlak syndrome (MIM 203300) is a genetically heterogeneous group of related autosomal recessive conditions described in humans and in mice. There are eight known human HPS genes that cause different subtypes of HPS (HPS 1–8) and at least 14 murine HPS genes, eight of which are orthologous to the human genes [118]. Defects in proteins encoded by these genes can affect the biogenesis and/or function of intracellular organelles found in specialized secretory cells, such as pigment cells (melanocytes and retinal pigment epithelial cells), platelets, T cells, neutrophils and lung type II epithelial cells. The organelles affected by HPS genes belong to the family of organelles known as lysosome-related organelles [118].
Hermansky–Pudlak syndrome is similar to albinism, but with additional clinical features. The pigment phenotype of HPS patients is extremely variable and can range from a minimal to a severe reduction in skin, hair and ocular pigmentation. In general, there is no tanning response after sunlight exposure in these patients [104]. Mutations in two of the HPS genes (HPS1 and HPS4) cause the most common and most severe clinical subtypes, in which affected individuals have OCA, prolonged bleeding (due to platelet storage pool deficiency) and can suffer morbidity from granulomatous colitis and premature mortality from pulmonary fibrosis [118].
The most common type of HPS (HPS1) is found in Puerto Rican patients and is caused by a 16-bp frameshift duplication in exon 15 [118]. The HPS1 protein is ubiquitous and is primarily localized to the cytosol, with a small proportion being membrane-associated.
As in other subtypes of HPS, HPS4 patients have great variability in the degree of hypopigmentation, and may present with a severe phenotype similar to that of HPS1 patients [118]. The similarity in phenotypes between HPS1 and HPS4 subtypes is explained by the finding that intracellular HPS1 and HPS4 proteins associate together in a protein complex termed biogenesis of lysosome-related organelle complex 3 (BLOC-3) [119]. The BLOC-3 complex regulates the biogenesis and/or function of lung lamellar bodies, as well as the platelet-dense bodies and melanosomes [118]. Recently, the specific interaction of BLOC-3 with Rab9a and Rab9b isoforms was reported, which suggests that BLOC-3 functions as a Rab9 effector [120].
Hermansky–Pudlak syndrome subtype 2 is caused by mutations in the AP3B1 gene that encodes the β3A subunit of the heterotetrameric adaptor protein complex AP-3. AP-3 plays a role in mediating cargo protein selection into transport vesicles and in trafficking those membrane proteins to lysosomes [118,121,122]. The HPS2 subtype may be clinically distinguished from the other forms of HPS as it is unique in causing immunodeficiency and manifests with neutropenia and susceptibility to recurrent respiratory illnesses.
The HPS3, HPS5 and HPS6 subtypes are clinically similar. They may present with ocular albinism and bruising, but without pulmonary fibrosis or colitis. The HPS3 protein associates with HPS5 and HPS6 proteins in yet another multimeric protein complex, termed BLOC-2. Immunofluorescent imaging of melanocytes derived from HPS3 patients demonstrated that molecules normally targeted to late melanosomes (e.g., the melanogenic enzymes TYR and TYRP1) are mislocalized. By contrast, the steady-state distribution of molecules targeted to early melanosomes (e.g., silver/Pmel17/gp100 and melan-a/MART1) were found to be normal. DOPA staining detected melanogenic enzymatic activity in melanosomes, suggesting that melanogenic enzymes can access melanosomes via an HPS3-independent pathway. In addition, it has been suggested that the lack of melanin detected in late melanosomes in HPS3 cells is due to the limiting quantity of another (presumably mis-trafficked) molecule [118,123]. HPS5 is a rare type of HPS [118] that encodes a cytoplasmic protein of unknown function that interacts with the HPS-6 protein [111]. All HPS5 patients have been reported to have elevated cholesterol levels, with several also having mildly elevated triglyceride levels. The significance of these elevated lipid levels, and whether they are a result of an underlying membrane trafficking defect, is not yet known [118].
The DTNBP1 gene, which is defective in HPS7, encodes the dysbindin protein. A single patient has been reported with HPS7, who presented with OCA, easy bruisability, bleeding tendency In patients with HPS8, the and a decreased lung compliance defective gene is BLOC1S3. Patients present with OCA and mild platelet dysfunction with easy bruising, epistaxis and a bleeding tendency [118]. HPS7 and HPS8 proteins are both subunits of BLOC-1. In mice, BLOC-1 functions in a transport pathway from early endosomes to maturing melanosomes, and that transport pathway is obligatory, at least for TYRP1. BLOC-2 seems to function downstream of BLOC-1 in this pathway, but AP-3 seems to regulate different cargo [124]. In mice, BLOC-1 also regulates copper-dependent TYR activity in melanosomes via ATP7A transport [125]. These new insights should be helpful to further understand the pathology of HPS7 and HPS8.
