Abstract
The complex picture of inflammation and coagulation alterations comes to life in acute stroke phases. Increasing evidence points to a strong interaction and extensive crosstalk between the inflammation and coagulation systems: the interest towards this relationship has increased since recent experimental research showed that the early administration of antithrombin III (ATIII) decreases the volume of ischemia in mice and might be neuroprotective, playing an antiinflammatory role.
We aimed to establish the extent of the relationship among markers of inflammation (S100B and IL-18) and procoagulant and fibrinolytic markers (ATIII, thrombin-antithrombin III complex (TAT), Fibrin Degradation Products (FDP), D-dimer) in 13 comatose patients affected by focal cerebral ischemia.
Plasma levels of TAT, D-dimer and FDP, IL18 and S100B were increased. IL-18 and S100B high serum levels in ischemic patients suggest an early activation of the inflammatory cascade in acute ischemic injury.
The basic principles of the interaction between inflammatory and coagulation systems are revised, from the perspective that simultaneous modulation of both coagulation and inflammation, rather than specific therapies aimed at one of these systems could be more successful in stroke therapy.
Key words: IL-18, S100B, Stroke, ATIII.
Introduction
Thrombin antithrombin complex in brain ischemia
Procoagulant or impaired fibrinolytic states as well as inflammatory reactions mediated by cytokines are very likely involved in the pathogenesis of acute ischemic stroke1 (Table 1).
Table 1. Thrombin-antithrombin III complex in literature.
| Authors | Journal | Type of study | Results | Conclusions |
|---|---|---|---|---|
| Kataoka S2 | Neurol Sci (2000) | Coagulation markers In atherothrombotic infarct (ATI), cardioembolic infarct (CEI) and lacunar infarct (LI) | CEI:>TAT, FpA, D-dimer, <ATIII ATI: >TAT, FpA, D-dimer, ATIII normal LI: No significant >TAT, d-dimer, FpA | Alterations in thrombotic and fibrinolytic markers should contribute to the clinical diagnosis of brain infarct subtypes |
| Ince B3 | Thromb Res (1999) | Coagulation and fibrinolysis in ischemic stroke of undetermined etiology | >TAT and FpA >>D-dimer | Hemostatic abnormalities have a primary role in the pathogenesis |
| Haapaniemi E4 | Acta Neurol Scand (2004) | Coagulation and fibrinolytic markers in acute and convalescent phase of ischemic stroke | >D-dimer TAT stable | Minor changes of the fibrinolytic and coagulation system activity in the patients with mild ischemic stroke |
| Ilzecka S5 | Neurol Neurochir Pol (2001) | TAT in ischemic stroke patients | >>TAT | Intense thrombinogenesis |
| Haapaniemi E6 | Acta Neurol Scand (2002) | ATIII in mild and moderate ischemic stroke | >>ATIII No correlation with etiology of stroke, any stroke risk factor or neurological scores | Natural anticoagulants levels did not deliver useful information |
FpA, fibrinopeptide A.
Toll-like receptors (TLRs) are critical components of the innate immune system that have been shown recently to mediate ischemic injury.7,8 Host-endogenous molecules associated with damaged cellls and tissues have been demostrated to activate TRLs. TLRs are located on antigen presenting cells such as B cells, dendritic cells, monocytes/macrophages and microglia.9–11 The TLRs signal through common intracellular pathways leading to transcription factor activation and the generation of cytokines and chemokines.12,13 TRL2 and TRL4 have been shown to play a role in cerebral ischemic damage. The TLR endogenous ligands HSP 60, HSP70 and HMGB1 have been found in the brain following injury;14–16 hence, these molecules may activate TLR2 and TLR4 within the brain itself, leading to the generation of inflammatory mediators such as TNFα, IL1, IL6, and I NOS, all known to be associated with stroke damage.