Chediak–Higashi syndrome
Chediak–Higashi syndrome (MIM 214500) is a rare autosomal recessive disorder characterized by OCA and a silvery sheen to the hair. CHS is also characterized by a bleeding tendency, progressive primary neurological impairment and severe immune deficiency due to a lack of natural killer cell function, which results in recurrent pyogenic infection. In addition, it causes a severe hemophagocytic lymphoproliferative syndrome caused by uncontrolled T-cell and macrophage activation. CHS is characterized by massive cytoplasmic lysosomal and nonlysosomal inclusions in granule-containing cells, which are probably responsible for most of the impaired functions in CHS cells. Melanocytes containing giant melanosomes seem to account for the hypopigmentation. Most cases are fatal unless treated by bone marrow transplantation. CHS has been linked to the human gene CHS1/LYST. The CHS1 protein is predicted to be a cytosolic protein with a role in vesicular transport, although no specific function for this protein has been elucidated despite intense efforts of many groups [126].
Griscelli syndrome
Griscelli syndrome is a rare autosomal recessive disorder characterized by pigmentary dilution of the skin, a silver-gray sheen to the hair, large clumps of pigment within hair shafts and the accumulation of large and abnormal end-stage melanosomes in the center of melanocytes [111]. GS has been linked with defects of the Rab27a–Mlph–MyoVa protein complex formation in melanocytes, which is important for melanosomes to connect to the actin network [111]. Patients with GS can be categorized into three types. Type 1 (GS1; MIM 214450) is manifested with albinism and severe primary neurological impairment, with developmental delay and mental retardation. It has been attributed to mutations of the myosin 5a gene (MYO5a) that encodes an organelle motor protein, Myo5a [127].
GS type 2 (GS2; MIM 607624) presents with albinism and is associated with potentially lethal immune defects and a hemophagocytic syndrome. Bone marrow transplantation is currently the only curative treatment. GS2 is caused by mutations in RAB27A, which encodes a small GTPase protein (Rab27a) that is involved in the function of the intracellular-regulated secretory pathway [127].
Griscelli syndrome type 3 (GS3; MIM 609227) results from mutations in the gene that encodes melanophilin (MLPH). Unlike GS1 and GS2, GS3 has only dermatological manifestations [127,128].
Expert commentary
Many research groups are still attempting to elucidate the mechanisms of melanogenesis, with the goal to develop cosmetics and/or treatments for hypopigmentary and/or hyperpigmentary disorders, with the ultimate goal of therapies leading to melanoma treatment. We have briefly reviewed updates in our understanding of melanogenesis and pigmentary disorders related to human melanogenesis dysfunction. There is a vast literature on these topics, and interested readers should refer to the original articles and/or recent reviews for full bibliographic citations. The transcription factor MITF plays a key role in regulating signaling pathways in melanogenesis. Identification of factors that regulate MITF, MITF target genes and the MITF-dependent transcriptome is significant to better understand the mechanisms involved. With regard to regulating MITF, factors derived not only from keratinocytes, but also from fibroblasts, play important roles. Melanosome regulation by pH is important and, in fact, pH regulation via ion transporters has been known to be related to the functions of various organelles for some time. In melanosomes, V-ATPase plays a major role in regulating pH, but other important ion transporters have also been identified. The potassium-dependent sodium/calcium exchanger NCKX5 (encoded by SLC24A5) has been implicated in regulating pigmentation, as has an NAADP-dependent calcium ion transporter TPC2 (encoded by TPCN2). Finally, P and SLC45A2 presumably play some roles as ion transporter proteins, although their specific functions are still being actively characterized.
Five-year view
In the next 5 years, our understanding of factors involved with regulating melanogenesis will be further improved since there are still many factors, proteins and genes with unknown functions that dramatically affect melanogenesis, melanosome transport and visible pigmentation of tissues. Although biochemically the mechanism of switching between eumelanogenesis and pheomelanogenesis depends on TYR activity, as well as the availability of tyrosine and cysteine in vitro, the mechanism that regulates that switch in vivo is still unsolved. Additional signaling pathways that regulate MITF will provide further understanding, not only of melanogenesis, but also of pigmentary disorders and melanoma. With regard to ion transporters that regulate melanosome pH, characterizing the functions of the P and SLA45A2 proteins will provide us with new insights about their mechanism of action.