During focal cerebral ischemia, alterations in the hemostatic system, platelet activaction and changes in the plasminogen activactorinhibitor axis take place, particularly in the acute phase when there is an elevation of thrombin activity and a depressed fibrinolytic activity.17,18
The conversion of prothrombin into active thrombin is a key event within the coagulation cascade. Thrombin is a serine protease and an essential component in the coagulation cascade. Direct infusion of large doses of thrombin into the brains of animals causes inflammatory-cell infiltration, mesenchymal-cell proliferation, scar formation, brain oedema formation, and seizures.19–26 Thus, early oedema formation involves activation of the coagulation cascade and thrombin production.22
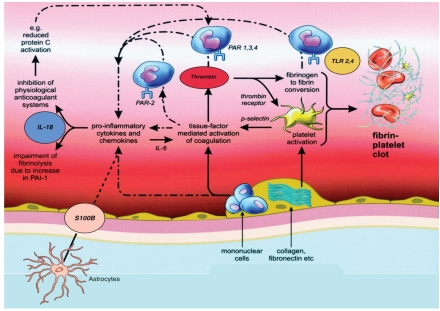
Thrombin acts on various physiological substrates (fibrinogen, protein C, platelets, etc.) and is inhibited by antithrombin III, resulting in an inactive proteinase/inhibitor complex (TAT complex) (Figure 1).
Figure 1.
Schematic rappresentation of activation of coagulation and inflammation during cerebral ischemia. Exposure of tissue factor-bearing inflammatory cells to blood results in thrombin generation and subsequent fibrinogen to fibrin conversion. Simultaneously, activation of platelets occurs, both by thrombin and by exposure of collagen (and other subendothelial platelet-activating factors) to blood. Binding of tissue factor, thrombin, and other activated coagulation proteases to specific PARs on inflammatory cells may affect inflammation by inducing release of proinflammatory cytokines (such as IL-18), which will subsequently further modulate coagulation and fibrinolysis. Fibrinogen and fibrin can directly stimulate expression of proinflammatory cytokines on mononuclaer cells and induce production of chemokines by endothelial cells and fibroblast. The effects of fibrin (and fibrinogen) on mononuclear cells are at least in part mediated by toll-like receptors (TLR2-4), which are also the receptors of endotoxin. S100 β at supraphysiological concentration can stimulate astrocytes and microglia to produce proinflammatory cytokine. Coagulation pathways are indicated by straight arrows, inflammatory mechanisms by dashed arrows.
ATIII is an important endogenous inhibitor of serine proteases that are generated within the coagulation cascade. Clinically, it has been widely used in patients admitted to ICU as treatment for ATIII deficiency, treatment of sepsis or disseminated intravascular coagulation. Recently, it has been demonstrated that ATIII may have other functions in addition to its role in inhibiting clotting. O 'Reilly et al.27 have shown its remarkable antiangiogenetic and antitumor activities. In addition, some investigators have reported that ATIII suppresses leukocyte infiltration and subsequent tissue damage in endotoxin-induced vascular injury28 or ischemia-reperfusion injury.29,30 In the field of septic shock, the suppressive effects of ATIII on inflammation have been highlighted.31,32 In patients with severe sepsis, treatment with ATIII improved lung function and prevented septic liver and kidney failure.33 ATIII has attracted a great deal of attention as a possible treatment for these various conditions; because no adverse side effects have been reported in human trials.34 However, little information is available about the effects of ATIII on cerebral ischemia and the mechanisms of its antiinflammatory action.
When generated endogenously or administered intravenously, ATIII is tethered to the wall of the microvasculature with the help of heparin and it is then bound to the active site on thrombin to suppress its functions. In addition to its hemostatic role, thrombin has the ability to induce interactions between endothelial cells and blood-flowing cells, such as leukocytes35–37 or tumor cells.38,39 Because these interactions are thought to be the initial step in leukocyte infiltration, tumor invasion, or metastasis,40,41 the antiinflammatory and antitumor properties of ATIII may be based partially on its suppressive effects on these interactions.
In addiction, recent experimental research showed that the administration of ATIII decreases the volume of ischemia in mice and might be neuroprotective.42 Detecting and monitoring activation of the coagulation system in ischemic stroke with conventional laboratory tests is difficult because the tests lack adequate sensitivity and specificity. The recent development of immunochemical assays has allowed the detection of intermediate breakdown products of fibrin formation and fibrinolysis.43,44 Among these, thrombin-antithrombin III (TAT) complex reflects thrombin activation and fibrin formation, while D-dimer marks plasmin activity and fibrinolysis.45–48
IL18
IL-18, previously termed IFN-alpha-inducing factor, is a member of the IL-1 family, a large group of pro-inflammatory cytokines. It is synthesized as an inactive 24-kDa precursor protein (pro-IL-18) that is subsequently processed by caspase-1 into its mature and biologically active form, which has a molecular weight of 18kDa. The secreted pro-IL-18 can also be processed into its active form by various extracellular enzymes constitutively expressed by leukocytes, such as proteinase-3. The active form of IL-18 induces signal transduction by binding to its heterocomplex IL-18α/β receptor expressed on diverse cell-types These include cells resident in the CNS, such as hypothalamic neurons and murine glia.
Beyond the CNS, the biological effects of IL-18 binding to IL-18R include induction of Th1 and Th2 helper T-cell responses and of cytotoxic activity by natural killer cells, in addition to propagation of intrinsic and extrinsic pathways of apoptosis. IL-18 is a ‘key’ cytokine in the CNS, controlling two distinct immunological regulatory pathways of cytotoxic and inflammatory responses under neuropathological conditions (Table 2).
Table 2. IL18 in literature.
| Authors | Journal | Type of study | Results | Conclusions |
|---|---|---|---|---|
| Zaremba J50 | Neurol Sci (2003) | IL-18 in stroke patients | Elevated serum IL18 levels in stroke patients | IL-18 is involved in stroke-induced inflammation |
| Mallat Z51 | Circulation (2001) | IL18 mRNA in stable and unstable human carotid atherosclerotic plaques | Significantly higher levels of IL-18 mRNA were found in symptomatic (unstable) plaques than asymptomatic (stable) plaques | Major role for IL-18 in atherosclerotic plaque destabilization leading to acute ischemic syndromes |
| Yuen CM52 | Circ J (2007) | IL-18 in ischemic stroke patients | Elevated IL18 levels | Evaluation of circulating IL-18 level might improve the prediction of unfavorable clinical outcome following ischemic stroke |
S100B
S100B, a low-molecular-weight calcium-binding protein, is most abundant in glial cells of the central nervous system, predominately in astrocytes. Initially these proteins were thought to be brain-specific, but later studies have shown that S100 proteins are found in other tissues, albeit in much lower concentrations than those found in the CNS53 (Table 3).
Table 3. S100 β in literature.
| Authors | Journal | Type of study | Results | Conclusions |
|---|---|---|---|---|
| Jackson RG54 | Clin Chem Lab Med (2000) | S100 β in traumatic brain injury | Levels of S-100 β fell rapidly after its release following traumatic brain injury | S100 β is released after brain insults and serum levels are positively correlated with the degree of injury |
| Piazza O55 | Minerva Chir (2005) | S100B in cardiac arrest | S100β levels are elevated after cardiac arrest | Increased levels of S100 β 12 h after a cardiac arrest might be expression of a still amendable brain damage |
| Piazza O56 | Br J Anaesth (2007) | S100B in severe sepsis | Elevated S100 β levels | An increase in S100 β does not allow the physicians to distinguish patients with severe impairment of consciousness from those with milder derangements or to prognosticate neurological recovery |
Astrocytes dominate the cellular regulation of homeostasis and are immediately activated after primary brain injury. S100B is involved specifically in this process by regulation of calcium fluxes and stimulation of astrocyte proliferation.
Intracellulary, S100B is involved in signal transduction via the inhibition of protein phoshorylation, regulation of enzyme activity and by affecting the calcium homeostasis. S100B serum level is increased in a variety of CNS disorders: in cerebral ischemia it has been associated to infarct size, neurovascular status on admission and outcome.54 Although in many instances its release may be an effect of the condition rather than the cause, it is nonetheless strongly implicated, and can be considered to be a strong candidate as a surrogate marker of CNS injury.
S100B, is involved in cytokine cascade53 (Figure 1). Secreted S100B can therefore be compared to the circulating pro-inflammatory cytokines, since, like interleukin 1 (IL1), it promotes neuronal survival at low concentrations but is neurotoxic at high levels.