We still understand little about melanosome transfer from melanocytes to keratinocytes and between keratinocytes compared with what we know about the critical signaling pathways involved in regulating melanocyte function. Although it remains unproven at this time, the filopodial–phagocytosis model might be an important advance in this area. There are still many cases of OCA and other pigmentary disorders that have not been associated with any genes, although at this time, only approximately 50% of the over 200 known pigment genes have been associated with human pigmentary functions, and thus many remain to be characterized. Many pathogenic genes remain to be characterized, and those should eventually provide more insights into the complex mechanisms that regulate melanogenesis in mammals.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1•.Ito S, Wakamatsu K. Chemistry of Melanins. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne JP, editors. The Pigmentary System. Physiology and Pathophysiology. 2. Blackwell Publishing; Oxford, UK: 2006. pp. 282–310. Good overview of the chemical background of melanogenesis. [Google Scholar]
- 2.Land EJ, Ito S, Wakamatsu K, et al. Rate constants for the first two chemical steps of eumelanogenesis. Pigment Cell Res. 2003;16:487–493. doi: 10.1034/j.1600-0749.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, d’Ischia M, Prota G. Tyrosinase-promoted oxidation of 5,6-dihydroxyindole-2-carboxylic acid to melanin. Isolation and characterization of oligomer intermediates. Tetrahedron. 1987;43:4203–4206. [Google Scholar]
- 4.Palumbo A, Solano F, Misuraca G, et al. Comparative action of dopachrome tautomerase and metal ions on the rearrangement of dopachrome. Biochim Biophys Acta. 1991;1115:1–5. doi: 10.1016/0304-4165(91)90003-y. [DOI] [PubMed] [Google Scholar]
- 5.Tsukamoto K, Jackson IJ, Urabe K, et al. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992;11:519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edge R, d’Ischia M, Land EJ, et al. Dopaquinone redox exchange with dihydroxyindole and dihydroxyindole-2-carboxylic acid. Pigment Cell Res. 2006;19:443–450. doi: 10.1111/j.1600-0749.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 7.Olivares C, Jiménez-Cervantes C, Lozano JA, et al. The 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem J. 2001;354:131–139. doi: 10.1042/0264-6021:3540131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiménez-Cervantes C, Solano F, Kobayashi T, et al. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1) J Biol Chem. 1994;269:17993–18001. [PubMed] [Google Scholar]
- 9.Kobayashi T, Urabe K, Winder AJ, et al. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994;13:5818–5825. doi: 10.1002/j.1460-2075.1994.tb06925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boissy RE, Sakai C, Zhao H, et al. Human tyrosinase-related protein-1 (TRP-1) does not function as a DHICA oxidase activity in contrast to murine TRP-1. Exp Dermatol. 1998;7:198–204. doi: 10.1111/j.1600-0625.1998.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 11.Lamoreux ML, Wakamatsu K, Ito S. Interaction of major coat color gene functions in mice as studied by chemical analysis of eumelanin and pheomelanin. Pigment Cell Res. 2001;14:23–31. doi: 10.1034/j.1600-0749.2001.140105.x. [DOI] [PubMed] [Google Scholar]
- 12.Ozeki H, Ito S, Wakamatsu K, et al. Chemical characterization of hair melanins in various coat-color mutants of mice. J Invest Dermatol. 1995;105:361–366. doi: 10.1111/1523-1747.ep12320792. [DOI] [PubMed] [Google Scholar]
- 13.Theos AC, Truschel ST, Raposo G, et al. The Silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Res. 2005;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napolitano A, Di Donato P, Prota G, et al. Transient quinonimines and 1,4-benzothiazines of pheomelanogenesis: new pulse radiolytic and spectrophotometric evidence. Free Radic Biol Med. 1999;27:521–528. doi: 10.1016/s0891-5849(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 15.Napolitano A, Costantini C, Crescenzi O, et al. Characterization of 1,4- benzothiazine intermediates in the oxidative conversion of 5-S-cysteinyldopa to pheomelanins. Tetrahedron Lett. 1994;35:6365–6368. [Google Scholar]
- 16.Napolitano A, Di Donato P, Prota G. New regulatory mechanisms in the biosynthesis of pheomelanins: rearrangement vs. redox exchange reaction routes of a transient 2H-1,4-benzothiazine-o-quinonimine intermediate. Biochim Biophys Acta. 2000;1475:47–54. doi: 10.1016/s0304-4165(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 17.Wakamatsu K, Ohtara K, Ito S. Chemical analysis of late stages of pheomelanogenesis: conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment Cell Melanoma Res. 2009;22:474–486. doi: 10.1111/j.1755-148X.2009.00580.x. [DOI] [PubMed] [Google Scholar]