Materials and Methods
We aimed to establish the relationship between markers of the inflammatory response (IL-18 and S100B) and procoagulant and fibrinolytic markers (AT III, TAT, D-dimer, FDP), in patients with cerebral ischemia, and to investigate the association of brain inflammatory markers (S100B and IL-18) and severity of coma. Only a few markers were chosen because of particular interest, but this choice cannot imply a completely exhaustive presentation of the topic.
An audit of stroke outcome and procedures was designed in ICU patients; the study protocol was reviewed and approved by the Hospital Ethics Committee (University hospital of Naples, “Federico II”).
Thirteen comatose patients following acute stroke admitted in our ICU in one year time were studied.
The inclusion criteria were the following:
no prior history of ischemic stroke (defined as a rapid developing focal neurological deficit with no apparent signs other than a vascular origin);
ICU admission within 24h after stroke onset;
lesion confirmed by neuroradiological examination (brain CT).
To assess the natural course of coagulatory changes following brain infarct, and to avoid the enrollement of patients with concurrent diseases or conditions interfering with the expression of inflammatory mediators, the following exclusion criteria were applied:
presence of infections, inflammatory, autoimmune, hamatologic or malignant diseases;
severe renal or liver failure;
thrombolisis,
abnormalities induced by deep vein thrombosis, disseminated intravascular coagulation and collagen vascular disease.
thrombolitic treatment.
In all patients, we measured the plasma levels of: thrombin-antithrombin III complex (TAT), D-dimer, Fibrin Degradation Products (FDP), percent activity of ATIII and IL-18 at the 1st and 5th (two time points) day after the stroke onset, S100B serum levels within the first 24 h (one time point). All patients were evaluated using Simplified Acute Physiologic Score (SAPS) III on admission day, Sequential Organ Failure Assessment (SOFA) and Glasgow Coma Scale (GCS) every day.
Patients were divided in two groups according to coma severity:
Group 1) GCS≤8
Group 2) GCS>8
Data were expressed as mean ± standard deviation; the significance of the differences among the groups was assessed using ANOVA test and a randomness level of P<0.05 was considered statistically significant.
Measurement of coagulation markers
Plasma concentrations of TAT were determined by enzyme-linked immunoassay (EIA)/Enzygnost TAT micro, Behring, Marburg, Germany). The concentrations of D-dimer and FDP were determined by latex photometria immunoassay (LPIA) (DIA-IATRON, Tokyo, Japan). The measurements of ATIII biological activity were performed by the amidolytic method. Venous blood was sampled from the antecubital vein and then measured within 3h after blood sampling. TAT, ATIII and D-dimer were collected in tubes containing 3.8% trisodium citrate. These plasma samples were separated by centrifugation at 2300xg for 15 min at room temperature and then stored at −80° until needed for assay.
Measurement of IL-18
The samples obtained by the antecubital venipuncture were allowed to clot at room temperature for 30 min, and after being centrifuged for 10 min, the serum was immediately frozen. IL-18 serum levels were quantified by ELISA (MBL, Naka-ku Nagoya, Japan) according to the manufacturer's instructions (normal value 155.7±51.4 pg/mL50 Value of our control group of 10 volunteers, age 28±1.3, 20% women, is 168.9±40 pg/mL).
S100B analysis
A peripheral blood sample of S100B was analyzed in duplicate using a monoclonal two-site immunoradiometric assay (LIAISON® Sangect® 100). The lower limit detection was 0.02 µg/L (normal value <0.15 µg/L).17
Results
The study group was composed of 13 patients with atherothrombotic infarcts (7 males/6 females, age 73.6±6.75 years, SAPS III 63.9±7.23). Demographic data, mortality and biochemical markers of study groups are shown in Table 4.
Table 4. Demographic data and severity scores.
| Mortality % (dead/alive) | 58/42 |
| SAPSIII | 63.9±7.23 |
| SOFA day 1 | 6.90±3.04 |
| SOFA day 5 | 6.88±3.51 |
| GCS day 1 | 7.25±3.30 |
| S100B day 1 ( g/mL) | 1.11±1.48 |
| IL18 day 1 (pg/mL) | 355.42±105.94 |
| IL18 day 5 (pg/mL) | 350.42±236.36 |
| ATIII day 1 (%) | 92.08±10.63 |
| ATIII day 5 (%) | 89.30±12.80 |
| TAT day 1 (ng/mL) | 11.63±13.67 |
| TAT day 5 (ng/mL) | 8.37±10.38 |
| D-d day 1 (ng/mL) | 4.54±6.00 |
| D-d day 5 (ng/mL) | 6.24±6.99 |
| FDP day 1 (ng/mL) | 2.09±0.70 |
| FDP day 5 (ng/mL) | 1.70±0.82 |
In all the patients plasma levels of TAT increased at all time points, but no significant differences were observed between day 1 and day 5. These constantly elevated levels were associated with increased D-dimer and FDP plasma levels at all time points.
Percent activity of ATIII was not decreased in ischemic patients on day 1 and 5 after the ischemic onset.
No correlation was found between inflammatory response (IL-18 and S100B) and procoagulant and fibrinolytic markers (ATIII, TAT, D-dimer and FDP).
IL-18 plasma levels were significantly (P<0.05) higher at day 1 (309.4±58.4 pg/mL) and at day 5 (246.7±73.6 pg/mL) compared to reference value.
S100B serum levels were significantly higher (P=0.004) in stroke patients compared to normal value.
No correlation was found between IL-18 serum levels in focal ischemic patients and GCS on admission. S100B serum levels were higher (1.5772 µg/mL) in those patients who had the poorer neurological examination (GCS≤8) (P=0.003) (Table 5).
Table 5. Biomarkers in the two GCS-related groups.
| N° of patients | S100 β | IL-18 | Mortality (dead/alive) | |
|---|---|---|---|---|
| GCS ≤ 8 | 9 | 1.5772 µg/mL | 296 pg/ML | 78% |
| GCS > 8 | 4 | 0.2005 µg/mL | 334 pg/mL | 0% |
Discussion
In our case series, in contrast to other available literature data,49–52 the increase of TAT is not followed by the reduction of plasma ATIII activity. The elevation of D-dimer and FDP without a depletion of ATIII-activity may indicate the presence of active thrombotic lesions promoting the endogenous fibrinolysis.
Increase in serum IL-18 levels of ischemic stroke patients within the first 24–48 hours after disease onset may suggest cytokine upregulation during acute phase of ischemia.
No correlation was found between IL-18 serum levels in focal ischemic patients and GCS on admission, suggesting that IL-18 could be considered an early but aspecific marker of brain injury and its role as a predictive factor for ischemic outcome should be better investigated.
High S100B serum levels are correlated with the GCS score.58 The ischemic-induced cerebral S100B release could be the result of a passive release by damaged cerebral cells and of an active release by stimulated cerebral cells initiating repair mechanisms,55–59 but S100B serum levels might not reflect the “cerebral” S100B levels because of extra-cerebral sources of this protein.60
Conclusions
Thrombin production may be directly related to thrombus formation or may reflect secondary hemostatic activation due to inflammation and endothelial injury. Antithrombin III is the most important physiological inhibitor of blood coagulation as it interferes with the clotting process at various levels and its deficiency has been suggested to have an important role in the pathogenesis and in the evolution of cerebral ischemia and might be neuroprotective, playing an anti inflammatory role.
Activation of coagulation and impairment of fibrinolysis is mediated by cytokines, with an enhancement of coagulation. IL-18 is a proinflammatory cytokine considered an important marker for monitoring severe inflammation conditions and it is involved in early stroke-induced inflammation: increase in serum IL-18 levels of ischemic stroke patients within the first 24–48 hours after disease onset suggests cytokine upregulation during acute phase of ischemia. The use of biochemical markers of cerebrovascular injury in ischemia, such as the Calcium-binding protein S100B, could improve patients care but, unfortunately, up to now, none of these biochemical markers can be considered specific enough to guide clinical decisions.