- 18.Greco G, Wakamatsu K, Panzella L, et al. Isomeric cysteinyldopas provide a (photo) degradable bulk component and a robust structural element in red human hair pheomelanin. Pigment Cell Melanoma Res. 2009;22:319–327. doi: 10.1111/j.1755-148X.2009.00561.x. [DOI] [PubMed] [Google Scholar]
- 19•.Ito S, Wakamatsu K. Chemistry of mixed melanogenesis – pivotal roles of dopaquinone. Photochem Photobiol. 2008;84:582–592. doi: 10.1111/j.1751-1097.2007.00238.x. Interesting for the three-step pathway of mixed melanogenesis. [DOI] [PubMed] [Google Scholar]
- 20•.Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. General overview of signal pathways of melanogenesis. [DOI] [PubMed] [Google Scholar]
- 21.Bentley NJ, Eisen T, Goding CR. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994;14:7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasumoto K, Yokoyama K, Shibata K, et al. Microphthalmia associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol. 1994;14:8058–8070. doi: 10.1128/mcb.14.12.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertolotto C, Busca R, Abbe P, et al. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol Cell Biol. 1998;18:694–702. doi: 10.1128/mcb.18.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasumoto K, Yokoyama K, Takahashi K, et al. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J Biol Chem. 1997;272:503–509. doi: 10.1074/jbc.272.1.503. [DOI] [PubMed] [Google Scholar]
- 25.Nylander K, Bourdon JC, Bray SE, et al. Transcriptional activation of tyrosinase and TRP-1 by p53 links UV irradiation to the protective tanning response. J Pathol. 2000;190:39–46. doi: 10.1002/(SICI)1096-9896(200001)190:1<39::AID-PATH492>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Khlgatian MK, Hadshiew IM, Asawanonda P, et al. Tyrosinase gene expression is regulated by p53. J Invest Dermatol. 2002;118:126–132. doi: 10.1046/j.0022-202x.2001.01667.x. [DOI] [PubMed] [Google Scholar]
- 27.Schallreuter KU, Kothari S, Hasse S, et al. In situ and in vitro evidence for DCoH/HNF-1 α transcription of tyrosinase in human skin melanocytes. Biochem Biophys Res Commun. 2003;301:610–616. doi: 10.1016/s0006-291x(02)03076-0. [DOI] [PubMed] [Google Scholar]
- 28.Hou L, Arnheiter H, Pavan WJ. Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc Natl Acad Sci USA. 2006;103:9081–9085. doi: 10.1073/pnas.0603114103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sturm RA, O’Sullivan BJ, Box NF, et al. Chromosomal structure of the human TYRP1 and TYRP2 loci and comparison of the tyrosinase-related protein gene family. Genomics. 1995;29:24–34. doi: 10.1006/geno.1995.1211. [DOI] [PubMed] [Google Scholar]
- 30.Galibert MD, Yavuzer U, Dexter TJ, et al. Pax3 and regulation of the melanocyte-specific tyrosinase-related protein-1 promoter. J Biol Chem. 1999;274:26894–26900. doi: 10.1074/jbc.274.38.26894. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama K, Yasumoto K, Suzuki H, et al. Cloning of the human DOPAchrome tautomerase/tyrosinase-related protein 2 gene and identification of two regulatory regions required for its pigment cell-specific expression. J Biol Chem. 1994;269:27080–27087. [PubMed] [Google Scholar]
- 32.Britsch S, Goerich DE, Riethmacher D, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potterf SB, Mollaaghababa R, Hou L, et al. Analysis of SOX10 function in neural crest-derived melanocyte development: SOX10-dependent transcriptional control of dopachrome tautomerase. Dev Biol. 2001;237:245–257. doi: 10.1006/dbio.2001.0372. [DOI] [PubMed] [Google Scholar]
- 34.Jiao Z, Mollaaghababa R, Pavan WJ, et al. Direct interaction of Sox10 with the promoter of murine dopachrome tautomerase (DCT) and synergistic activation of DCT expression with MITF. Pigment Cell Res. 2004;17:352–362. doi: 10.1111/j.1600-0749.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 35.Ludwig A, Rehberg S, Wegner M. Melanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and MITF transcription factors. FEBS Lett. 2004;556:236–244. doi: 10.1016/s0014-5793(03)01446-7. [DOI] [PubMed] [Google Scholar]
- 36.Stolt CC, Lommes P, Hillgartner S, et al. The transcription factor Sox5 modulates Sox10 function during melanocyte development. Nucleic Acids Res. 2008;36:5427–5440. doi: 10.1093/nar/gkn527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murisier F, Beermann F. Genetics of pigment cells: lessons from the tyrosinase gene family. Histol Histopathol. 2006;21:567–578. doi: 10.14670/HH-21.567. [DOI] [PubMed] [Google Scholar]