References
- 1.Vila N, Reverter JC, Yagüe J, Chamorro A. Interaction between interleukin-6 and the natural anticoagulant system in acute stroke. J Interferon Cytokine Res. 2000;20:325–9. doi: 10.1089/107999000312478. [DOI] [PubMed] [Google Scholar]
- 2.Kataoka S, Hirose G, Hori A, et al. Activation of thrombosis and fibrinolysis following brain infarction. J Neurol Sci. 2000;181:82–8. doi: 10.1016/s0022-510x(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 3.Ince B, Bayram C, Harmanci H, Ulutin T. Hemostatic markers in ischemic stroke of undetermined etiology. Thromb Res. 1999;96:169–74. doi: 10.1016/s0049-3848(99)00097-3. [DOI] [PubMed] [Google Scholar]
- 4.Haapaniemi E, Soinne L, Syrjala M. Serial changes in fibrinolysis and coagulation acivation markers in acute and convalescent phase of ischemic stroke. Act Neurol Scand. 2004;110:242–7. doi: 10.1111/j.1600-0404.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 5.Ilzecka J, Stelmasiak Z, Dobosz B. Thrombogenesis in patients with ischemic stroke. Neurol Neurochir Pol. 2001;35:793–801. [PubMed] [Google Scholar]
- 6.Haapaniemi E, Tatlisumak T, Soinne L, et al. Natural anticoagulants (antithrombin III, protein C, and protein S) in patients with mild to moderate ischemic stroke. Acta Neurol Scand. 2002;105:107–14. doi: 10.1034/j.1600-0404.2002.1o112.x. [DOI] [PubMed] [Google Scholar]
- 7.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–21. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 8.Marsh BJ, Williams-Karnesky, Stenzel-Poore MP. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience. 2009;158:1007–20. doi: 10.1016/j.neuroscience.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Jack CS, Arbour N, Manusow J, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–30. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 11.Bsibsi M, Persoon-Deen C, Verwer RW, et al. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–95. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 12.Vogel SN, Fitzgerald KA, Fenton MJ. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Intervent. 2003;3:466–77. doi: 10.1124/mi.3.8.466. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 14.Kinouchi H, Sharp FR, Hill MP, et al. Induction of 70-kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1993;13:105–15. doi: 10.1038/jcbfm.1993.13. [DOI] [PubMed] [Google Scholar]
- 15.Faraco G, Fossati S, Bianchi ME, et al. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem. 2007;103:590–603. doi: 10.1111/j.1471-4159.2007.04788.x. [DOI] [PubMed] [Google Scholar]
- 16.Lehnardt S, Schott E, Trimbuch T, et al. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–31. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 18.Kataoka S, Hirose G, Hori A, et al. Activation of thrombosis and fibrinolysis following brain infarction. J Neurol Sci. 2000;181:82–8. doi: 10.1016/s0022-510x(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 19.Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–6. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- 20.Xi G, Wagner KR, Keep RF. The role of blood clot formation on early edema development following experimental intracerebral hemorrhage. Stroke. 1998;29:2580–6. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 21.Lee KR, Colon GP, Betz AL, et al. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996;84:91–6. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- 22.Lee KR, Kawai N, Kim S, et al. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86:272–8. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- 23.Lee KR, Betz AL, Keep RF, et al. Intracerebral infusion of thrombin as a cause of brain edema. J Neurosurg. 1995;83:1045–50. doi: 10.3171/jns.1995.83.6.1045. [DOI] [PubMed] [Google Scholar]