- 38.Fryer JP, Oetting WS, King RA. Identification and characterization of a DNase hypersensitive region of the human tyrosinase gene. Pigment Cell Res. 2003;16:679–684. doi: 10.1046/j.1600-0749.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 39.Regales L, Giraldo P, Garcia-Diaz A, et al. Identification and functional validation of a 5′ upstream regulatory sequence in the human tyrosinase gene homologous to the locus control region of the mouse tyrosinase gene. Pigment Cell Res. 2003;16:685–692. doi: 10.1046/j.1600-0749.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 40•.Hoek KS, Schlegel NC, Eichhoff OM, et al. Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell Melanoma Res. 2008;21:665–676. doi: 10.1111/j.1755-148X.2008.00505.x. Interesting study on novel MITF targets. [DOI] [PubMed] [Google Scholar]
- 41.Nagle DL, Karim MA, Woolf EA, et al. Identification and mutation analysis of the complete gene for Chediak–Higashi syndrome. Nat Genet. 1996;14:307–311. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 42.Wei ML. Hermansky–Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 43••.Levy C, Khaled M, Robinson KC, et al. Lineage-specific transcriptional regulation of DICER by MITF in melanocytes. Cell. 2010;141:994–1005. doi: 10.1016/j.cell.2010.05.004. Excellent study demonstrating that DICER plays a crucial role in melanocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 45.Rousseau K, Kauser S, Pritchard LE, et al. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007;21:1844–1856. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. Good general review of hormonal signaling in melanocytes. [DOI] [PubMed] [Google Scholar]
- 47.Lazova R, Pawelek JM. Why do melanomas get so dark? Exp Dermatol. 2009;18:934–938. doi: 10.1111/j.1600-0625.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- 48.Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis controversies and new concepts. Exp Dermatol. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 49.Sato-Jin K, Nishimura EK, Akasaka E, et al. Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J. 2008;22:1155–1168. doi: 10.1096/fj.07-9080com. [DOI] [PubMed] [Google Scholar]
- 50.Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone-proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20:2539–2547. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui R, Widlund HR, Feige E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 52.Slominski A, Tobin DJ, Paus R. Does p53 regulate skin pigmentation by controlling proopiomelanocortin gene transcription? Pigment Cell Res. 2007;20:307–308. doi: 10.1111/j.1600-0749.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- 53.Clément K, Dubern B, Mencarelli M, et al. Unexpected endocrine features and normal pigmentation in a young adult patient carrying a novel homozygous mutation in the POMC gene. J Clin Endocrinol Metab. 2008;93:4955–4962. doi: 10.1210/jc.2008-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hida T, Wakamatsu K, Sviderskaya EV, et al. Agouti protein, mahogunin, and attractin in pheomelanogenesis and melanoblast-like alteration of melanocytes: a cAMP-independent pathway. Pigment Cell Melanoma Res. 2009;22:623–634. doi: 10.1111/j.1755-148X.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aberdam E, Bertolotto C, Sviderskaya EV, et al. Involvement of microphthalmia in the inhibition of melanocyte lineage differentiation and of melanogenesis by agouti signal protein. J Biol Chem. 1998;273:19560–19565. doi: 10.1074/jbc.273.31.19560. [DOI] [PubMed] [Google Scholar]
- 56.Le Pape E, Passeron T, Giubellino A, et al. Microarray analysis sheds light on the dedifferentiating role of agouti signal protein in murine melanocytes via the Mc1r. Proc Natl Acad Sci USA. 2009;106:1802–1807. doi: 10.1073/pnas.0806753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schallreuter KU, Hasse S, Rokos H, et al. Cholesterol regulates melanogenesis in human epidermal melanocytes and melanoma cells. Exp Dermatol. 2009;18:680–688. doi: 10.1111/j.1600-0625.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi Y, Itami S, Watabe H, et al. Mesenchymal-epithelial interactions in the skin: increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol. 2004;165:275–285. doi: 10.1083/jcb.200311122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi Y, Passeron T, Hoashi T, et al. Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/β-catenin signaling in keratinocytes. FASEB J. 2008;22:1009–1020. doi: 10.1096/fj.07-9475com. [DOI] [PubMed] [Google Scholar]
- 60.Choi W, Wolber R, Gerwat W, et al. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J Cell Sci. 2010;24:3102–3111. doi: 10.1242/jcs.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaggioli C, Buscà R, Abbe P, et al. Microphthalmia-associated transcription factor (MITF) is required but is not sufficient to induce the expression of melanogenic genes. Pigment Cell Res. 2003;16:374–382. doi: 10.1034/j.1600-0749.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 62.Newton RA, Cook AL, Roberts DW, et al. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol. 2007;127:2216–2227. doi: 10.1038/sj.jid.5700840. [DOI] [PubMed] [Google Scholar]
- 63.Bellei B, Maresca V, Flori E, et al. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J Biol Chem. 2010;285:7288–7299. doi: 10.1074/jbc.M109.070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuller BB, Spaulding DT, Smith DR. Regulation of the catalytic activity of preexisting tyrosinase in black and Caucasian human melanocyte cell cultures. Exp Cell Res. 2001;262:197–208. doi: 10.1006/excr.2000.5092. [DOI] [PubMed] [Google Scholar]
- 65.Basrur V, Yang F, Kushimoto T. Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J Proteome Res. 2003;2:69–79. doi: 10.1021/pr025562r. [DOI] [PubMed] [Google Scholar]
- 66••.Cheli Y, Luciani F, Khaled M, et al. αMSH and cyclic AMP elevating agents control melanosome pH through a protein kinase A-independent mechanism. J Biol Chem. 2009;284:18699–18706. doi: 10.1074/jbc.M109.005819. Excellent study of αMSH regulating melanosome pH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 68.Lamason RL, Mohideen MA, Mest JR, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 69•.Ginger RS, Askew SE, Ogborne RM, et al. SLC24A5 encodes a trans-Golgi network protein with potassium-dependent sodium–calcium exchange activity that regulates human epidermal melanogenesis. J Biol Chem. 2008;283:5486–5495. doi: 10.1074/jbc.M707521200. Good study demonstrating that SLC24A5 functions as a potassium-dependent sodium–calcium transporter in melanogenesis. [DOI] [PubMed] [Google Scholar]
- 70.Newton JM, Cohen-Barak O, Hagiwara N. Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am J Hum Genet. 2001;69:981–988. doi: 10.1086/324340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puri N, Gardner JM, Brilliant MH. Aberrant pH of melanosomes in pink-eyed dilution (p) mutant melanocytes. J Invest Dermatol. 2000;115:607–613. doi: 10.1046/j.1523-1747.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- 72.Brilliant MH. The mouse p (pink-eyed dilution) and human P genes, oculocutaneous albinism type 2 (OCA2), and melanosomal pH. Pigment Cell Res. 2001;14:86–93. doi: 10.1034/j.1600-0749.2001.140203.x. [DOI] [PubMed] [Google Scholar]
- 73.Ancans J, Tobin DJ, Hoogduijn MJ, et al. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res. 2001;268:26–35. doi: 10.1006/excr.2001.5251. [DOI] [PubMed] [Google Scholar]
- 74.Ancans J, Thody AJ. Activation of melanogenesis by vacuolar type H(+)-ATPase inhibitors in amelanotic, tyrosinase positive human and mouse melanoma cells. FEBS Lett. 2000;478:57–60. doi: 10.1016/s0014-5793(00)01795-6. [DOI] [PubMed] [Google Scholar]
- 75.Saeki H, Oikawa A. Stimulation by ionophores of tyrosinase activity of mouse melanoma cells in culture. J Invest Dermatol. 1985;85:423–425. doi: 10.1111/1523-1747.ep12277091. [DOI] [PubMed] [Google Scholar]
- 76•.Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009;18:9–17. doi: 10.1093/hmg/ddp003. Good recent review of genes regulating melanogenesis. [DOI] [PubMed] [Google Scholar]
- 77••.Calcraft PJ, Ruas M, Pan Z, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. Excellent study of NAADP-dependent calcium ion transporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu XS, Rao K, Zhang H, et al. Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 79.Lambert J, Vancoillie G, Naeyaert JM. Molecular motors and their role in pigmentation. Cell Mol Biol. 1999;45:905–918. [PubMed] [Google Scholar]
- 80.Robertson AM, Allan VJ. Brefeldin A-dependent membrane tubule formation reconstituted in vitro is driven by a cell cycle-regulated microtubule motor. Mol Biol Cell. 2000;11:941–955. doi: 10.1091/mbc.11.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vallee RB, Wall JS, Paschal BM, et al. Microtubule-associated protein 1C from brain is a two-headed cytosolic dynein. Nature. 1988;332:561–563. doi: 10.1038/332561a0. [DOI] [PubMed] [Google Scholar]
- 82.Schnapp BJ, Reese TS. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Byers HR, Yaar M, Eller MS, et al. Role of cytoplasmic dynein in melanosome transport in human melanocytes. J Invest Dermatol. 2000;114:990–997. doi: 10.1046/j.1523-1747.2000.00957.x. [DOI] [PubMed] [Google Scholar]
- 84.Hara M, Yaar M, Byers HR, et al. Kinesin participates in melanosomal movement along melanocyte dendrites. J Invest Dermatol. 2000;114:438–443. doi: 10.1046/j.1523-1747.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 85.Wu X, Hammer JA., III Making sense of melanosome dynamics in mouse melanocytes. Pigment Cell Res. 2000;13:241–247. doi: 10.1034/j.1600-0749.2000.130405.x. [DOI] [PubMed] [Google Scholar]