- 24.Lee KR, Betz AL, Kim S, et al. The role of the coagulation cascade in brain edema formation after intracerebral hemorrhage. Acta Neuochir. 1996;138:396–400. doi: 10.1007/BF01420301. [DOI] [PubMed] [Google Scholar]
- 25.Lee KR, Drury I, Vitarbo E, Hoff JT. Seizures induced by intracerebral injection of thrombin: a model of intracerebral hemorrhage. J Neurosurg. 1997;87:73–8. doi: 10.3171/jns.1997.87.1.0073. [DOI] [PubMed] [Google Scholar]
- 26.Nishino A, Suzuki M, Othani H. Thrombin may contribute to the pathophysiology of central nervous system injury. J Neurotrauma. 1993;10:167–79. doi: 10.1089/neu.1993.10.167. [DOI] [PubMed] [Google Scholar]
- 27.O'Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science. 1999;285:1926–8. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 28.Okajima K. Antithrombin prevents endotoxin-induced pulmonary vascular injury by inhibiting leukocyte activation. Blood Coagul Fibrinolysis. 1998;9:S25–S37. [PubMed] [Google Scholar]
- 29.Harada N, Okajima K, Kishimoto S, et al. Antithrombin reduces ischemia/reperfusion injury of rat liver by increasing the hepatic level of prostacyclin. Blood. 1999;93:157–64. [PubMed] [Google Scholar]
- 30.Ostrovsky L, Woodman RC, Payne D, et al. Antithrombin III prevents and rapidly reverses leukocyte recruitment in ischemia/reperfusion. Circulation. 1997;96:2302–10. doi: 10.1161/01.cir.96.7.2302. [DOI] [PubMed] [Google Scholar]
- 31.Minnema MC, Chang AC, Jansen PM, et al. Recombinant human antithrombin III improves survival and attenuates inflammatory responses in baboons lethally challenged with Escherichia coli. Blood. 2000;95:1117–23. [PubMed] [Google Scholar]
- 32.Dickneite G, Leithauser B. Influence of antithrombin III on coagulation and inflammation in porcine septic shock. Arterioscler Thromb Vasc Biol. 1999;19:1566–72. doi: 10.1161/01.atv.19.6.1566. [DOI] [PubMed] [Google Scholar]
- 33.Inthorn D, Hoffmann JN, Hartl WH, et al. Effect of antithrombin III supplementation on inflammatory response in patients with severe sepsis. Shock. 1998;10:90–6. doi: 10.1097/00024382-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Carrell RW. How serpins are shaping up. Science. 1999;285:1861–63. doi: 10.1126/science.285.5435.1861. [DOI] [PubMed] [Google Scholar]
- 35.Vu TH, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–68. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman BJ, Paulson JC, Arrhenius TS, et al. Thrombin receptor peptide-mediated leukocyte rolling in rat mesenteric venules: roles of P-selectin and sialyl Lewis X. Am J Physiol. 1994;267:H1049–H1053. doi: 10.1152/ajpheart.1994.267.3.H1049. [DOI] [PubMed] [Google Scholar]
- 37.Toothill VJ, Van-Mourik JA, Niewenhuis HK, et al. Characterization of the enhanced adhesion of neutrophil leukocytes to thrombin-stimulated endothelial cells. J Immunol. 1990;145:283–91. [PubMed] [Google Scholar]
- 38.Klepfish A, Greco MA, Karpatkin S. Thrombin stimulates melanoma tumorcell binding to endothelial cells and subendothelial matrix. Int J Cancer. 1993;53:978–82. doi: 10.1002/ijc.2910530620. [DOI] [PubMed] [Google Scholar]
- 39.Wojtukiewicz MZ, Tang DG, Ciarelli JJ, et al. Thrombin increases the metastatic potential of tumor cells. Int J Cancer. 1993;54:793–806. doi: 10.1002/ijc.2910540514. [DOI] [PubMed] [Google Scholar]
- 40.Eugene CB. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 41.Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990;62:3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- 42.Cuomo O, Pignataro G, Gala R, et al. Antithrombin reduces ischemic volume, ameliorates neurologic deficits, and prolongs animal survival in both transient and permanent focal ischemia. Stroke. 2007;38:3272–9. doi: 10.1161/STROKEAHA.107.488486. [DOI] [PubMed] [Google Scholar]
- 43.Fisher M, Meiselman HJ. Hemorheological factors in cerebral ischemia. Stroke. 1991;22:1164–9. doi: 10.1161/01.str.22.9.1164. [DOI] [PubMed] [Google Scholar]
- 44.Tohgi H, Kawashima M, Tamura K, Suzuki H. Coagulation-fibrinolysis abnormalities in acute and chronic phases of cerebral thrombosis and embolism. Stroke. 1990;21:1663–7. doi: 10.1161/01.str.21.12.1663. [DOI] [PubMed] [Google Scholar]
- 45.Altes A, Abellan MT, Mateo J, et al. Hemostatic disturbances in acute ischemic stroke: A study of 86 patients. Acta Haematol. 1995;94:10–5. doi: 10.1159/000203964. [DOI] [PubMed] [Google Scholar]
- 46.Fon EA, Mackey A, Cote R, et al. Hemostatic marker in acute transient ischemic attacks. Stroke. 1994;25:282–6. doi: 10.1161/01.str.25.2.282. [DOI] [PubMed] [Google Scholar]
- 47.Francis CW, Doughney K, Brenner B, et al. Increased immunoreactivity of plasma after fibrinolytic activation in anti-DD ELISA system. Circulation. 1989;79:666–73. doi: 10.1161/01.cir.79.3.666. [DOI] [PubMed] [Google Scholar]
- 48.Marder VJ. What does the “dimertest” test? Circulation. 1990;82:1514–5. doi: 10.1161/01.cir.82.4.1514. [DOI] [PubMed] [Google Scholar]
- 49.Felderhoff-Mueser U, Schmidt OI, Oberholzer A, et al. IL-18: a key player in neuroinflammation and neurodegeneration? Trends Neurosci. 2005;28:487–93. doi: 10.1016/j.tins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Zaremba J, Losy J. Interleukin-18 in acute ischaemic stroke patients. Neurol Sci. 2003;24:117–24. doi: 10.1007/s10072-003-0096-0. [DOI] [PubMed] [Google Scholar]
- 51.Mallat Z, Corbaz A, Scoazec A, et al. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 52.Yuen CM, Chiu CA, Chang LT, et al. Level and value of interleukin-18 after acute ischemic stroke. Circ J. 2007;71:1691–6. doi: 10.1253/circj.71.1691. [DOI] [PubMed] [Google Scholar]
- 53.Kleindienst A, Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma. 2006;23:1185–200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- 54.Jackson RG, Samra GS, Radcliffe J, et al. The early fall in levels of S-100 beta in traumatic brain injury. Clin Chem Lab Med. 2000;38:1165–7. doi: 10.1515/CCLM.2000.179. [DOI] [PubMed] [Google Scholar]
- 55.Piazza O, Cotena S, Esposito G, et al. S100B is a sensitive but not specific prognostic index in comatose patients after cardiac arrest. Minerva Chir. 2005;60:477–80. [PubMed] [Google Scholar]
- 56.Piazza O, Russo E, Cotena S, et al. Elevated S100B levels do not correlate with the severity of encephalopathy during sepsis. Br J Anaesth. 2007;99:518–21. doi: 10.1093/bja/aem201. [DOI] [PubMed] [Google Scholar]
- 57.Cotena S, Piazza O, Storti M. The S100B protein and traumatic brain injury. J Neurosurg. 2006;104:435–6. doi: 10.3171/ped.2006.104.6.435. [DOI] [PubMed] [Google Scholar]
- 58.Feinberg WM, Bruck DC, Ring ME, Corrigan JJ. Hemostatic markers in acute stroke. Stroke. 1989;20:592–7. doi: 10.1161/01.str.20.5.592. [DOI] [PubMed] [Google Scholar]
- 59.Jauch EC, Lindsell C, Broderick J, et al. NINDS rt-PA Stroke Study Group. Association of serial biochemical markers with acute ischemic stroke. Stroke. 2006;37:2508–13. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- 60.Kögel D, Peters M, König HG, et al. S100B potently activates p65fc-Rel transcriptional complex in hippocampal neurone: clinical implications for the role of S100B in excitotoxic brain injury. Neuroscience. 2004;127:913–20. doi: 10.1016/j.neuroscience.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Piazza O, Russo E, Cotena S, et al. Elevated S100B levels do not correlate with the severity of encephalopathy during sepsis. Br J Anaesth. 2007;99:518–21. doi: 10.1093/bja/aem201. [DOI] [PubMed] [Google Scholar]