- 86.Vancoillie G, Lambert J, Haeghen YV, et al. Colocalization of dynactin subunits P150Glued and P50 with melanosomes in normal human melanocytes. Pigment Cell Res. 2000;13:449–457. doi: 10.1034/j.1600-0749.2000.130607.x. [DOI] [PubMed] [Google Scholar]
- 87.Watabe H, Valencia JC, Le Pape E, et al. Involvement of dynein and spectrin with early melanosome transport and melanosomal protein trafficking. J Invest Dermatol. 2007;128:162–174. doi: 10.1038/sj.jid.5701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukuda M, Kuroda TS. Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J Biol Chem. 2002;277:43096–43103. doi: 10.1074/jbc.M203862200. [DOI] [PubMed] [Google Scholar]
- 89.Goud B. How Rab proteins link motors to membranes. Nat Cell Biol. 2002;4:E77–E78. doi: 10.1038/ncb0402-e77. [DOI] [PubMed] [Google Scholar]
- 90.Strom M, Hume AN, Tarafder AK, et al. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 2002;277:25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- 91.Westbroek W, Lambert J, Bahadoran P, et al. Interactions of human Myosin Va isoforms, endogenously expressed in human melanocytes, are tightly regulated by the tail domain. J Invest Dermatol. 2003;120:465–475. doi: 10.1046/j.1523-1747.2003.12068.x. [DOI] [PubMed] [Google Scholar]
- 92.Wu X, Sakamoto T, Zhang F, et al. In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va. FEBS Lett. 2006;580:5863–5868. doi: 10.1016/j.febslet.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 93.Kuroda TS, Fukuda M. Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat Cell Biol. 2004;6:1195–1203. doi: 10.1038/ncb1197. [DOI] [PubMed] [Google Scholar]
- 94.Van Den Bossche K, Naeyaert JM, Lambert J. The quest for the mechanism of melanin transfer. Traffic. 2006;7:769–778. doi: 10.1111/j.1600-0854.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 95.Berens W, Van Den Bossche K, Yoon TJ, et al. Different approaches for assaying melanosome transfer. Pigment Cell Res. 2005;18:370–381. doi: 10.1111/j.1600-0749.2005.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seiberg M, Paine C, Sharlow E, et al. The protease-activated receptor 2 regulates pigmentation via keratinocyte–melanocyte interactions. Exp Cell Res. 2000;25:25–32. doi: 10.1006/excr.1999.4692. [DOI] [PubMed] [Google Scholar]
- 97.Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- 98.Virador VM, Muller J, Wu X, et al. Influence of alpha-melanocyte stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes. FASEB J. 2002;16:105–107. doi: 10.1096/fj.01-0518fje. [DOI] [PubMed] [Google Scholar]
- 99.Singh SK, Nizard C, Kurfurst R, et al. The silver locus product (Silv/gp100/Pmel17) as a new tool for the analysis of melanosome transfer in human melanocyte–keratinocyte co-culture. Exp Dermatol. 2008;17:418–426. doi: 10.1111/j.1600-0625.2008.00702.x. [DOI] [PubMed] [Google Scholar]
- 100.Scott G, Leopardi S, Printup S, et al. Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci. 2002;115:1441–1451. doi: 10.1242/jcs.115.7.1441. [DOI] [PubMed] [Google Scholar]
- 101.Zhang RZ, Zhu WN, Xia MY. Morphology of cultured human epidermal melanocytes observed by atomic force microscopy. Pigment Cell Res. 2004;17:62–65. doi: 10.1046/j.1600-0749.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 102••.Singh SK, Kurfurst R, Nizard C, et al. Melanin transfer in human skin cells is mediated by filopodia – a model for homotypic and heterotypic lysosome-related organelle transfer. FASEB J. 2010;24(10):3756–3769. doi: 10.1096/fj.10-159046. Interesting study of the melanosome transport model, the filopodia-phagocytosis model. [DOI] [PubMed] [Google Scholar]
- 103•.Dessinioti C, Stratigos AJ, Rigopoulos D, et al. A review of genetic disorders of hypopigmentation: lessons learned from the biology of melanocytes. Exp Dermatol. 2009;18:741–749. doi: 10.1111/j.1600-0625.2009.00896.x. Good recent general overview of pigmentary disorders. [DOI] [PubMed] [Google Scholar]
- 104.Boissy RE, Nordlund JJ. Molecular basis of congenital hypopigmentary disorders in humans: a review. Pigment Cell Res. 1997;10:12–24. doi: 10.1111/j.1600-0749.1997.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 105.Tomita Y, Suzuki T. Genetics of pigmentary disorders. Am J Med Genet C Semin Med Genet. 2004;131C:75–81. doi: 10.1002/ajmg.c.30036. [DOI] [PubMed] [Google Scholar]
- 106.Giebel LB, Tripathi RK, Strunk KM, et al. Tyrosinase gene mutations associated with type 1B (‘yellow’) oculocutaneous albinism. Am J Hum Genet. 1991;48:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 107.Matsunaga J, Dakeishi-Hara M, Tanita M, et al. A splicing mutation of the tyrosinase gene causes yellow oculocutaneous albinism in Japanese patients with a pigmented phenotype. Dermatology. 1999;199:124–129. doi: 10.1159/000018218. [DOI] [PubMed] [Google Scholar]
- 108.King RA, Townsend D, Oetting W, et al. Temperature-sensitive tyrosinase associated with peripheral pigmentation in oculocutaneous albinism. J Clin Invest. 1991;87:1046–1053. doi: 10.1172/JCI115064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giebel LB, Tripathi RK, King RA, Spritz RA. A tyrosinase gene missense in temperature-sensitive type 1 oculocutaneous albinism. A human homologue to the Siamese cat and the Himalayan mouse. J Clin Invest. 1991;87:1119–1122. doi: 10.1172/JCI115075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berson JF, Frank DW, Calvo PA, et al. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J Biol Chem. 2000;275:12281–12289. doi: 10.1074/jbc.275.16.12281. [DOI] [PubMed] [Google Scholar]
- 111.Spritz RA, Chiang PW, Oiso N, Alkhateeb A. Human and mouse disorders of pigmentation. Curr Opin Genet Dev. 2003;13:284–289. doi: 10.1016/s0959-437x(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 112.King RA, Willaert RK, Schmidt RM, et al. MC1R mutations modify the classic phenotype of oculocutaneous albinism type 2 (OCA2) Am J Hum Genet. 2003;73:638–645. doi: 10.1086/377569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sitaram A, Piccirillo R, Palmisano I, et al. Localization to mature melanosomes by virtue of cytoplasmic dileucine motifs is required for human OCA2 function. Mol Biol Cell. 2009;20:1464–1477. doi: 10.1091/mbc.E08-07-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rooryck C, Roudaut C, Robine E, et al. Oculocutaneous albinism with TYRP1 gene mutations in a Caucasian patient. Pigment Cell Res. 2006;19:239–242. doi: 10.1111/j.1600-0749.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 115.Chiang P-W, Spector E, Scheuerle A. A case of Asian Indian OCA3 patient. Am J Med Genet. 2009;149:1578–1580. doi: 10.1002/ajmg.a.32930. [DOI] [PubMed] [Google Scholar]
- 116.Kobayashi T, Imokawa G, Bennett DC, Hearing VJ. Tyrosinase stabilization by TYRP1 (the brown locus protein) J Biol Chem. 1998;273:31801–31805. doi: 10.1074/jbc.273.48.31801. [DOI] [PubMed] [Google Scholar]
- 117•.Suzuki T, Tomita Y. Recent advances in genetic analyses of oculocutaneous albinism types 2 and 4. J Dermatol Sci. 2008;51:1–9. doi: 10.1016/j.jdermsci.2007.12.008. Recent OCA2 and OCA4 overview including classification of Japanese patients with oculocutaneous albinism. [DOI] [PubMed] [Google Scholar]
- 118.Wei ML. Hermansky–Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 119.Chiang PW, Oiso N, Gautam R, et al. The Hermansky–Pudlak syndrome 1 (HPS1) and HPS4 proteins are components of two complexes, BLOC-3 and BLOC-4, involved in the biogenesis of lysosome-related organelles. J Biol Chem. 2003;278:20332–20337. doi: 10.1074/jbc.M300090200. [DOI] [PubMed] [Google Scholar]
- 120.Kloer DP, Rojas R, Ivan V, et al. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem. 2010;2285:7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chapuy B, Tikkanen R, Mühlhausen C, et al. AP-1 and AP-3 mediate sorting of melanosomal and lysosomal membrane proteins into distinct post-Golgi trafficking pathways. Traffic. 2008;9:1157–1172. doi: 10.1111/j.1600-0854.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 122.Dell’Angelica EC. AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol. 2009;21:552–559. doi: 10.1016/j.ceb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 123.Richmond B, Huizing M, Knapp J, et al. Melanocytes derived from patients with Hermansky–Pudlak syndrome types 1, 2, and 3 have distinct defects in cargo trafficking. J Invest Dermatol. 2005;124:420–427. doi: 10.1111/j.0022-202X.2004.23585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Setty SR, Tenza D, Truschel ST, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2008;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Setty SR, Tenza D, Sviderskaya EV, et al. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1146. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huizing M, Helip-Wooley A, Westbroek W, et al. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127•.Van Gele M, Dynoodt P, Lambert J. Griscelli syndrome: a model system to study vesicular trafficking. Pigment Cell Melanoma Res. 2009;22:268–282. doi: 10.1111/j.1755-148X.2009.00558.x. Good recent review of Griscelli syndrome. [DOI] [PubMed] [Google Scholar]
- 128.Ménasché G, Hsuan HH, Sanal O, et al. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5AF-exon deletion (GS1) J Clin Invest. 2003;112:450–456. doi: 10.1172/JCI18264. [DOI] [PMC free article] [PubMed] [Google Scholar]